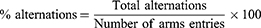Back to Journals » Drug Design, Development and Therapy » Volume 17
Effect of Gabapentin-Fluoxetine Derivative GBP1F in a Murine Model of Depression, Anxiety and Cognition
Authors Gohar A, Ali G , Rashid U, Rauf K , Arif M, Khan MS, Alkahramaan YM, Sewell RDE
Received 8 March 2023
Accepted for publication 26 May 2023
Published 16 June 2023 Volume 2023:17 Pages 1793—1803
DOI https://doi.org/10.2147/DDDT.S407229
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Aneela Gohar,1 Gowhar Ali,2 Umer Rashid,3 Khalid Rauf,1 Mehreen Arif,1 Muhammad Sona Khan,1 Yasser MSA Alkahramaan,1 Robert DE Sewell4
1Department of Pharmacy, COMSATS University Islamabad, Abbottabad Campus, Abbottabad, Pakistan; 2Department of Pharmacy, University of Peshawar, Peshawar, Pakistan; 3Department of Chemistry, COMSATS University Islamabad Abbottabad Campus, Abbottabad, Pakistan; 4Cardiff School of Pharmacy and Pharmaceutical Sciences, Cardiff University, Cardiff, UK
Correspondence: Khalid Rauf, Department of Pharmacy, COMSATS University Islamabad, Abbottabad Campus, Abbottabad, Khyber Pakhtoonkhwa, 22060, Pakistan, Email [email protected]
Background and Objective: Gabapentin is a commonly prescribed antiepileptic agent for seizures, which is also used for pain and addiction management. Due to growing evidence of its abuse liability, there has been an incentive to synthesise potentially useful gabapentin derivatives devoid of adverse effects. A gabapentin adduct with a fluoxetine moiety, GBP1F, was assessed for any sedative, cognitive, anxiolytic, or antidepressant-like actions in murine behavioral models.
Materials and Methods: Selected groups of mice were used for each behavioral paradigm, and the effect of GBP1F (5, 10, and 15 mg/kg) was assessed using spontaneous locomotor activity, the tail suspension test, elevated plus maze test, and the Y maze test models. Immediately following behavioral experiments, postmortem striatal and hippocampal tissues were evaluated for the effect of GBP1F on concentrations of dopamine, DOPAC, HVA, serotonin, 5-HIAA, vitamin C, and noradrenaline using high performance liquid chromatography with electrochemical detection.
Results: GBP1F induced a mild suppression of locomotor activity, ameliorated anxiety and depression-like behavior, did not alter cognitive behavior, and raised serotonin and 5-HIAA concentrations in the hippocampus and striatum. GBP1F also positively enhanced dopamine and vitamin C tissue levels in the striatum. Thus, GBP1F represents a compound with anxiolytic- and antidepressant-like effects though further studies are warranted at the molecular level to focus on the precise mechanism(s) of action.
Keywords: depression, anxiety, DOPAC, HVA, 5-HIAA, gabapentin, cognition
Introduction
Gabapentin is a clinically used anticonvulsant with broad utility in the management of neuropathic pain, anxiety, alcohol withdrawal, and bipolar disorders.1,2 It exerts pharmacological activity mediated via interactions with molecular targets located within the CNS. These include the auxiliary α2δ subunit of voltage-gated calcium channels, inhibition of inward rectifying potassium channels,3 nitric oxide signaling,4 and serotonergic pathways.3 Gabapentin has been reported to decrease immobility time in animal models of depression through modulation of serotonergic, nitrergic and inward rectifying potassium channels.5 It has also been documented to enhance blood serotonin levels in healthy volunteers.6 Despite its clinical approval and off-label use in diverse populations, there has been increased prescribing of gabapentin globally, and over the last two decades there is emerging evidence of gabapentin misuse, abuse and dependence.5,7–9 Due to its safety, tolerability and abuse liability profile, there has been an incentive to synthesise potentially useful gabapentin derivatives by addition of diverse chemical moieties such as thiols,10 Schiff bases,11 salicylaldehyde12 and sulfonamides.13 These derivatives have been evaluated for their possible application as antioxidants, anticancer agents, anticonvulsants and also against neuropathic pain.14 Acknowledging the promise of positive outcomes associated with the addition of pharmacologically and chemically diverse moieties to gabapentin, this study was designed to explore the potential of a new gabapentin adduct with fluoxetine (GBP1F; Figure 1) for any possible sedative, anxiolytic or antidepressant-like activity in murine behavioral models. A simultaneous correlative neurochemical study was undertaken in postmortem hippocampal and striatal tissues for levels of vitamin C, the neurotransmitters, noradrenaline, serotonin and dopamine, as well as the metabolites 3, 4-dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA) and 5-hydroxyindole acetic acid (5-HIAA).
