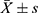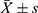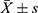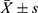Back to Journals » Journal of Inflammation Research » Volume 16
Effect of Hyperbaric Oxygen Intervention on Oxidative Stress and Expression of Nerve Growth Factor in Patients with Craniocerebral Injury
Authors Ren B, Ye H, Shan W, Tao X, Ye Z
Received 24 May 2023
Accepted for publication 10 October 2023
Published 30 October 2023 Volume 2023:16 Pages 4925—4932
DOI https://doi.org/10.2147/JIR.S422790
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Bingyan Ren,1,* Hanbin Ye,2,* Wenyuan Shan,3 Xuelei Tao,4 Zi Ye2
1Department of Emergency, the Second Affiliated Hospital of Nantong University, Nantong First People’s Hospital, Nantong, People’s Republic of China; 2Department of Neurosurgery, the Second Affiliated Hospital of Nantong University, Nantong First People’s Hospital, Nantong, People’s Republic of China; 3Department of Neurosurgery, the Fourth Affiliated Hospital of Nantong University, Nantong Fourth People’s Hospital, Nantong, People’s Republic of China; 4Department of Neurosurgery, Nantong Second People’s Hospital, Nantong, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zi Ye, Department of Neurosurgery, the Second Affiliated Hospital of Nantong University, Nantong First People’s Hospital, No. 6 of North of Haierxiang Road, Hepingqiao District, Nantong, 216000, People’s Republic of China, Email [email protected] Xuelei Tao, Department of Neurosurgery, Nantong Second People’s Hospital, No. 43 of Xinglong Road, Nantong, 100730, People’s Republic of China, Email [email protected]
Objective: To examine the impact of hyperbaric oxygen intervention on oxidative stress and nerve growth factor in patients with craniocerebral injury.
Methods: Using the random number table method, 40 patients with craniocerebral injury who were treated at the First People’s Hospital of Nantong were randomly assigned to either the control group or the hyperbaric oxygen group, with 20 patients in each group. The control group received routine intervention for clinical traumatic brain injury, while the hyperbaric oxygen group received additional hyperbaric oxygen intervention during the 7 to 30 days of routine intervention. Indicators of oxidative stress and nerve growth factor levels were compared between the two groups at the time of admission and 30 days after therapy.
Results: The serum levels of superoxide dismutase, endothelium-derived relaxing factor—nitric oxide, and nerve growth factor in the hyperbaric oxygen group increased more significantly than in the control group. The serum malondialdehyde concentration was also significantly reduced in the hyperbaric oxygen group.
Conclusion: Hyperbaric oxygen intervention can successfully lower systemic oxidative stress response and increase the expression level of nerve growth factor in patients with craniocerebral injury.
Keywords: craniocerebral injury, hyperbaric oxygen, nerve growth factor, oxidative stress
Background
Severe acute traumatic brain injury is a severe brain injury usually caused by external forces, resulting in abnormal brain function. Patients with severe acute traumatic brain injury often accompany severe brain contusion, brain edema, malignant intracranial hypertension, and other symptoms.1 The overall mortality rate is approximately 30%; among survivors of mild injury, 10% have permanent disabilities, and the disability rates for moderate and severe injury patients are as high as 66% and 100%, respectively.2
The pathophysiological changes in severe acute traumatic brain injury are complex and involve inflammatory reactions, cell apoptosis, brain edema, and blood-brain barrier disruption in brain tissue. These pathophysiological changes further aggravate brain damage and may continue to develop within a time window after the injury, causing irreversible effects on neurologic recovery.3 Neurotrophic factors play a crucial role in acute brain injury diseases. These factors are important in the development and maintenance of the nervous system, promoting the growth, survival, and connectivity of neurons, as well as regulating signal transmission between neurons. In acute brain injury, neurotrophic factors may be impaired or inhibited, leading to neuronal damage and functional impairment. Therefore, studying the changes and mechanisms of neurotrophic factors in acute brain injury can enhance our understanding of the pathophysiological processes underlying brain injury and provide new targets for disease treatment.
