Back to Journals » Drug Design, Development and Therapy » Volume 17
Effect of Intraoperative Intravenous Lidocaine on Postoperative Delirium in Elderly Patients with Hip Fracture: A Prospective Randomized Controlled Trial
Authors Li X, Wu J, Lan H, Shan W, Xu Q , Dong X, Duan G
Received 24 September 2023
Accepted for publication 11 December 2023
Published 15 December 2023 Volume 2023:17 Pages 3749—3756
DOI https://doi.org/10.2147/DDDT.S437599
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Tin Wui Wong
Xiaofen Li,1 Jimin Wu,2 Haiyan Lan,2 Weifeng Shan,2 Qiaomin Xu,2 Xiaoli Dong,2 Gongchen Duan2
1Nursing Department, Lishui People’s Hospital, The Sixth Affiliated Hospital of Wenzhou Medical University, Lishui, People’s Republic of China; 2Department of Anesthesiology, Lishui People’s Hospital, The Sixth Affiliated Hospital of Wenzhou Medical University, Lishui, People’s Republic of China
Correspondence: Gongchen Duan, Department of Anesthesiology, Lishui People’s Hospital, The Sixth Affiliated Hospital of Wenzhou Medical University, No. 1188, Liyang Street, Lishui, Zhejiang, 323000, People’s Republic of China, Tel/Fax +8618957093030, Email [email protected]
Purpose: This study was performed to evaluate the effects of intraoperative intravenous lidocaine on postoperative delirium in elderly patients with hip fracture.
Patients and methods: In total, 100 elderly patients undergoing hip fracture surgery were randomized to the lidocaine group (Group L) or saline (control) group (Group C). Before anesthetic induction, Group L received lidocaine at 1 mg/kg for more than 10 minutes followed by continuous infusion at 1.5 mg/kg/h until the end of surgery. Group C received normal saline, and the injection methods were consistent with those in Group L. General anesthesia was induced with propofol, sufentanil, and cis-atracurium. Anesthesia was maintained by propofol and remifentanil. The primary outcome was the incidence of postoperative delirium in the first 7 postoperative days. The secondary outcomes included the severity of delirium, onset and duration of delirium, emergence agitation, adverse events, total propofol dose, intraoperative opioid dosage, length of post-anesthesia care unit stay, extubation time, and patient satisfaction with postoperative pain management.
Results: All 100 patients completed the study. The incidence of postoperative delirium was lower in Group L than in Group C (14% vs 36%, P = 0.011). The delirium severity scores were lower in Group L (3 [3– 4]) than in Group C (4 [4– 5]) (P = 0.017). In addition, the incidences of hypertension, tachycardia, and emergence agitation were significantly lower in Group L than in Group C. No cases of local anesthetic toxicity occurred in either group.
Conclusion: Patients received lidocaine at 1 mg/kg for more than 10 minutes followed by continuous infusion at 1.5 mg/kg/h until the end of surgery, which can reduce the incidence of postoperative delirium in elderly patients undergoing hip fracture. In addition, the used regimen of lidocaine would not increase the risk of local anesthetic toxicity.
Keywords: lidocaine, postoperative delirium, hip fracture, elderly patients
Introduction
Hip fracture is a common type of fracture in elderly patients, and most patients with hip fracture receive surgical treatment. Postoperative delirium (POD) is an acute neuropsychiatric syndrome that affects postoperative recovery, increases the hospital stay and medical costs, and even increases the risk of mortality.1 The prevalence of POD in elderly patients undergoing surgery varies from 20% to 45%.2 Patients undergoing hip fracture surgery are at higher risk of POD than patients undergoing other types of surgery.3 This may be related to their older age, higher incidence of comorbidities, and greater physical weakness.4 Avoiding perioperative benzodiazepines, BIS-guided anesthesia, and treating pain are effective strategies to reduce POD.5 While intraoperative hypotension, postoperative persistent hypoxia or hypercapnia are considered as risk factors and predisposing factors for POD.6 Lidocaine is a commonly used amide local anesthetic, but it also has special analgesic and anti-inflammatory properties.7 Intravenous lidocaine is associated with lower opioid demand, reduced nausea and vomiting, and a shorter time to resumption of diet.8 However, few studies have focused on the effect of lidocaine on postoperative cognitive function. Among very early studies, some scholars demonstrated the cerebral protective effect of lidocaine in a feline model of cerebral artery gas embolism.9 Additionally, clinical studies have proven that lidocaine has a protective effect on patients’ postoperative cognitive function.10 However, the effect of systemic lidocaine on POD in older patients undergoing major surgery remains inconclusive. This study was performed to evaluate the effects of intraoperative intravenous lidocaine on POD in elderly patients undergoing hip fracture surgery.