 |
Figure 1 Structure of the Gabapentin-fluoxetine derivative GBP1F. |
Materials and Methods
Acquisition of Animals
Female BALB/c mice (n=6/group) weighing 25–30g were acquired from the animal care facility of the Department of Pharmacy COMSATS University Islamabad, Abbottabad campus. The animals were housed in standardized environmental conditions at a temperature of 24 ± 1°C, and a 12:12-hour light and dark cycle (lights on at 8:00 am and off at 8:00 pm). Standardized recommended laboratory-prepared food and water were provided ad libitum. Ethical guidelines developed by the ethical care committee of COMSATS University Islamabad, Abbottabad Campus were followed during experimentation under an allotted number of PHM.Eth/CS-M01-019-2901. Guidelines followed were compliant with the Animal Care Act (1986) of the United Kingdom.
Drugs
GBP1F was donated by Prof. Dr. Umar Rashid Department of Chemistry CUI Abbottabad.
Animal Grouping (N= 6/Group)
Group No. 1: Normal saline (10ml/kg orally)
Group No. 2: GBP1F (5 mg/kg, orally)
Group No. 3: GBP1F (10 mg/kg, orally)
Group No. 4: GBP1F (15 mg/kg, orally)
Behavioral Tests
The GBP1F compound was freshly prepared as a suspension in 10% carboxymethylcellulose (CMC) and administered at doses of 5, 10, and 15mg/kg orally (P.O.) and 60 minutes later, the animals were subjected to different behavioral tests.
Locomotor Activity
In order to evaluate exploratory locomotor behavior, mice were subjected to an open field test.15–17 Locomotor activity was assessed in locomotor activity boxes (45.6×45.6×30 cm) with the floor divided into quadrants (22.8×22.8 cm). In between animal trials, the open field activity box was cleaned with 70% ethanol. Sixty minutes after dosing, locomotor activity was recorded for individual animal using a video camera mounted 230 cm above the open field arena. The number of line crossings was noted by an observer who was blinded to the treatments, and data was analysed accordingly.18,19
Elevated Plus Maze (EPM)
To assess anxiety-like behavior, animals were subjected to the elevated plus maze (EPM). The EPM apparatus was comprised of a total of four arms; two open (16 × 5 cm2) and two closed (16 × 5 cm2). The closed arms had 12 cm high walls that were painted black. However, the open arms were devoid of walls. Between the open and closed arms, there was a central arena of 5×5 cm where animals were gently placed at the start of testing. The maze was elevated 25 cm above the laboratory floor and a video camera mounted 100 cm above the apparatus recorded subject behavior during each individual 5-minute test session. During individual sessions, the time expended in each arm was determined and the criterion for arm occupancy was established when all four limbs were present inside the specific arm.20,21
Tail Suspension Test (TST)
The tail suspension method was applied for the evaluation of any possible antidepressant-like effect of the test compound. Each animal was gently suspended by the tail with the help of adhesive tape attached to a wooden apparatus located 59 cm above the floor for a total of 6 minutes. The mean mobility time of animal groups was documented after one minute of habituation and the data was statistically analysed.22–25
Forced Swim Test (FST)
In order to evaluate depression-like behavior and behavioral despair, the Porsolt forced swim test was used. Each FST apparatus (individually screened from each other, but in quadruplicate) consisted of a Plexiglas cylinder (20 cm in height × 10 cm in diameter) surrounded by a squared area with 3 white acrylic walls (20 × 40 × 60 cm) and one open wall for recording. The cylinder was filled with water to a depth of 7.5 cm and the temperature was mainatained at 23.0 °C. In each test session, mice were placed in the water for a total of 6 min and the time of behavioural immobility was recorded by video camera.26
Y-Maze Test
The Y-maze consisted of three equal-length arms (21 cm long × 8.5 cm wide × 40 cm height) positioned at an angle of 120°. Individual animals were placed in the center of the maze and allowed to habituate for 5 mins. Animal arm entries were recorded by video camera when both hind paws were present in any specific arm. Maze deodorization between sessions was performed with 70% ethanol. For analysis of spontaneous Y-maze activity, the number of alternations, entries in each arm, and percentage alternations were determined. The following formula was used for the calculation of percentage alternations.26,27