Hyperbaric oxygen therapy has attracted attention as a potential treatment. This therapy utilizes high-concentration pure oxygen to treat brain injury patients. By providing high oxygen pressure, hyperbaric oxygen therapy aims to improve brain oxygenation, reduce brain damage caused by cerebral hypoxia, and potentially promote the repair of damaged neural tissue. The basic principle of this therapy is to increase the dissolved oxygen concentration in the blood by creating a high-oxygen environment, thereby enhancing the oxygen supply to the brain tissue.4
However, other clinical studies have failed to observe significant improvement with hyperbaric oxygen therapy. These studies have a skeptical attitude towards the efficacy of hyperbaric oxygen therapy, suggesting that it may have limitations and restrictions in practical application. For example, hyperbaric oxygen therapy may carry a risk of severe oxygen toxicity, leading to the generation of oxygen free radicals and vascular damage. Additionally, the therapeutic effects of hyperbaric oxygen therapy may be influenced by factors such as oxygen therapy duration, treatment duration, and timing. The impact of these factors may result in variations in the clinical outcomes of hyperbaric oxygen therapy. Therefore, further research is needed to explore the therapeutic effects and mechanisms of hyperbaric oxygen therapy in severe acute traumatic brain injury. In this study, hyperbaric oxygen therapy was employed to treat patients with brain injuries, and the effects of hyperbaric oxygen therapy on oxidative stress and the production of nerve growth factors (NGF) were investigated, aiming to lay a foundation for future clinical practices.
Material and Methods
General Material
Clinical Data
We selected 40 patients with severe acute craniocerebral injury who were hospitalized in the First People’s Hospital of Nantong between February 2019 and August 2020 and randomly assigned them to either the control group or the HBO group (20 patients in each group) using the random number table method. The patients in the control group received routine intervention for craniocerebral injury, whereas those in the HBO group received HBO intervention based on routine intervention. At present, HBO therapy is not administered to patients with contraindications, such as active bleeding, unstable vital signs, cerebrospinal fluid leakage, or skull base fractures. Inclusion criteria: All patients were admitted to the hospital within 24 hours of sustaining an injury, as confirmed by imaging. Before treatment, all patients had Glasgow coma scale (GCS) ratings between 5 and 8, except those with serious damage to the thoracic and abdominal organs and open craniocerebral injury. All of the patients who underwent surgery to remove the hematoma exhibited stable vital signs and had no history of cerebrovascular or significant systemic diseases.
Main Equipment and Reagents
We utilized microplate readers manufactured by Thermo Fisher Scientific. We purchased superoxide dismutase (SOD) (A001-3-2), malondialdehyde (MDA)(A003-1-2), and nitric oxide (NO) (A012-1-2) from Nanjing Jiancheng Institute of Biotechnology, and NGF (CSB-RA288635A0HU) from Cusabio in Wuhan. All patients were instructed to fast before the administration of hyperbaric oxygen therapy. On the day prior to the therapy, venous blood samples were collected from the patients on an empty stomach for SOD/MDA/NO analysis. Following the completion of oxygen therapy, venous blood samples were collected again on the first day of therapy, while the patients were in a fasting state, for subsequent SOD/MDA/NO analysis.
Methods
Intervention methods
Routine treatment: All patients were effectively cured after undergoing surgery to remove the hematoma, and reexamination of CT scans revealed that the hematoma was successfully removed. Postoperatively, symptomatic treatments such as hemostasis, dehydration to lower intracranial pressure, antibiotics to prevent infection, improvement of water, electrolytes, acid-base balance disorders, blood pressure control, fluid infusion, and other medications were administered.
Atmospheric pressure oxygen treatment group (control group): A normobaric oxygen mask was used to inhale pure oxygen, administer oxygen for 30 minutes. Rest for 5 minutes, and then administer oxygen for another 30 minutes.