Methods
Ethics and Registration
This study was registered in the Chinese Clinical Trial Registry (www.chictr.org.cn, registration number: ChiCTR2300072987). The study was approved by the medical ethics committee of The Sixth Affiliated Hospital of Wenzhou Medical University (approval no. 2023–095), and all enrolled patients provided written informed consent.
Patient Inclusion and Exclusion Criteria
This study involved 106 elderly patients who were admitted to The Sixth Affiliated Hospital of Wenzhou Medical University (Lishui People’s Hospital) for hip fracture surgery from June 2023 to September 2023.
The inclusion criteria were (1) elective hip arthroplasty or proximal femoral nail antirotation, (2) age of 65 to 90 years, (3) an American Society of Anesthesiologists physical status of II or III, and (4) a body mass index of 18 to 30 kg/m2. The exclusion criteria were (1) preoperative mental disorders, cognitive dysfunction or communication difficulties, (2) obvious sinus bradycardia (heart rate of <50 beats per minute) or other serious cardiovascular diseases, (3) symptomatic cerebrovascular disease (such as previous stroke), (4) history of liver and kidney dysfunction, and (5) allergy to lidocaine.
Randomization and Masking
An independent anesthesiologist randomly divided the patients into 2 groups of 53 patients each using computer-generated random numbers (Group L and Group C). We discreetly placed the randomization results in envelopes until the end of the study. Both lidocaine and saline are colorless liquids, and they were digitally encoded so that the researchers who were responsible for postoperative follow-up and data processing were blinded to the group allocation during the whole study period. All patients were also blinded to the group allocation.
Perioperative Management and Interventions
No premedication was administered. Upon arrival in the operating room, all patients underwent pulse oxygen saturation, noninvasive blood pressure, and electrocardiogram monitoring using a Carestation 620 A2 monitor (GE Healthcare, Chicago, IL, USA). The patients were also monitored with a bispectral index (BIS) sensor (Canwell Medical Co., Ltd., Jinhua, Zhejiang, China). After the patients had lain down for 10 minutes, their vital signs were set as the baseline values (ie, when the patients were quiet and the values were stable). Before anesthetic induction, Group L was given 1 mg/kg (0.15 mL/kg) 1% lidocaine (Hunan Kelun Medicine Trade Co., Ltd., Hunan, China); the infusion time was 10 minutes, and the lidocaine was continuously infused with a micropump for 0.15 mL/(kg·h) until the end of surgery. Group C was given a normal saline bolus and infusion, and the volume and rate changes were the same as those in Group L. Anesthesia was induced with 0.3 to 0.4 μg/kg sufentanil (Yichang Humanwell Pharmaceutical Co., Ltd., Yichang, China), 0.2 mg/kg cis-atracurium (Jiangsu Hengrui Pharmaceutical Co., Ltd., Jiangsu, China) and 1.0 to 1.5 mg/kg propofol (Beijing Fresenius Kabi Pharmaceutical Co., Ltd., Beijing, China). When the BIS value was <60, tracheal intubation was performed.
All patients underwent controlled mechanical ventilation, and the target end-expiratory carbon dioxide partial pressure was set at 35 to 45 mmHg. Anesthesia was maintained with propofol (4–12 mg/kg/h) and remifentanil (0.1–0.3) μg/kg/min (Yichang Humanwell Pharmaceutical Co., Ltd., Yichang, China), and the depth of anesthesia was maintained to achieve a BIS value of 40 to 60. Vasoactive drugs were used as needed intraoperatively to maintain the heart rate and blood pressure within 20% of the pre-induction baseline. After surgery, the patients were transferred to the post-anesthesia care unit (PACU) for close observation. Patient-controlled intravenous analgesia was used for postoperative analgesia, and the total amounts of sufentanil and tropisetron were 1.5 μg/kg and 10 mg, respectively, diluted to 100 mL with normal saline. The background infusion rate was 2 mL/h, and the method of rescue analgesia was a bolus injection of 2 mL with a locking interval of 15 min. Adverse events recorded during the observation period included hypotension, bradycardia, hypertension, tachycardia, and local anesthetic toxicity. If these events occurred, we treated them with intravenous injection of ephedrine, urapidil, atropine, and esmolol, respectively. In patients with local anesthetic toxicity, emergency treatment was carried out according to the established rescue process.