Quantification of Neurotransmitters and Their Metabolites Using HPLC
Animals were killed by decapitation at the end of behavioral experiments and whole brains were excised and immediately placed on an ice-chilled plate. The striatum and hippocampus, were extracted, stored in labeled Eppendorf tubes. Prior to analysis, they were homogenized in ice-chilled 0.2% perchloric acid. Brain samples were then stored at −80°C to preclude neurotransmitter degradation. Using high performance liquid chromatography (HPLC) dopamine, 3, 4-dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA), serotonin, 5-hydroxyindole acetic acid (5-HIAA), noradrenaline and vitamin C were quantified from stored brain samples. HPLC analysis was performed using the method outlined by Rauf et al.28 A Shimadzu HPLC system coupled to an electrochemical detector was used for analysis and separation was achieved using analytical columns (MD_150; 3 × 150, 3 um), and an electrochemical detector (ESA Choulchem III model 5300) coupled with an analytical cell (model 5011 A). Electrodes A and B of the analytical cell were kept at +200 and −200 mV respectively, with a selected sensitivity of 2 μA, and the guard cell (model 5020) was maintained at 500 mV. Freshly prepared and filtered mobile phase with 2.3 mM sodium 1-octane sulphonic acid, 94 mM sodium dihydrogen orthophosphate, 40 mM citric acid, 50 µM EDTA and 10% acetonitrile (pH 3).
Statistical Analysis
Data were presented as mean ± SEM values. Behavioral and neurochemical data was analyzed using one-way ANOVA followed by post hoc Dunnett’s test for multiple comparisons. The threshold for statistical significance was assumed at P<0.05 and GraphPad Prism (version 9) was used for all analyses.
Results
Activity of the Gabapentin Derivative, GBP1F on Locomotor Activity
GBP1F administered at the lowest dose (5 mg/kg) did not statistically modify animal locomotor activity. However, there was a dose related mild suppression of activity at the two higher doses by up to 71.0% at 15 mg/kg in comparison with the saline vehicle treated control group (Figure 2).
 |
Figure 2 Action of orally administered GBP1F (5, 10 and 15 mg/kg) on spontaneous locomotor activity in mice. (**P<0.01 and *P<0.05). |
Activity of the Gabapentin Derivative, GBP1F in the Elevated Plus Maze (EPM)
GBP1F did not modify the occupancy time on the center of the maze. Conversely, all three GBP1F doses markedly increased the time spent in the maze open arm while simultaneously diminishing the occupancy period in the closed arm when compared to the control animals. These outcomes may well reflect an overall anxiolytic-like behaviour induced by GBP1F (Figure 3).
 |
Figure 3 Anxiolytic-like activity in the elevated plus maze (EPM) of orally administered GBP1F (5, 10 and 15 mg/kg) in mice. ***P<0.001. |
Activity of the Gabapentin Derivative, GBP1F in the Tail Suspension Test (TST)
GBP1F at the two higher doses (10 and 15 mg/kg) dose-dependently reduced the group mean immobility time respectively by 69.0% and 45.0% versus controls in the TST (Figure 4). Since, immobility is considered as an index of “depression-like behavior”,23 the converse interpretation of an antidepressant-like effect may be adopted where a GBP1F-induced decrement in immobility time is observed (Figure 4).22
 |
Figure 4 Antidepressant-like activity in the tail suspension test (TST) of orally administered GBP1F (5, 10 and 15 mg/kg) in mice. ***P<0.001 and *P<0.05. |
Activity of the Gabapentin Derivative, GBP1F in the Forced Swim Test (FST)
GBP1F administered at all three dose levels (5, 10 and 15 mg/kg) generated a dose-related inhibition of immobility time in the FST causing a 52.0% decrease in this parameter at the highest dose, compared to saline vehicle treated controls (Figure 5). This measure is thought to be predictive of depression-like behavior,26 and it is apparent that GPB1F has inhibitory activity against this behavioral element.