HBO group: Standard methods commonly used in China and internationally were adopted.5 The pressure was applied at 0.2 MPa with a 20-minute pressurization period. The patients breathed pure oxygen through a mask for 60 minutes in the pressurization chamber, followed by a 5-minute break and a 20-minute decompression period. The therapy was administered daily. The state of consciousness and vital signs of the patients were closely monitored during treatment.
Both the HBO group and the control group patients received a total of 10 days of treatment. After completing the entire 10-day course, serum samples were extracted for laboratory testing.
SOD Assay
In accordance with the experimental procedures provided in the kit’s instructions, a control well, a blank control well, and a blank assay well were prepared. The corresponding enzyme and substrate were then added to the serum of each group, incubated at 37 °C for 20 minutes after being evenly mixed, and the absorbance value was measured at 450 nm.
MDA Assay
In accordance with the experimental procedures provided in the kit’s instructions, a blank tube and a standard tube were set, along with a control tube and an assay tube for each sample. The reaction solutions were added as needed, mixed evenly, placed in a 100 °C water bath for 80 minutes, followed by cooling with flowing water, and the absorbance was measured at 532 nm.
NO Assay
In accordance with the experimental procedures provided in the kit’s manual, a blank well and a standard well were prepared. The corresponding reagents were added to the serum of each group, mixed evenly, left to stand for 10 minutes, then centrifuged at 4000 rpm for 15 minutes. The supernatant was then mixed with a coloring agent and left to react for 15 minutes. The absorbance value was measured at 550 nm.
NGF Assay
Each well of the ELISA plate was filled with 100μL reference substances and samples and incubated for 2 hours at 37 °C. The supernatant was then discarded, followed by the addition of 100 μL biotin-labeled antibodies and incubation at 37 °C for 1 h, followed by washing three times for two minutes each; 100 μL HRP-labeled avidin was then added and incubated for 1 h at 37 °C, followed by washing three times for two minutes each; 90 μL substrate was then added and incubated for 0.5 h at 37 °C in the dark. Immediately following the addition of 50 μL stop solution, absorbance was measured at 450 nm.
Statistical Analysis
STATA V 16.0 statistical software was used to process all experimental measurement data, while GraphPad Prism 5 statistical analysis software was used for statistical mapping. The data results are expressed as mean ± standard deviation. A two-independent sample t-test was used to compare the continuous variables between the HBO group and the control group. A significance level of p < 0.05 was considered statistically significant.
Results
Determination of SOD and MDA Content
The age range of the included patients was 40 to 70 years, with a mean GCS score of 6.40±1.14. Each group comprised of 5 male participants (50.0%) and 5 female participants (50.0%). The content of SOD (Figure 1) and MDA (Figure 2) in the control group and the HBO group were detected and analyzed before and after hospitalization. Neither SOD nor MDA levels differed significantly between the two groups at the time of admission (P > 0.05). After treatment, the levels of SOD in both groups were higher than those at admission, and the difference was statistically significant (P < 0.05). The HBO group had significantly increased SOD levels compared to the control group (P < 0.05). After treatment, the MDA levels in both groups declined and the difference was statistically significant (P < 0.05) compared to their levels at admission, with the HBO group experiencing a greater decrease than the control group (P < 0.05).
Determination of NO Content
We detected and analyzed the NO content in the control group and the HBO group prior to and following admission (Figure 3). At the time of admission, there was no significant difference in NO levels between the two groups (P > 0.05). This difference was statistically significant (P < 0.01, P < 0.05, respectively) for both groups, the NO levels were greater after therapy than at admission. In addition, the level of NO in the HBO group was much higher than in the control group, but there was no statistically significant difference between the two groups.
 |
Figure 3 Determination of NO content (n = 20, |
Determination of NGF Content
The standard curve was plotted using an NGF ELISA kit (Figure 4). We detected and analyzed the NGF content in the control group and the HBO group before and after hospitalization (Figure 5). At the time of admission, there was no significant difference in NGF levels between the two groups (P > 0.05). The difference in the NGF levels in the two groups before and after therapy was statistically significant (P < 0.01). The HBO group experienced a more significant increase than the control group (P < 0.01).
