Back to Journals » Drug Design, Development and Therapy » Volume 17
Effect of Food on the Pharmacokinetics of Tenofovir Amibufenamide: A Phase I, Randomized, Open-Label, Two-Period Crossover Trial in Healthy Adult Subjects
Authors Liu J , Wu M, Kai J, Lin M, Zheng Y, Jiang Y, Huang Q, Zhai Y , Qiu Y
Received 18 May 2023
Accepted for publication 23 September 2023
Published 9 October 2023 Volume 2023:17 Pages 3061—3072
DOI https://doi.org/10.2147/DDDT.S419084
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Tuo Deng
Jian Liu,1,2 Minglan Wu,1,2 Jiejing Kai,1,2 Meihua Lin,1,2 Yunliang Zheng,1,2 Yiya Jiang,1,2 Qian Huang,1,2 You Zhai,1,2 Yunqing Qiu2,3
1Department of Clinical Pharmacy, the First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang Province, People’s Republic of China; 2Zhejiang Provincial Key Laboratory for Drug Evaluation and Clinical Research, Hangzhou, Zhejiang Province, People’s Republic of China; 3State Key Laboratory for Diagnosis and Treatment of Infectious Disease, the First Affiliated Hospital, Zhejiang University, School of Medicine, Hangzhou, Zhejiang Province, People’s Republic of China
Correspondence: Yunqing Qiu, Zhejiang Provincial Key Laboratory for Drug Evaluation and Clinical Research, Hangzhou, Zhejiang Province, 310003, People’s Republic of China, Tel/Fax +86 571 87236606, Email [email protected]
Purpose: Tenofovir amibufenamide (TMF) is a novel nucleotide reverse transcriptase inhibitor. The aim of this study was to investigate the effect of food on the single-dose pharmacokinetic properties of TMF.
Patients and Methods: In this open-label, randomized, crossover study, after an overnight fast, eligible subjects received a single 25 mg dose of TMF tablet, either under fasted conditions or following consumption of a high-fat, high-calorie meal, followed by a two-week washout period. Blood samples were collected until 144 h after administration. TMF and its metabolite, tenofovir (TFV), were analyzed using validated liquid chromatography-tandem mass spectrometry methods. The geometric mean ratio (GMR) and the corresponding 90% confidence interval (CI) values of AUC0–t, AUC0–∞, and Cmax were acquired for analysis. The absence of an effect of food was indicated if the 90% CI values were within the predefined equivalence limits of 80%– 125%. Safety and tolerability were also assessed.