 |
Figure 5 Antidepressant-like activity in the forced swim test (FST) of orally administered GBP1F (5, 10 and 15 mg/kg) in. ***P<0.001 and *P<0.05. |
Activity of the Gabapentin Derivative, GBP1F on Cognitive Performance in the Y-Maze
GBP1F (5–15 mg/kg) did not modify animal performance in the Y-maze. This outcome was signified by a lack of any significant change evoked by the compound on total arm entries, number of alternations or percentage alternations, suggesting that there was no incitement of a spatial memory deficit (Figure 6).
 |
Figure 6 Cognitive activity in the y-maze of orally administered GBP1F (5, 10 and 15 mg/kg) in mice. |
Action of the Gabapentin Derivative, GBP1F on Hippocampal Concentrations of Monoamine Neurotransmitters and Their Metabolites Plus Vitamin C
The hippocampal serotonin tissue concentration was augmented by treatment with gabapentin (45 mg/kg) as well as the highest dose of GBP1F (15 mg/kg). Correspondingly, gabapentin and all three doses of GBP1F increased hippocampal 5-HIAA levels compared to the saline-treated group. There was also a notable increase in hippocampal noradrenaline tissue levels with gabapentin. However, treatment with the gabapentin derivative, GBP1F did not disclose any difference from the saline-treated group (Table 1A). Hippocampal tissue dopamine, DOPAC and HVA levels were all elevated by gabapentin while GBP1F did not modify either dopamine or HVA. However, the highest dose of GBP1F did induce an upsurge in DOPAC concentration in the hippocampus (Table 1B). Treatment with neither gabapentin nor GBP1F altered the concentration of vitamin C in the hippocampus (data not shown).
 |
Table 1 Action of the Gabapentin Derivative, GBP1F on Hippocampal Concentrations of Monoamine Neurotransmitters and Their Metabolites Plus Vitamin C |
Action of the Gabapentin Derivative, GBP1F on Striatal Concentrations of Monoamine Neurotransmitters and Their Metabolites Plus Vitamin C
The striatal serotonin tissue concentration was enhanced by treatment with either gabapentin or the two higher doses of GBP1F (10 and 15 mg/kg). Similarly, gabapentin and the two higher doses of GBP1F elevated striatal levels of 5-HIAA. In addition, there was a marked rise in striatal noradrenaline in response to gabapentin though GBP1F administration did not change the concentration of this neurotransmitter in the striatum (Table 2A). The striatal tissue dopamine concentration was also elevated by gabapentin though only the highest dose of GBP1F augmented tissue dopamine in the striatum. Moreover, neither gabapentin nor GBP1F modified striatal levels of DOPAC or HVA compared to the saline-treated animals. There was also a statistically significant increase in the striatal tissue concentration of vitamin C caused by the highest dose of GBP1F though this effect did not extend to gabapentin (Table 2B).
 |
Table 2 Action of the Gabapentin Derivative, GBP1F on Striatal Concentrations of Monoamine Neurotransmitters and Their Metabolites Plus Vitamin C |
Discussion
Gabapentin has extensive off-label use in neuropsychiatric disorders apart from epilepsy, primarily as a treatment for alcohol withdrawal and alcohol use disorder, as well as a third-line treatment for social anxiety and severe panic disorder.29 Although there are no trials reporting clinical effectiveness of gabapentin in mood disorders30 one retrospective study has outlined the efficacy of gabapentin as adjuvant therapy for multi-drug resistant depression.31 In preclinical studies in vitro, gabapentin has been shown to exert effects through inhibition of P/Q and L-type Ca2+ channels.32,33 Gabapentin exerts antidepressant effects in murine models through the NO/cGMP pathway.4 It is pertinent to mention that apart from depression, an interaction between nNOS and GABAergic pathways has also been implicated in epilepsy.34 Gabapentin has diverse targets in the brain and it has been reported to exert antidepressant-like activity by interacting with ATP-sensitive potassium channels.3 Such inherent actions are positively conducive towards amelioration of the emotional component of pain, in addition to a core effectiveness in relieving pain centrally via transient receptor channels and voltage-gated Ca2+ channel subunits.35,36 In our study, GBP1F had a behavioral antidepressant-like effect at all doses studied. Additionally, there was evidence that the tissue concentration of dopamine was enhanced by GBP1F and gabapentin in the striatum and it is of note that dopamine has been implicated in modulating both glutamate and GABA via L-type calcium channels.37,38 Consequently, enhanced levels of dopamine and subsequently altered metabolism may have feasibly contributed to the antidepressant-like action of GBP1F, although more receptor-specific studies are warranted to fully elucidate this phenomenon. Dopamine is also involved with a number of crucial central neuronal activities that have key outcomes in thinking, mood, and behavior. In relation to this, drugs such as bupropion, which facilitates dopamine reuptake, has significant antidepressant effects39 and induces non-clinical antidepressant-like activity partly via modulation of dopaminergic tone.40,41 Moreover, patients displaying low levels of HVA have been found to exhibit treatment-resistant depression.42 So the gabapentin-induced hippocampal HVA upsurge observed in our study, may infer potentiality as an antidepressant by way of enhanced dopaminergic tone.