Outcomes
Primary Outcome
The primary outcome was the incidence of POD in the first 7 days after surgery. The Confusion Assessment Method (CAM), a screening tool suitable for non-psychiatrically trained clinicians, is widely used for POD assessment.11 Delirium can be diagnosed via interview using the CAM algorithm with the following four criteria: (1) acute onset or fluctuating course, (2) inattention, (3) disorganized thinking, and (4) altered level of consciousness. The investigator assessed the patient for POD twice a day (08:00 and 20:00). Delirium was diagnosed if both criteria 1 and 2 and either 3 or 4 were present.
Secondary outcomes
The secondary outcomes were as follows.
- The severity of delirium was assessed using the CAM-Severity (CAM-S).12 The sum of the CAM-S scores ranges from 0 to 7 (7 = most severe). Each symptom of delirium (except fluctuation) was rated as absent (0), mild (1), or marked (2). Acute onset or fluctuation was rated as absent (0) or present (1). The onset and duration of delirium were also recorded.
- Emergence agitation was assessed using the Riker Sedation–Agitation Scale during emergence. A score of >4 was defined as emergence agitation.13
- Perioperative adverse events were defined as hypotension (mean arterial pressure of ≤70% of baseline and/or <65 mmHg), hypertension (mean arterial pressure of >120% of baseline), bradycardia (heart rate of ≤45 beats/min), and tachycardia (heart rate of >120% of baseline).14 Adverse events of lidocaine were recorded using a daily questionnaire survey of local anesthetic toxicity symptoms, including hearing loss, metallic taste, slurred speech, perioral numbness, tinnitus, dizziness, and tremor.
- Two days after surgery, the patients’ satisfaction with pain management was assessed using an 11-point Likert scale (0 = completely dissatisfied, 10 = completely satisfied).
- Intraoperative and postoperative characteristics included the length of PACU stay, total propofol dose, extubation time, intraoperative opioid dosage, duration of surgery, blood loss, amount of intraoperative fluid, and urine volume.
Sample Size and Statistical Analysis
Forty patients were included in the pre-experiment. According to the results of the pre-experiment, the incidence of POD within 7 days after surgery was 40% in Group C and 15% in Group L. A sample size of 94 patients was needed to provide a power of 0.8 and a significance of 0.05. Considering a 10% attrition rate, we recruited 106 patients for the study (53 patients in each group).
The data processing and analyses were performed using SPSS 20.0 statistical software (IBM Corp., Armonk, NY, USA). All data are presented as mean ± standard deviation, number (percentage), or median (interquartile range) as appropriate. The measurement data were verified for normality using the Shapiro–Wilk test. Normally distributed data were compared between the groups using an independent-samples t-test, and non-normally distributed data were compared between the groups using the Mann–Whitney U-test. The chi-square test or Fisher’s exact test was used for comparison of enumeration data between the groups. The significance level for this analysis was set at α = 0.05.
Results
In total, 128 patients were initially screened for eligibility, and 22 were excluded. Six patients subsequently refused to undergo postoperative follow-up. Finally, data for 50 patients in Group L and 50 patients in Group C were analyzed (Figure 1).
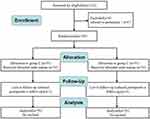 |
Figure 1 Flow chart of this study. |
Patient Characteristics and Operation Details
The patient characteristics and operation details were statistically similar between the two groups (P > 0.05) (Table 1).
 |
Table 1 Patient Characteristics and Operation Details |
The incidence of POD was lower in Group L than in Group C (14% vs 36%, P = 0.011). The delirium severity scores were lower in Group L (3 [3–4]) than in Group C (4 [4–5]) (P = 0.017). The incidence of emergence agitation was lower in Group L than in Group C (14% vs 34%, P = 0.019). There were no statistically significant differences in the onset time of delirium or duration of delirium between the two groups (P > 0.05) (Table 2).