 |
Figure 4 NGF standard curve. |
 |
Figure 5 Determination of NGF content (n = 20, |
Discussion
The pathological process of traumatic brain injury comprises a series of cascade events initiated by molecular and cellular triggers.6 Many substances, including endogenous opioid peptides and reactive oxygen species (ROS) are released due to a severe stress response induced by acute craniocerebral injury, leading to pathophysiological effects such as hypoxia-ischemia of brain tissue, decreased cerebral perfusion pressure, changes in cerebral blood flow, and aggravation of consciousness.7 NGF is one of the earliest neurotrophic factors to be found and investigated. When an acute craniocerebral injury occurs, there is an increase in the expression of NGF and its receptors at the damage site and in effector neurons, which is related to their protective effects.8
In recent years, HBO therapy has progressively evolved into a new discipline. The use of HBO in the adjuvant treatment of severe craniocerebral trauma was primarily motivated by the following two factors: Firstly, it can shorten cerebral vessels, reduce cerebral blood flow, reduce intracranial pressure, stabilize the blood-brain barrier, and reduce cerebral edema;9 secondly, partial pressure of oxygen and blood oxygen content are increased, together with the oxygen diffusion radius of capillaries in brain tissue, which can improve the hypoxic state of brain tissue, improve the function of mitochondria, and promote aerobic metabolism of neurons.10 In this study, the effect of HBO on the condition of patients was further investigated from the aspects of intracranial oxidative stress and the production of NGF after severe craniocerebral trauma. The results of this study indicate that there was no significant difference in NGF levels between the two groups upon admission (P > 0.05). However, there was a statistically significant difference in NGF levels between the two groups before and after treatment (P < 0.01), with a significantly greater increase observed in the hyperbaric oxygen group compared to the control group (P < 0.01). This suggests that the use of hyperbaric oxygen therapy is more beneficial in promoting nerve growth compared to conventional treatment methods, thereby exerting a positive effect on improving the patient’s condition.
In the early stage of craniocerebral injury, the level of oxidative stress increased significantly, but the severity of oxidative stress varies greatly with the severity of brain injury.11 In acute traumatic brain injury, the impact of external trauma on cells can lead to excessive production of free radicals, resulting in intensified oxidative stress. These highly reactive free radicals can cause damage to important molecules such as cell membranes, proteins, and DNA. The presence of superoxide dismutase (SOD) is crucial for controlling free radical damage. SOD catalyzes the dismutation of superoxide anions, converting them into oxygen and hydrogen peroxide, effectively reducing the generation of free radicals and alleviating cellular damage.12 Additionally, SOD indirectly participates in the degradation of other nitric oxide-related free radicals, further protecting brain tissue. Therefore, it is widely believed that increasing SOD activity or supplementing with exogenous SOD can mitigate inflammation caused by brain injury. This study suggests a significant increase in SOD levels in patients after receiving hyperbaric oxygen therapy, indicating that this treatment method is beneficial in regulating oxidative stress levels in patients.
Free radicals of oxygen attack polyunsaturated fatty acids in the biofilm, resulting in lipid peroxidation and the formation of lipid peroxides. For example, the aldehyde group (malondialdehyde (MDA)), and the concentration of MDA in serum can reflect the severity of brain injury.13 Lipid peroxidation can not only transform ROS into active molecules, but also accelerate the effect of ROS through chain or chain-branched chain reaction. Therefore, even if there is only one ROS at the beginning, it eventually leads to the formation of many lipolysis products, some of which are harmless, while others can cause cell metabolism and dysfunction, and even death. Therefore, the content of MDA can often reflect the degree of lipid peroxidation in the body, thus indirectly reflecting the degree of cell damage. In this study, following the administration of HBO therapy, there was a notable improvement in the patients’ condition, indicating a superior curative impact of HBO therapy. Furthermore, the analysis of SOD and MDA results provided valuable insights into the clinical therapeutic effect of HBO therapy.