Noradrenaline also plays a substantial role in depression, and patients with refractory depression may display lower central noradrenaline levels.42 In both humans and animals, noradrenaline regulates mood, cognition, social behavior, decision making, and to some degree neuropathic pain.43 Furthermore, it has been reported that the antidepressant-like effect of Vitamin B6 in the forced swim test is also mediated via noradrenaline and its adrenoceptors.44 In addition, noradrenaline given by direct microinjection into the periaqueductal grey (PAG) area induces anxiolytic-like activity in the EPM.45 Hence, our finding, that GBP1F yielded a similar anxiolytic-like effect in the EPM, may be at least partly attributable to an enhancement of central noradrenaline concentration. It is also noteworthy that noradrenaline has been shown to induce neurogenesis by controlling TRK B signaling and augmenting BDNF levels.46,47 In contrast, decreased neurogenesis and a subsequent smaller hippocampal size due to downgraded neurogenesis are hallmarks of clinical depression.48 Attenuation of serotonergic and noradrenergic transmission in the cortex and hippocampus is associated with memory impairment49 and in our case, there was no behavioral evidence that GBP1F caused any memory detriment or a learning deficit.50 SSRI and MAO inhibitors have been shown to exert antidepressant effects primarily by increasing serotonin levels in key brain areas, and natural as well as synthetic compounds that enhance brain serotonin levels have been reported to possess antidepressant activity.51,52 We found that GBP1F enhanced serotonin levels in the hippocampus, so it may be a conceivable deduction that this serotonin elevation was a participating factor in the identified behavioral antidepressant-like activity.
There is a positive correlation between the concentration of Vitamin C and catecholamine levels in the brain, there being an overall involvement in the regulation of mood and learning. Indeed, it appears that low levels of vitamin C facilitate the progression of multiple neuropsychiatric illnesses including anxiety and depression.53 In light of this, our finding that GBP1F raised vitamin C levels, albeit in the striatum, may possibly suggest not only that a neuroprotective antioxidant effect prevailed, but also that there was an anxiolytic- and/or antidepressant-like propensity.
In conclusion, over the dose range studied, GBP1F produced a mild suppression of locomotor activity, ameliorated anxiety and depression-like behavior, did not alter cognition, and raised hippocampal and striatal serotonin and 5-HIAA concentrations while simultaneously increasing the tissue levels of striatal dopamine and vitamin C. It was deduced that GBP1F represents a compound with both anxiolytic/antidepressant-like effects though further studies are warranted at the molecular level to focus on the precise mechanism(s) of action.
Disclosure
The authors report no conflict of interest in this work.