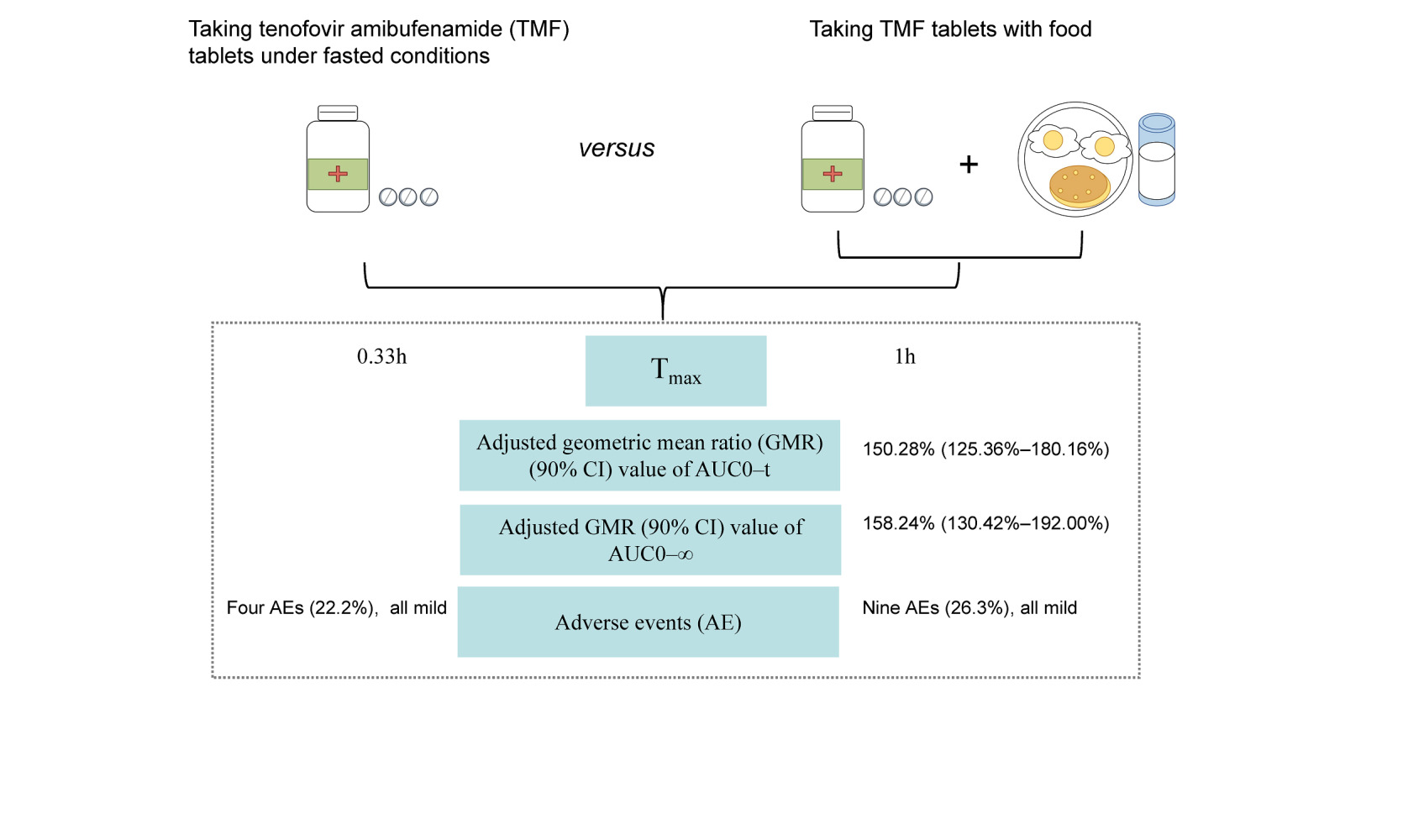
Results: For TMF, adjusted GMR (90% CI) values for the fed versus fasted states were 150.28% (125.36%– 180.16%), 158.24% (130.42%– 192.00%), and 57.65% (45.68%– 72.76%) for AUC0–t, AUC0–∞, and Cmax, respectively. For TFV, the GMR (90% CI) of Cmax was 82.00% (74.30%– 90.49%) after administration under fed conditions, slightly outside the bioequivalence boundary of 80%– 125%, while the corresponding values for AUC0–t and AUC0–∞ were within range. The absorption of TMF was delayed by food, with median Tmax values of 0.33 and 1.00 h in fasted and fed conditions, respectively. The adverse events observed in subjects were all mild.
Conclusion: Our results demonstrated that TMF tablets were well-tolerated in healthy volunteers. When TMF tablets were taken with food, Tmax was delayed and exposures of TMF and TFV were higher than under fasted conditions. The modest changes observed are not considered clinically relevant, so TMF can be taken with or without food.
Plain Language Summary: Tenofovir amibufenamide (TMF) is the first innovative, oral anti-hepatitis B drug to be developed in China. To better understand the effect of food on TMF bioavailability, 25 mg of TMF was given with or without food randomly to twenty healthy volunteers. Plasma samples were collected up to 144 h after dosing to measure the concentrations of TMF and its metabolite, tenofovir (TFV).
The rate of drug absorption was measured by determining the maximum plasma concentration of drug (Cmax) and the time to Cmax (Tmax). The amount of drug absorbed was measured using the area under the plasma concentration-time curve (AUC). When subjects were fed, the AUC values for TMF and TFV were higher than when they fasted. The Cmax values for TMF and TFV under fed conditions were lower compared with fasted conditions. The Tmax of TMF was delayed when subjects were fed compared with fasted. The modest changes observed are not considered clinically relevant. The adverse events (AEs) occurring in volunteers were all mild. Our findings suggested that TMF can be taken with or without food.