 |
Table 2 Postoperative Delirium Related Outcomes |
The total dosage of sufentanil, remifentanil, and propofol was significantly lower in Group L than in Group C (P < 0.05). The patient satisfaction with pain management after surgery was significantly better in Group L than in Group C (P < 0.05). There were no significant difference in the length of PACU stay, extubation time, duration of surgery, blood loss, amount of intraoperative fluid, urine volume between the two groups (P > 0.05) (Table 3).
 |
Table 3 Intra- and Postoperative Characteristics |
No cases of local anesthetic toxicity occurred in either group. The incidence of hypertension and tachycardia was significantly lower in Group L than in Group C (P < 0.05). There was no significant difference in the incidence of hypotension and bradycardia between the two groups (P > 0.05) (Table 4).
 |
Table 4 Perioperative Adverse Events |
Discussion
Lidocaine is a classic local anesthetic with a long history, and it has been mostly used for local anesthesia. Although some clinical studies have focused on intravenous lidocaine, they have faced challenges because of concerns regarding local anesthetic toxicity. However, some studies in recent years have shown that with an appropriate dose and infusion speed, lidocaine intravenous injection will not cause serious local anesthetic toxicity.15–17 In the present study, we chose continuous intravenous lidocaine to explore whether systemic lidocaine can reduce the incidence of POD in elderly patients. The loading dose and continuous infusion dose were determined with reference to the international consensus statement.18 The results of this study show that systemic lidocaine during surgery can reduce the incidence of POD in elderly patients with hip fracture. Neuroinflammation is one hypothesis for the pathogenesis of POD. The body produces a large number of inflammatory mediators, such as interleukin-6, interleukin-1β, and tumor necrosis factor-α, when undergoing surgery and trauma.19 These inflammatory factors can cause central inflammation through the blood–brain barrier, leading to synaptic dysfunction and neuronal apoptosis and ultimately resulting in cognitive dysfunction such as POD.20,21 However, intravenous lidocaine has been proven to inhibit the release of peripheral inflammatory factors and thus reduce the concentration of central inflammatory factors, thereby protecting brain function.7 One study showed that lidocaine may be an effective neuroprotective agent in treating early postoperative cognitive dysfunction in elderly patients undergoing spine surgery.10 Another study showed that compared with general anesthesia alone, intravenous infusion of lidocaine could effectively reduce patients’ surgical stress and inflammatory response.22 Although lidocaine reduced the incidence of delirium 2 days after surgery (13.3% vs 30.0%), there was no statistical significance. This may have been related to the small sample size (n = 30 in each group).
The incidence of POD is also closely related to the assessment method and time point. POD is divided into hypoactive delirium, hyperactive delirium, and mixed delirium, among which hypoactive delirium has the highest occurrence rate.3 Because of the absence of overt distress or disturbance in patients with hypoactive delirium, hypoactive delirium is more likely to be overlooked than hyperactive delirium.23 It is easy to be mistaken for fatigue or sleepiness and should receive more attention. POD tends to occur in the morning and evening, and assessments should be performed at these time points to obtain more accurate results. To achieve a comprehensive evaluation of POD in this study, we informed the researchers in advance to pay close attention to the patients’ postoperative mental state, and we chose to spend two time periods each day after surgery carefully evaluating the patients.
Our results also showed that the incidences of hypertension, tachycardia, and emergence agitation were significantly lower in the lidocaine group, which may have been related to the anti-stress response effect of lidocaine. Systemic lidocaine is antinociceptive in both acute and chronic pain states, especially in acute postoperative and chronic neuropathic pain, and opioids and lidocaine synergistically potentiate anti-nociception.7 Therefore, lidocaine can reduce the total amount of opioid drugs used during the perioperative period. This is consistent with the results of our study, in which we not only reduced the total dosage of sufentanil and remifentanil but also reduced the total dosage of propofol. A randomized controlled study of intravenous lidocaine in children undergoing laparoscopic appendectomy had similar results.24 A systematic review showed that patients who received perioperative intravenous lidocaine had improved visual analogue scale scores at 1 to 4 hours and 24 hours after surgery. However, no pain relief benefit associated with this intervention was observed at 48 hours after surgery.25 Therefore, the effect of lidocaine may be limited to the intraoperative period and shortly after surgery. A mechanistic, multicenter randomized clinical trial showed that intraoperative infusion of lidocaine did not alter overall or disease-free survival.26 However, by improving early postoperative cognitive function and reducing the incidence of adverse reactions, lidocaine can definitely promote early postoperative recovery. Nevertheless, further research is needed to confirm patients’ long-term functional recovery.