References
1. Binder MR. Gabapentin—the popular but controversial anticonvulsant drug may be zeroing in on the pathophysiology of disease. AJCEM. 2021;9(4):122–134. doi:10.11648/j.ajcem.20210904.15
2. Zullino DF, Khazaal Y, Hattenschwiler J, Borgeat F, Besson J. Anticonvulsant drugs in the treatment of substance withdrawal. Drugs Today. 2004;40(7):603–620. doi:10.1358/dot.2004.40.7.850478
3. Ostadhadi S, Akbarian R, Norouzi-Javidan A, et al. Possible involvement of ATP-sensitive potassium channels in the antidepressant-like effects of gabapentin in mouse forced swimming test. Can J Physiol Pharmacol. 2017;95(7):795–802. doi:10.1139/cjpp-2016-0292
4. Ostadhadi S, Kordjazy N, Haj-Mirzaian A, Ameli S, Akhlaghipour G, Dehpour A. Involvement of NO/cGMP pathway in the antidepressant-like effect of gabapentin in mouse forced swimming test. Naunyn-Schmiedebergs Archiv Pharmacol. 2016;389:393–402. doi:10.1007/s00210-015-1203-5
5. Evoy KE, Peckham AM, Covvey JR, Tidgewell KJ. Gabapentinoid pharmacology in the context of emerging misuse liability. J Clin Pharmacol. 2021;61:S89–S99. doi:10.1002/jcph.1833
6. Rao ML, Clarenbach P, Vahlensieck M, Krätzschmar S. Gabapentin augments whole blood serotonin in healthy young men. J Neural Transm. 1988;73:129–134. doi:10.1007/BF01243384
7. Kapil V, Green JL, Le Lait M-C, Wood DM, Dargan PI. Misuse of the γ-aminobutyric acid analogues baclofen, gabapentin and pregabalin in the UK. Br J Clin Pharmacol. 2014;78(1):190. doi:10.1111/bcp.12277
8. Khave LJ, Noori M, Rahimi-Movaghar A, Noroozi A. Management of gabapentin misuse in a patient with previous history of opioid use disorder: case report. Asian J Psychiatr. 2023;80:103322. doi:10.1016/j.ajp.2022.103322
9. Venkatesh G, Kalaiyarasi C, Ramanathan M. Antidepressant like effect of gabapentin decreases the immobility time in despair animal models in mice: roll of serotonergic system in it. Res J Pharma Technol. 2011;4(11):1702–1706.
10. Türk S, Tok F, Erdoğan Ö, et al. Synthesis, anticancer evaluation and in silico ADMET studies on urea/thiourea derivatives from gabapentin. Phosphorus Sulfur Silicon Relat Elem. 2020;196(4):382–388. doi:10.1080/10426507.2020.1845678
11. Saleem MF, Khan MA, Ahmad I, Aslam N, Khurshid U. Synthesis and characterization of some new Schiff base derivatives of gabapentin, and assessment of their antibacterial, antioxidant and anticonvulsant activities. Trop J Pharma Res. 2021;20(1):145–153. doi:10.4314/tjpr.v20i1.21
12. Ahmad N, Subhan F, Islam NU, et al. Pharmacological evaluation of the gabapentin salicylaldehyde derivative, gabapentsal, against tonic and phasic pain models, inflammation, and pyrexia. Naunyn-Schmiedebergs Archiv Pharmacol. 2021;394:2033–2047. doi:10.1007/s00210-021-02118-x
13. Kanwal N, Khan IU, Sharif S, Hussain EA, Mehmood A, Sahin O. Efficient syntheses, crystal structure and thermal properties of gabapentin 4-acetamido, 2-mesitylene and 2, 4-dinitro sulfonamides derivatives. J Chem Crystallogr. 2019;49:162–168. doi:10.1007/s10870-018-00765-2
14. Papagiouvannis G, Theodosis-Nobelos P, Tziona P, Gavalas A, Kourounakis PN, Rekka EA. Gabapentin antioxidant derivatives with anti-inflammatory and neuroprotective potency. Lett Drug Des Discov. 2022;19(7):579–590. doi:10.2174/1570180818666211210161922
15. Dingman MA, Gyekis JP, Whetzel CA, Klein LC, Vandenbergh DJ. Age-specific locomotor response to nicotine in yellow and mottled yellow A vy/a mice. BMC Res Notes. 2013;6(1):497. doi:10.1186/1756-0500-6-497
16. Hall CS. Emotional behavior in the rat. I. Defecation and urination as measures of individual differences in emotionality. J Comp Psychol. 1934;18(3):385. doi:10.1037/h0071444
17. Lezak KR, Missig G, Carlezon WA. Behavioral methods to study anxiety in rodents. Dialogues Clin Neurosci. 2022;2022:154.