Keywords: tenofovir amibufenamide, food, pharmacokinetics, safety
Graphical Abstract:
Introduction
Hepatitis B is a potentially life-threatening liver infection caused by the hepatitis B virus (HBV). Currently, two types of drugs are used to treat chronic hepatitis B virus. One treatment is based on interferon (IFN, including normal and pegylated IFN).1,2 The other is based on nucleoside (acid) analogues (NAs), primarily lamivudine, adefovir dipivoxil, telbivudine, entecavir (ETV), tenofovir disoproxil fumarate (TDF) and tenofovir alafenamide (TAF). Compared with IFN, advantages including better safety, sustained and potent antiviral suppression of HBV, convenience of administration (once daily oral administration), tolerability, and affordability make the NAs preferred alternatives to IFN for treating chronic hepatitis B.1,3
The 2017 guidelines from the European Association for the Study of the Liver,2 the American Association for the Study of Liver Diseases 2018 guidelines,1 and the expert consensus for the management of chronic HBV infection in Asian Americans 20184 recommend ETV, TDF, or TAF as the preferred first-line options for management of chronic HBV infection. TDF, a prodrug of tenofovir (TFV), is a second-generation NA that was approved for treatment of chronic hepatitis B (CHB) in March 2008. As a nucleoside monophosphoric acid drug, TDF bypasses the requirement for in vivo monophosphorylation (the most difficult step of activation in vivo) and improves in vivo absorption, resulting in good clinical outcomes, but its use can result in renal toxicity and bone loss in patients with HBV infection.5
TAF, a structural modification of TDF, was approved by the Food and Drug Administration (FDA) to treat CHB infection in 2016. Results from two Phase III clinical trials demonstrated that at 48 weeks, the proportion of subjects with HBV DNA < 29 IU/mL in the 25 mg TAF group was comparable to that of subjects receiving 300 mg TDF, but the TAF group was superior to the TDF group in terms of spinal and hip bone mineral density (BMD) and creatinine safety indicators.6,7 Compared with TDF, TAF delivered the active metabolite more efficiently to hepatocytes with less systemic exposure,8,9 thus reducing bone and renal adverse effects.10,11
Tenofovir amibufenamide (TMF, codename: HS-10234) is a novel nucleotide reverse transcriptase inhibitor (NRTI) that contains an additional methyl group compared with TAF and is also a prodrug of TFV. Of the three prodrugs (TDF, TAF, and TMF), TMF exhibits the highest bioavailability. It gave much higher plasma concentrations as an ester form than TAF or TDF.12 When absorbed into hepatocytes, TMF is hydrolyzed by cathepsin A and carboxylesterase 1 to generate TFV, which is then phosphorylated to give TFV diphosphate. The higher stability of TMF in plasma guarantees better target loading, higher antiviral efficacy, and safety. TMF and TAF exhibited significantly stronger inhibition of HBV DNA replication than TDF in HBV-positive HepG2.2.15 cells. The anti-HBV activity of TMF was slightly stronger than TAF after 9 days of treatment (EC50 7.29 ± 0.71 vs 12.17 ± 0.56 nM).12
Food can affect the bioavailability of a drug by delaying gastric emptying, stimulating bile flow, changing gastrointestinal pH, increasing splanchnic blood flow, modulating the activity of drug-metabolizing enzymes and drug transporters, and interacting with drug ingredients or formulation.13 When conducting clinical trials, it is essential to evaluate how food affects the pharmacokinetic (PK) behavior of a drug to adapt dosing decisions and provide information for the clinical pharmacology, dosing and administration sections of the drug label. The Cmax of TAF was 82% higher, and AUC0–∞ was 20% lower, in fasted compared with fed conditions. The TAF AUC0–t was comparable under both conditions. Also, the mean maximum TAF concentrations were reached earlier in fasted compared with fed conditions.14 There is no unambiguous evidence that food affects the PK of TMF. This study aimed to assess the potential impact of a high-fat, high-calorie breakfast on the relative bioavailability of a single dose of TMF to provide evidence for its clinical use with or without food.
Materials and Methods
Study Population
Healthy Chinese males and females aged 18–45 years, with body mass indices (BMI) of 19–28 kg/m2 and weights greater than 45 or 55 kg, respectively, were recruited in this study. The trial was conducted between 8 July 2019 and 19 August 2019. All subjects were fully informed and voluntarily signed informed consent forms before the study. Medical history was taken, and complete physical examination, vital signs examination, 12-lead electrocardiogram (ECG), and clinical laboratory examination were performed to evaluate the health status of subjects during the screening period. Every healthy subject agreed to use effective contraception during the trial and for three months afterwards. Subjects with clinically significant abnormalities at screening were excluded from the study. Other major exclusions included: any history of serious disease (including, but not limited to, digestive, cardiovascular, respiratory, urinary, musculoskeletal, endocrine, nervous, hematologic, immune system, or metabolic disorder); susceptibility to allergic reactions or allergy to the components or analogs of the drug; a history of alcohol or drug abuse; excessive smoking or drinking (tea, coffee and caffeinated beverages); blood donation or acute blood loss in the previous three months; participation in other drug clinical trials within the previous three months; dysphagia or any history of gastrointestinal disorders affecting drug uptake; use of prescription or non-prescription medications in the two weeks before first dosing; surgery that would affect in vivo drug disposition; special dietary requirements. Female subjects who were breastfeeding or pregnant during the screening period were also excluded.