This study had certain limitations. First, it was a single-center study. We anticipate the performance of a more comprehensive multicenter clinical study in the future. Second, we did not measure the serum concentration of lidocaine during the perioperative period. The neurotoxicity caused by lidocaine accumulation is still a concern in patients undergoing continuous infusion. Finally, all patients in this study had an American Society of Anesthesiologists physical status of II or III; we did not enroll high-risk elderly patients, especially those with liver and kidney dysfunction. This represents another future research direction.
Conclusions
Intraoperative intravenous lidocaine can reduce the incidence of POD and emergence agitation in elderly patients with hip fracture within 7 days after surgery. Moreover, because of the unique anti-stress reaction of lidocaine, this drug can reduce the incidence of hypertension and tachycardia during surgery. Therefore, patients received lidocaine at 1 mg/kg for more than 10 minutes followed by continuous infusion at 1.5 mg/kg/h until the end of surgery, which is a safe and beneficial intervention for elderly patients undergoing hip fracture surgery.
Data Sharing Statement
The original data analyzed in this study are included in the article; further inquiries can be directed to the corresponding author.
Ethics Approval and Consent to Participate
This trial was performed in accordance with the Declaration of Helsinki and the Chinese Clinical Trial Specifications. The study was approved by the Medical Ethics Committee of The Sixth Affiliated Hospital of Wenzhou Medical University (approval No. 2023-095), and was registered in the Chinese Clinical Trial Registry (www.chictr.org.cn; registration number: ChiCTR2300072987). Written informed consent was obtained from all participants. The study protocol followed the CONSORT guidelines. The study protocol was performed in the relevant guidelines.
Acknowledgments
We thank the patients who participated in this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors declare that they have no conflicts of interest.
References
1. Chen Y, Liang S, Wu H, et al. Postoperative delirium in geriatric patients with Hip fractures. Front Aging Neurosci. 2022;14:1068278. doi:10.3389/fnagi.2022.1068278
2. Daiello LA, Racine AM, Yun Gou R, et al. Postoperative delirium and postoperative cognitive dysfunction: overlap and divergence. Anesthesiology. 2019;131(3):477–491. doi:10.1097/ALN.0000000000002729
3. Albrecht JS, Marcantonio ER, Roffey DM, et al. Stability of postoperative delirium psychomotor subtypes in individuals with Hip fracture. J Am Geriatr Soc. 2015;63(5):970–976. doi:10.1111/jgs.13334
4. Li T, Li J, Yuan L, et al. Effect of regional vs general anesthesia on incidence of postoperative delirium in older patients undergoing hip fracture surgery: the RAGA randomized trial. JAMA. 2022;327(1):50–58. doi:10.1001/jama.2021.22647
5. Swarbrick CJ, Partridge JSL. Evidence-based strategies to reduce the incidence of postoperative delirium: a narrative review. Anaesthesia. 2022;77(1):92–101. doi:10.1111/anae.15607
6. Bilotta F, Lauretta MP, Borozdina A, Mizikov VM, Rosa G. Postoperative delirium: risk factors, diagnosis and perioperative care. Minerva Anestesiol. 2013;79(9):1066–1076.