18. Arif M, Rauf K, Rehman NU, Tokhi A, Ikram M, Sewell RD. 6-methoxyflavone and donepezil behavioral plus neurochemical correlates in reversing chronic ethanol and withdrawal induced cognitive impairment. Drug Des Devel Ther. 2022;1573–1593. doi:10.2147/DDDT.S360677
19. Rehman NU, Abbas M, Al-Rashida M, et al. Effect of 4-fluoro-N-(4-sulfamoylbenzyl) benzene sulfonamide on acquisition and expression of nicotine-induced behavioral sensitization and striatal adenosine levels. Drug Des Devel Ther. 2020;14:3777. doi:10.2147/DDDT.S270025
20. Walia V, Garg C, Garg M. NO-sGC-cGMP signaling influence the anxiolytic like effect of lithium in mice in light and dark box and elevated plus maze. Brain Res. 2019;1704:114–126. doi:10.1016/j.brainres.2018.10.002
21. Bertagna NB, Dos Santos PGC, Queiroz RM, Fernandes GJD, Cruz FC, Miguel TT. Involvement of the ventral, but not dorsal, hippocampus in anxiety-like behaviors in mice exposed to the elevated plus maze: participation of CRF1 receptor and PKA pathway. Pharmacol Rep. 2021;73(1):57–72. doi:10.1007/s43440-020-00182-3
22. Mayorga AJ, Dalvi A, Page ME, Zimov-Levinson S, Hen R, Lucki I. Antidepressant-like behavioral effects in 5-hydroxytryptamine1A and 5-hydroxytryptamine1B receptor mutant mice. J Pharmacol Exp Therap. 2001;298(3):1101–1107.
23. Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology. 1985;85(3):367–370. doi:10.1007/BF00428203
24. Vadnie CA, DePoy LM, McClung CA. Measuring the effects of circadian rhythm-related manipulations on depression-like behavior in rodents: forced swim and tail suspension tests. In: Circadian Clocks. Springer; 2021:69–78.
25. Perez E, De Biasi M. Assessment of affective and somatic signs of ethanol withdrawal in C57BL/6J mice using a short-term ethanol treatment. Alcohol. 2015;49(3):237–243. doi:10.1016/j.alcohol.2015.02.003
26. Abi I, Ashiekaa M, Abi E, Adeniyi OS, Saalu LC. High fat diet alteration of gut microbiota impacts learning, memory and anxiety response in mice: cannabidiol and omega 3 possible remedies. Adv Alzheimers Dis. 2022;11(1):1–9. doi:10.4236/aad.2022.111001
27. Prieur EA, Jadavji NM. Assessing spatial working memory using the spontaneous alternation Y-maze test in aged male mice. Bio-Protocol. 2019;9(3). doi:10.21769/BioProtoc.3162
28. Rauf K, Subhan F, Sewell RD. A bacoside containing Bacopa monnieri extract reduces both morphine hyperactivity plus the elevated striatal dopamine and serotonin turnover. Phytother Res. 2012;26(5):758–763. doi:10.1002/ptr.3631
29. Martin JC, Gainer D. Psychiatric uses of gabapentin. Innov Clin Neurosci. 2022;19(7–9):55–60.
30. Berlin RK, Butler PM, Perloff MD. Gabapentin therapy in psychiatric disorders: a systematic review. Prim Care Companion CNS Disord. 2015;17(5):27293.
31. Yasmin S, Carpenter LL, Leon Z, Siniscalchi JM, Price LH. Adjunctive gabapentin in treatment-resistant depression: a retrospective chart review. J Affect Disord. 2001;63(1–3):243–247. doi:10.1016/S0165-0327(00)00187-7
32. Oka M, Itoh Y, Wada M, Yamamoto A, Fujita T. Gabapentin blocks L-type and P/Q-type Ca 2+ channels involved in depolarization-stimulated nitric oxide synthase activity in primary cultures of neurons from mouse cerebral cortex. Pharm Res. 2003;20:897–899. doi:10.1023/A:1024078704020
33. Oka M, Itoh Y, Wada M, Yamamoto A, Fujita T. A comparison of Ca2+ channel blocking mode between gabapentin and verapamil: implication for protection against hypoxic injury in rat cerebrocortical slices. Br J Pharmacol. 2003;139(2):435–443. doi:10.1038/sj.bjp.0705246
34. Rajasekaran K, Jayakumar R, Venkatachalam K. Increased neuronal nitric oxide synthase (nNOS) activity triggers picrotoxin-induced seizures in rats and evidence for participation of nNOS mechanism in the action of antiepileptic drugs. Brain Res. 2003;979(1–2):85–97. doi:10.1016/S0006-8993(03)02878-6
35. Bang S, Yoo S, Hwang SW. Gabapentin attenuates the activation of transient receptor potential A1 by cinnamaldehyde. Exp Neurobiol. 2009;18(1):1–7. doi:10.5607/en.2009.18.1.1
36. Kukkar A, Bali A, Singh N, Jaggi AS. Implications and mechanism of action of gabapentin in neuropathic pain. Arch Pharm Res. 2013;36:237–251. doi:10.1007/s12272-013-0057-y
37. Bergquist F, Jonason J, Pileblad E, Nissbrandt H. Effects of local administration of L‐, N‐, and P/Q‐type calcium channel blockers on spontaneous dopamine release in the striatum and the substantia nigra: a microdialysis study in rat. J Neurochem. 1998;70(4):1532–1540. doi:10.1046/j.1471-4159.1998.70041532.x