Study Procedures
This was a single-center, randomized, two-period, two-sequence, crossover clinical trial conducted in healthy adult Chinese subjects. The study protocol was approved by the independent Ethical Committee of The First Affiliated Hospital, Zhejiang University School of Medicine. The study was conducted following the Guidelines for Good Clinical Practice,15 Technical Guidelines for Clinical Pharmacokinetic Studies of Chemical Drugs by the China National Medical Products Administration (NMPA),16 Guidance for Industry: Food-Effect Bioavailability and Fed Bioequivalence Studies issued by the US FDA,17 International Conference on Harmonization Good Clinical Practice guidelines,18 and the Declaration of Helsinki (as revised, Fortaleza, 2013).19 Before undergoing any study procedures, all subjects were informed of the nature, objectives, procedures, possible risks, and requirements of the study. The study was registered at www.chictr.org.cn (ChiCTR1900027135).
The study was performed at the Phase I Clinical Research ward of the Research Center in Clinical Pharmacy. All subjects were hospitalized the day before dosing and randomly assigned to group AB or BA. Group AB received a single 25 mg oral dose of TMF tablet (25 mg per tablet, batch number 20190101, expiration date 2020/12, obtained from Jiangsu Hansoh Pharmaceutical Group Co. Ltd., Jiangsu, China) in the first treatment period, conducted under fasted conditions. Subjects received the same dose in the second period, conducted under fed conditions (within 30 min of a standardized high-fat breakfast). Subjects in Group BA received the same dose but the treatment periods were reversed. The randomization table was generated by the statistical unit using SAS (version 9.4) with a ratio between groups of 1:1. There was a two-week washout phase between the two treatment periods. The standardized high-fat, high-calorie breakfast [973.3 kcal; 61.7 g fat (555.3 kcal), 37.5 g protein (150 kcal), and 67 g carbohydrate (268 kcal)] consisted of two eggs fried in 30 g of mixed oil, one pancake made with 75 g flour, 50 g lean meat and 10 g of mixed oil, and 250 mL of whole milk. All volunteers were required to fast overnight for at least 10 h before drug administration. On the first day of period 1, subjects under fed conditions were required to eat a standardized high-fat breakfast 30 min before treatment. TMF was taken orally with 240 mL water in each group. Drinking water was allowed as desired except for 1 h before and after drug administration. The same standard lunches and dinners were served to each group 4 and 10 h after dosing. In the second period, which started on day 15, the treatments were the same for each group except that the dietary state was crossed over. All volunteers received standardized meals scheduled at the same time in each period of the study.
PK Assessment
Blood samples (4 mL) were collected from an indwelling venous catheter into coded heparinized tubes at pre-dose (baseline) and at 5 min, 10 min, 20 min, 30 min, 45 min, 1 h, 1.5 h, 2 h, 3 h, 4 h, 6 h, 8 h, 12 h, 24 h, 48 h, 72 h, 96 h, 120 h, and 144 h post drug administration. The samples were centrifuged at 2000 × g and 4 °C for 10 min to separate the plasma within 30 min. Plasma samples were temporarily stored at −20 ± 8 °C within 60 min and transferred to an ultra-low temperature freezer (−70 ± 10 °C) within 12 h for storage until analysis. All collection and processing procedures were performed in ice baths.
Safety and Tolerance Assessments
The investigators conducted safety and tolerability evaluations, including laboratory tests, physical examinations, 12-lead ECG, vital signs measurements, and recording of events spontaneously reported by volunteers during the study. Laboratory tests included urinalysis, hematology, blood chemistry, electrolyte, and coagulation function. Vital signs, such as blood pressure, pulse, temperature, and respiratory rate, were monitored before administration and at 2, 4, 12, 24, 48, 72, 96, 120, and 144 h after each drug administration. The severity of adverse events was determined according to CTCAE5.0.