Endothelium-derived relaxing factor—nitric oxide (NO) is a highly reactive free radical in organisms, which works as both a second messenger and a neurotransmitter; it is also an effector molecule that has a wide range of physiological effects on the body.14 NO can swiftly flow through the biofilm through simple diffusion without any medium or energy and can easily participate in the electron transfer reaction and the oxidation-reduction reaction of the body. Abnormal NO production is closely related to the occurrence and progression of various diseases. After the blood-brain barrier of patients with craniocerebral injury is damaged, the endothelial function of cerebral capillaries is reduced. NO can widen blood arteries, which is good for accelerating blood circulation to a certain extent. However, a large amount of NO can aggravate nerve cell damage.15 In this study, the NO content was comparatively low at the time of admission of patients. After treatment with atmospheric pressure oxygen and HBO, the condition improved, and there was a significant increase in NO content after HBO therapy. The results of SOD, MDA, and NO analysis revealed that HBO intervention could successfully lower the oxidative stress response in patients.
NGF is the most extensively researched neurotrophic factor currently. Existing research demonstrates that the expression of NGF is early, transient, and considerably up regulated after brain damage, demonstrating that NGF is associated with the modulation of neuronal response following brain injury.16 According to studies, the protective effects of NGF on neurons can be produced in the following ways: 1. Anti-oxygen free radical effect; 2. Increase the activity of free radical scavengers and reduce the damage to ischemic nerve cells; 3. Antagonize the neurotoxicity of excitatory amino acids; 4. Regulate the calcium ions in the neuronal cytoplasm, inhibit calcium ion overload, and protect injured neurons; 5. Inhibit programmed cell death.17 Currently, it is believed that NGF is a water-soluble macromolecule that is unable to penetrate the blood-brain barrier. Nonetheless, numerous studies on NGF in craniocerebral trauma have demonstrated that administration via different routes produces curative effects without obvious adverse effects.18 It is hypothesized that the administration of NGF through multiple routes and the use of medications to temporarily access the blood-brain barrier following cerebral trauma can ensure the maintenance of a high level of NGF in damaged brain tissue, thus exerting the neuroprotective effect of NGF. In this study, following HBO therapy, there was an improvement in the condition, and the NGF content saw a more significant increase. This, in turn, further enhanced the survival of damaged neurons.
Conclusion
In conclusion, HBO intervention for patients with craniocerebral injury following emergency surgery can effectively inhibit the oxidative stress response of the body, enhance the survival of damaged neurons and accelerate the recovery of clinical symptoms in patients. It provides new ideas for the clinical treatment strategy for patients with severe craniocerebral injury and is a dependable strategy to improve the treatment outcome for patients with craniocerebral injury.
Abbreviations
SOD, superoxide dismutase; NO, nitric oxide; NGF, nerve growth factor; MDA, Malondialdehyde; HBO, hyperbaric oxygen; TBI, Traumatic brain injury; GCS, Glasgow coma scale; BBB, blood-brain barrier.
Data Sharing Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
This study was conducted with approval from the Ethics Committee of the Nantong First People’s Hospital (2018KT096). This study was conducted in accordance with the declaration of Helsinki. Written informed consent was obtained from all participants.
Acknowledgments
We would like to acknowledge the hard and dedicated work of all the staff that implemented the intervention and evaluation components of the study.
Funding
1. Natural Science Project of Nantong Science and Technology Bureau (No. JC2021182); 2. Jiangsu Provincial Health Care Commission Guidance Project (No. Z2021017); 3. Nantong Municipal Health Commission Subjects (QN2023009 and QN2023012).