38. Fass DM. Regulation of L-Type Calcium (2+) Channels in Pituitary Cells. University of Pittsburgh; 1999.
39. Guiard BP, Mansari ME, Blier P. Prospect of a dopamine contribution in the next generation of antidepressant drugs: the triple reuptake inhibitors. Curr Drug Targets. 2009;10(11):1069–1084. doi:10.2174/138945009789735156
40. Prica C, Hascoet M, Bourin M. Is co-administration of bupropion with SSRIs and SNRIs in forced swimming test in mice, predictive of efficacy in resistant depression? Behav Brain Res. 2008;194(1):92–99. doi:10.1016/j.bbr.2008.06.028
41. Yamada J, Sugimoto Y, Yamada S. Involvement of dopamine receptors in the anti-immobility effects of dopamine re-uptake inhibitors in the forced swimming test. Eur J Pharmacol. 2004;504(3):207–211. doi:10.1016/j.ejphar.2004.09.057
42. Lambert G, Johansson M, Ågren H, Friberg P. Reduced brain norepinephrine and dopamine release in treatment-refractory depressive illness: evidence in support of the catecholamine hypothesis of mood disorders. Arch Gen Psychiatry. 2000;57(8):787–793. doi:10.1001/archpsyc.57.8.787
43. Terbeck S, Savulescu J, Chesterman LP, Cowen PJ. Noradrenaline effects on social behaviour, intergroup relations, and moral decisions. Neurosci Biobehav Rev. 2016;66:54–60. doi:10.1016/j.neubiorev.2016.03.031
44. Mesripour A, Sajadian S, Hajhashemi V. Antidepressant-like effect of vitamin B6 in mice forced swimming test and the possible involvement of the noradrenergic system. J Rep Pharma Sci. 2019;8(2):133. doi:10.4103/jrptps.JRPTPS_52_18
45. Estrada VB, Matsubara NK, Gomes MV, Corrêa FMA, Pelosi GG. Noradrenaline microinjected into the dorsal periaqueductal gray matter causes anxiolytic-like effects in rats tested in the elevated T-maze. Life Sci. 2016;152:94–98. doi:10.1016/j.lfs.2016.03.011
46. Rantamäki T, Hendolin P, Kankaanpää A, et al. Pharmacologically diverse antidepressants rapidly activate brain-derived neurotrophic factor receptor TrkB and induce phospholipase-Cγ signaling pathways in mouse brain. Neuropsychopharmacology. 2007;32(10):2152–2162. doi:10.1038/sj.npp.1301345
47. Kozisek ME, Middlemas D, Bylund DB. Brain-derived neurotrophic factor and its receptor tropomyosin-related kinase B in the mechanism of action of antidepressant therapies. Pharmacol Ther. 2008;117(1):30–51. doi:10.1016/j.pharmthera.2007.07.001
48. Kim IB, Park S-C. Neural circuitry–neurogenesis coupling model of depression. Int J Mol Sci. 2021;22(5):2468. doi:10.3390/ijms22052468
49. Yan Z, Rein B. Mechanisms of synaptic transmission dysregulation in the prefrontal cortex: pathophysiological implications. Mol Psychiatry. 2022;27(1):445–465. doi:10.1038/s41380-021-01092-3
50. Singh P, Mehdi MM. Functional foods, bioactives, and cognitive impairments during aging. In: Plant Bioactives as Natural Panacea Against Age-Induced Diseases. Elsevier; 2023:271–286.
51. Sharma K, Sundriyal A, Loshali A, Agrawal M, Krishna CG, Singh Y. Mechanism of action of antidepressants. In: How Synthetic Drugs Work. Elsevier; 2023:255–273.
52. Eloziia N, Kumar N, Kothiyal P, Deka P, Nayak BK. A review on antidepressant plants. J Pharma Res. 2017;11(5):382–396.
53. Yardımcı H, Demir G. The relationship of diet quality and body composition with depression level in young women. Acta Scientiarum Health Sci. 2023;45:e60410–e60410. doi:10.4025/actascihealthsci.v45i1.60410
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.