Bioanalytical Methods
Plasma concentrations of TMF and its metabolite, TFV, were determined using an LC-MS/MS method that was previously validated by Shanghai Frontage Lab Bio-technology Co., Ltd. (Shanghai, China). The sample analysis and determination method followed the guidelines of the FDA, Bioanalytical Method Validation-Guidance for Industry. The LC-MS/MS system included an LC-30AD high-performance liquid chromatography system (Shimadzu, Tokyo, Japan) and API4000 (for TMF) and 6500+ (for TFV) triple quadrupole mass spectrometer (AB Sciex, Ontario, Canada). Data were acquired using Analyst software, version 1.6.3. After performing protein precipitation, the compounds were detected by MS/MS in multiple reaction monitoring mode using electrospray ionization with positive polarity. The following ion transitions were monitored: m/z 491→270 for TMF and m/z 288→176 for TFV. The lower limit of quantification (LLOQ) was 1 ng/mL for TMF and 0.3 ng/mL for TFV. The linearity ranges of the detection method for TMF and TFV were 1–1000 ng/mL and 0.3–300 ng/mL, respectively. The inter-assay precision range (%CV) for TMF at 1, 3, 60, and 750 ng/mL was 2.0–4.5, and the range for TFV at 0.3, 0.9, 10, 100, and 225 ng/mL was 2.5–10.4. The inter-assay accuracy range (%RE) was −6.6 to −3.0 for TMF and 1.1 to 13.1 for TFV. Stability tests showed that plasma sample decomposition was <10% for all conditions examined (17 h in an ice bath, four freeze-thaw cycles, and after 56 days storage at −70 °C or −80 °C for TMF; 18 h in an ice bath, six freeze-thaw cycles, after 55 days storage at −80 °C or −20 °C, and after 59 days storage at −70 °C for TFV). All samples were analyzed within the storage stability window.
PK and Statistical Analysis
Pharmacokinetic parameters were calculated from the plasma concentration-time data by non-compartmental methods using Phoenix WinNonlin 8.1 software (Certara, St. Louis, MO). The primary variables were the area under the plasma concentration-time curve from time 0 to the last quantifiable concentration and infinity (AUC0–t and AUC0–∞) and the maximum drug concentration (Cmax). Additional pharmacokinetic parameters were the time to reach Cmax (Tmax), the terminal half-life (t1/2z), and the terminal elimination rate constant (λz). The Cmax and Tmax values were obtained directly from the observed data. The AUC0–t was calculated using the linear trapezoidal rule. The AUC0–∞ was calculated as AUC0–t + Ct/λz, where Ct is the last measurable concentration, and λz is the slope of the log-linear regression of the terminal concentration data points. The t1/2z was calculated as (ln2)/λz.
For Cmax, AUC0–t, and AUC0–∞, natural log-transformed data were analyzed for differences between fed and fasted conditions using an analysis of variance (ANOVA) model for a two-period crossover design, including sequence, subject within sequence, treatment, and period as fixed effects. The significance was assessed independently and tested (two 1-sided) for the difference at the 5% level. Results were expressed as least squares means, and the geometric means of fed/fasted ratios and the corresponding 90% confidence intervals (CIs) were determined. An absence of food effect was shown if the 90% CIs were within the predefined equivalence limits of 80%–125% for the ratios of fed/fasted based on guidance from the FDA.17 The Wilcoxon signed rank test was used for Tmax assessment.
Descriptive statistics were used to summarize baseline characteristics and demographic data of the participants for safety and tolerability. All safety data, including laboratory tests, vital signs, graded adverse events (AEs), and their incidence, were recorded. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Inc, Cary, North Carolina).