7. Hermanns H, Hollmann MW, Stevens MF, et al. Molecular mechanisms of action of systemic lidocaine in acute and chronic pain: a narrative review. Br J Anaesth. 2019;123(3):335–349. doi:10.1016/j.bja.2019.06.014
8. Ventham NT, Kennedy ED, Brady RR, et al. Efficacy of intravenous lidocaine for postoperative analgesia following laparoscopic surgery: a meta-analysis. World J Surg. 2015;39(9):2220–2234. doi:10.1007/s00268-015-3105-6
9. Evans DE, Kobrine AI, LeGrys DC, et al. Protective effect of lidocaine in acute cerebral ischemia induced by air embolism. J Neurosurg. 1984;60(2):257–263. doi:10.3171/jns.1984.60.2.0257
10. Chen K, Wei P, Zheng Q, et al. Neuroprotective effects of intravenous lidocaine on early postoperative cognitive dysfunction in elderly patients following spine surgery. Med Sci Monit. 2015;21:1402–1407. doi:10.12659/MSM.894384
11. Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941–948. doi:10.7326/0003-4819-113-12-941
12. Inouye SK, Kosar CM, Tommet D, et al. The CAM-S: development and validation of a new scoring system for delirium severity in 2 cohorts. Ann Intern Med. 2014;160(8):526–533. doi:10.7326/M13-1927
13. Jo JY, Jung KW, Kim HJ, et al. Effect of total intravenous anesthesia vs volatile induction with maintenance anesthesia on emergence agitation after nasal surgery: a randomized clinical trial. JAMA Otolaryngol Head Neck Surg. 2019;145(2):117–123. doi:10.1001/jamaoto.2018.3097
14. Amin SM, Hasanin A, ElSayed OS, et al. Comparison of the hemodynamic effects of opioid-based versus lidocaine-based induction of anesthesia with propofol in older adults: a randomized controlled trial. Anaesth Crit Care Pain Med. 2023;42(4):101225. doi:10.1016/j.accpm.2023.101225
15. Kaszyński M, Stankiewicz B, Pałko KJ, Darowski M, Pągowska-Klimek I. Impact of lidocaine on hemodynamic and respiratory parameters during laparoscopic appendectomy in children. Sci Rep. 2022;12(1):14038. doi:10.1038/s41598-022-18243-3
16. He C, Jin Y, Zhang Y, et al. The pharmacokinetics and safety of lidocaine in liver cancer patients undergoing hepatic resection. Eur J Clin Pharmacol. 2023;79(6):829–839. doi:10.1007/s00228-023-03498-0
17. Both CP, Thomas J, Bühler PK, Schmitz A, Weiss M, Piegeler T. Factors associated with intravenous lidocaine in pediatric patients undergoing laparoscopic appendectomy - A retrospective, single-centre experience. BMC Anesthesiol. 2018;18(1):88. doi:10.1186/s12871-018-0545-1
18. Foo I, Macfarlane AJR, Srivastava D, et al. The use of intravenous lidocaine for postoperative pain and recovery: international consensus statement on efficacy and safety. Anaesthesia. 2021;76(2):238–250. doi:10.1111/anae.15270
19. Xiao MZ, Liu CX, Zhou LG, Yang Y, Wang Y. Postoperative delirium, neuroinflammation, and influencing factors of postoperative delirium: a review. Medicine. 2023;102(8):e32991. doi:10.1097/MD.0000000000032991
20. Taylor J, Parker M, Casey CP, et al. Postoperative delirium and changes in the blood-brain barrier, neuroinflammation, and cerebrospinal fluid lactate: a prospective cohort study. Br J Anaesth. 2022;129(2):219–230. doi:10.1016/j.bja.2022.01.005
21. Yang S, Gu C, Mandeville ET, et al. Anesthesia and surgery impair blood-brain barrier and cognitive function in mice. Front Immunol. 2017;8:902. doi:10.3389/fimmu.2017.00902
22. Lai Y, Chen Q, Xiang C, Li G, Wei K. Comparison of the effects of dexmedetomidine and lidocaine on stress response and postoperative delirium of older patients undergoing thoracoscopic surgery: a randomized controlled trial. Clin Interv Aging. 2023;18:1275–1283. doi:10.2147/CIA.S419835
23. Oh ST, Park JY. Postoperative delirium. Korean J Anesthesiol. 2019;72(1):4–12. doi:10.4097/kja.d.18.00073.1
24. Kaszyński M, Lewandowska D, Sawicki P, Wojcieszak P, Pągowska-Klimek I. Efficacy of intravenous lidocaine infusions for pain relief in children undergoing laparoscopic appendectomy: a randomized controlled trial. BMC Anesthesiol. 2021;21(1):2. doi:10.1186/s12871-020-01218-0
25. Weibel S, Jokinen J, Pace NL, et al. Efficacy and safety of intravenous lidocaine for postoperative analgesia and recovery after surgery: a systematic review with trial sequential analysis. Br J Anaesth. 2016;116(6):770–783. doi:10.1093/bja/aew101
26. Zhang H, Qu M, Guo K, et al. Intraoperative lidocaine infusion in patients undergoing pancreatectomy for pancreatic cancer: a mechanistic, multicentre randomised clinical trial. Br J Anaesth. 2022;129(2):244–253. doi:10.1016/j.bja.2022.03.031
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