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Vella MA, Crandall ML, Patel MB. Acute management of traumatic brain injury. Surg Clin North Am. 2017;97(5):1015–1030. doi:10.1016/j.suc.2017.06.003
2. Capizzi A, Woo J, Verduzco-Gutierrez M. Traumatic brain injury: an overview of epidemiology, pathophysiology, and medical management. Med Clin North Am. 2020;104(2):213–238. doi:10.1016/j.mcna.2019.11.001
3. Kalra S, Malik R, Singh G, et al. Pathogenesis and management of traumatic brain injury (TBI): role of neuroinflammation and anti-inflammatory drugs. Inflammopharmacology. 2022;30(4):1153–1166. doi:10.1007/s10787-022-01017-8
4. Zhong X, Shan A, Xu J, et al. Hyperbaric oxygen for severe traumatic brain injury: a randomized trial. J Int Med Res. 2020;48(10):300060520939824. doi:10.1177/0300060520939824
5. Lu Y, Zhou X, Cheng J, et al. Early intensified rehabilitation training with hyperbaric oxygen therapy improves functional disorders and prognosis of patients with traumatic brain injury. Adv Wound Care. 2021;10(12):663–670. doi:10.1089/wound.2018.0876
6. Ng SY, Lee A. Traumatic brain injuries: pathophysiology and potential therapeutic targets. Front Cell Neurosci. 2019;13:528. doi:10.3389/fncel.2019.00528
7. Michinaga S, Koyama Y. Pathophysiological responses and roles of astrocytes in traumatic brain injury. Int J Mol Sci. 2021;22(12):6418. doi:10.3390/ijms22126418
8. Lin PH, Kuo LT, Luh HT. The roles of neurotrophins in traumatic brain injury. Life. 2021;12:1.
9. Balasubramanian P, Delfavero J, Nyul-Toth A, et al. Integrative role of hyperbaric oxygen therapy on healthspan, age-related vascular cognitive impairment, and dementia. Front Aging. 2021;2:678543. doi:10.3389/fragi.2021.678543
10. Daly S, Thorpe M, Rockswold S, et al. Hyperbaric oxygen therapy in the treatment of acute severe traumatic brain injury: a systematic review. J Neurotrauma. 2018;35(4):623–629. doi:10.1089/neu.2017.5225
11. Khatri N, Thakur M, Pareek V, et al. Oxidative stress: major threat in traumatic brain injury. CNS Neurol Disord Drug Targets. 2018;17(9):689–695. doi:10.2174/1871527317666180627120501
12. Zhang W, Hong J, Zhang H, et al. Astrocyte-derived exosomes protect hippocampal neurons after traumatic brain injury by suppressing mitochondrial oxidative stress and apoptosis. Aging. 2021;13(17):21642–21658. doi:10.18632/aging.203508
13. Xie BS, Wang Y-Q, Lin Y, et al. Inhibition of ferroptosis attenuates tissue damage and improves long-term outcomes after traumatic brain injury in mice. CNS Neurosci Ther. 2019;25(4):465–475. doi:10.1111/cns.13069
14. Sorokina EG, Semenova ZB, Reutov VP, et al. Brain biomarkers in children after mild and severe traumatic brain injury. Acta Neurochir Suppl. 2021;131:103–107.
15. Kozlov AV, Bahrami S, Redl H, et al. Alterations in nitric oxide homeostasis during traumatic brain injury. Biochim Biophys Acta Mol Basis Dis. 2017;1863(10 Pt B):2627–2632. doi:10.1016/j.bbadis.2016.12.020
16. Dadgostar E, Rahimi S, Nikmanzar S, et al. Aquaporin 4 in traumatic brain injury: from molecular pathways to therapeutic target. Neurochem Res. 2022;47(4):860–871. doi:10.1007/s11064-021-03512-w
17. Sims SK, Wilken-Resman B, Smith CJ, et al. Brain-derived neurotrophic factor and nerve growth factor therapeutics for brain injury: the current translational challenges in preclinical and clinical research. Neural Plast. 2022;2022:3889300. doi:10.1155/2022/3889300
18. Rejdak K, Sienkiewicz‐Jarosz H, Bienkowski P, et al. Modulation of neurotrophic factors in the treatment of dementia, stroke and TBI: effects of cerebrolysin. Med Res Rev. 2023;43:1668–1700. doi:10.1002/med.21960
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.