Results
Study Population
A total of 80 subjects were screened, and 20 healthy subjects were enrolled. One subject suffered from vomiting during the first treatment period after eating a high-fat meal and withdrew from the trial without taking medication. Another subject withdrew after a positive blood pregnancy test result on the day of admission to the second treatment period. Nineteen subjects were included in the safety and PK analysis after receiving at least one drug dose, and 18 subjects completed the two PK assessments. A flowchart showing conduct of the trial is illustrated in Figure 1.
 |
Figure 1 Flowchart showing conduct of the trial. |
The demographic characteristics of the 19 subjects (11 males and 8 females) included in the safety and PK analysis are summarized in Table 1. The mean age was 26.8 years (range 19–44), and the mean BMI was 22.49 kg/m2 (range 19.1–27.3). All participants were Chinese, 17 were Han, and two were Tujia and Manchu ethnic minorities.
 |
Table 1 Demographic Characteristics of Healthy Volunteers Included in Safety and PK Analysis |
Pharmacokinetics and Food-Effect Bioavailability Evaluation
The mean plasma concentration-time profiles of TMF and its metabolite, TFV, after administration of a single oral dose of 25 mg TMF are illustrated in Figure 2 (linear and semilogarithmic). Kinetic changes in individual plasma concentration-time profiles of TMF and TFV are illustrated in Figure 3. The plasma profile of TMF was moderately influenced by the concomitant administration of a high-fat, high-calorie meal. Absorption of TMF was delayed, and the degree of absorption was increased under fed conditions (high-calorie, high-fat meal). Tmax was delayed by 0.67 h (1.00 h vs 0.33 h), Cmax was reduced by about 42% (154.45 ng/mL vs 256.23 ng/mL), AUC was increased by about 50% (178.62 ng/mL vs 118.34 ng/mL for AUC0–t, 188.23 ng/mL vs 119.61 ng/mL for AUC0–∞), t1/2Z was prolonged by about 200% (1.32 h vs 0.44 h). The plasma TFV concentration-time profiles were comparable under the two conditions. Cmax was 18% lower in fed compared with fasted conditions, but the AUC was increased with no significant change under fed conditions. Table 2 provides a summary of the pharmacokinetic parameters of interest.
 |
Table 2 Plasma Pharmacokinetic Parameters of Tenofovir Amibufenamide (TMF) and Tenofovir (TFV) After Single-Dose Administration of TMF (25 mg) |
The geometric mean ratio (GMR) (90% CI) values of TMF for the fed versus fasted state were 150.28% (125.36%–180.16%), 158.24% (130.42%–192.00%), and 57.65% (45.68%–72.76%) for AUC0–t, AUC0–∞, and Cmax, respectively. All 90% CI values fell outside the bioequivalence boundaries of 80%–125%. The lower limit of the 90% CI of GMR for AUC was clearly above the bioequivalence boundary, and the upper limit of the 90% CI of GMR for Cmax was clearly below the bioequivalence boundary, indicating that TMF exposure parameters under fasted conditions were not bioequivalent to those under fed conditions. For TFV, the corresponding 90% CI of the GMR values for Cmax, 82.00% (74.30%–90.49%), were slightly outside the bioequivalence boundaries of 80%–125% after administration under fed conditions, whereas AUC0–t and AUC0–∞ were comparable under both conditions (90% CI of the GMR within the 80% to 125% interval) (Table 3).
 |
Table 3 Statistical Analysis of the Effect of Food on Tenofovir Amibufenamide (TMF) and Tenofovir (TFV) Pharmacokinetic Parameters |
The ANOVA indicated no sequence or period effects for any pharmacokinetic parameters of TMF or TFV. No significant differences were found in AUC0–t for TFV, but significant differences were found in Cmax, AUC0–t, AUC0–∞, and t1/2z for TMF, and Cmax, AUC0–∞, and t1/2z for TFV between fed and fasted conditions. The Tmax data demonstrated statistically significant differences between fed and fasted conditions for TMF and TFV.
Safety and Tolerability
The TMF tablets were generally well tolerated in both fasting and fed conditions. Nineteen volunteers who received at least a single dose of TMF were carefully evaluated for any AEs (Table 4). In fasted conditions, a total of four clinically relevant abnormalities, which were considered as treatment-emergent adverse events (TEAEs), were observed for laboratory values in four of the 18 subjects (22.2%) in the study, including three cases of elevated blood triglycerides (16.7%) and a single case of increased total bile acids (5.6%). In fed conditions, five out of 19 subjects (26.3%) experienced at least one TEAE in the study, including two cases of dizziness (10.5%), two cases of nausea (10.5%), one case of vomiting (5.3%), one case of elevated γ-glutamyl transferase (5.3%), one case of elevated serum creatine phosphokinase (5.3%), one case of elevated blood uric acid (5.3%), and one case of decreased neutrophil count (5.3%). These AEs were all mild and were considered grade 1 by the investigators. There were no discontinuations due to AEs, and no deaths or serious adverse events (SAEs) occurred during the study.
 |
Table 4 Summary of Treatment-Related Adverse Events (TEAEs) |
Discussion
TMF is a class 1 innovative antiviral drug independently developed by Jiangsu Hansoh Pharmaceutical Group Co., Ltd., and is the first oral anti-hepatitis B drug originally developed in China. This drug is a new generation of monophosphoramide monoester prodrug of TFV. It has been reported12 that, like TAF, TMF exhibited significantly stronger anti-HBV efficacy and correcting effects for disordered hepatic biochemical metabolism than TDF in vitro and in vivo, while TMF was superior to TAF. The tenofovir diphosphate (TFV-DP) level in hepatocytes produced by TFV prodrugs plays a key role in HBV inhibition. TMF produced the most TFV-DP (1.43-fold and 3.55-fold higher liver levels of TFV-DP in rats compared with TAF or TDF) in hepatocytes and thus exhibited the most potent HBV inhibitory effect. Liu et al published 48-week results from a large Phase III clinical study of TMF in CHB patients in China.20 The results indicated that efficacy in the experimental group was not inferior to that of the control group (TDF) with less than one-tenth of the dose (25 mg vs 300 mg), while bone and kidney safety in the experimental group were significantly better than in the control group. Since June 2021, TMF has been approved for use in mainland China, introducing a new treatment option for patients with chronic hepatitis B.
The dose (25 mg) of TMF used in this study was justified by data from the completed Phase 1a/b clinical study evaluating the pharmacokinetics and efficacy of TMF under fasting conditions,21 which demonstrated that 10–40 mg (10, 25, or 40 mg) were safe doses of TMF in healthy volunteers and patients with CHB infection. The Tmax values of TMF and TFV were 0.25–0.26 h and 0.75–1.0 h; the mean values of t1/2 were 0.27–0.69 h and 25.95–43.22 h, respectively. The PK profiles of TMF and TFV in plasma under fasted condition observed in this study after a 25 mg dose were consistent with previous reports.21
The administration of TMF tablets under fed conditions resulted in a 42% decrease in the mean Cmax and 50% increases in AUC0–t and AUC0–∞ values for TMF compared with those following administration under fasted conditions. The 90% CIs of the GMRs for Cmax and AUC were significantly below and above the bioequivalence boundaries of 80%–125%, respectively, indicating that the bioavailability of TMF following administration under fed conditions was not equivalent to that following administration under fasted conditions. This was also observed in previous studies with TAF.14 In contrast, the Cmax of TFV was slightly enhanced when TMF was administered after a high-calorie, high-fat meal. However, there was no effect on AUC values of TFV, even though the 90% CI of GMR for Cmax under fasted conditions was marginally lower (74.30%) than the lower limit of the bioequivalence boundary (80%). The findings of the present food-effect bioavailability study in respect of TFV were consistent with those of an earlier investigation, which assessed the pharmacokinetics of TFV after a single dose of TDF (300 mg) under fasted and fed conditions and showed that there was a minimal effect of food on TFV exposure.22 A significant increase (p < 0.05) of 0.67 h in median Tmax was observed for TMF under fed conditions, possibly due to delayed gastric emptying. According to this finding, food delays gastrointestinal absorption, and the rate-limiting step in absorption may be passage of the drug from the stomach into the intestine.
Food usually has the greatest effect on bioavailability when a drug is taken immediately after a meal.23 In accordance with Guidelines of the NMPA16 and FDA,17 a high-fat, high-calorie breakfast was chosen for this study, since such a meal can be expected to provide the greatest impact on the physiology of the gastrointestinal tract and absorption of the test drug. Under these extreme conditions, the PK parameters changed moderately. Although the package insert of TMF recommends that the medication is taken with food, this study provides evidence that TMF can be taken with a standard or light meal, or without food.
TMF tablets were generally well tolerated both under fed and fasted conditions. No grade 3/4 or SAEs occurred. The most frequently reported AEs during the study were elevated blood triglycerides, total bile acids, γ-glutamyl transferase, serum creatine phosphokinase, and blood uric acid, dizziness, decreased neutrophil count, nausea, and vomiting. These AEs were mild and transient, and they were tolerated by subjects without intervention.
The results from this study are the first to report effects of food on the pharmacokinetic characteristics of TMF in healthy volunteers, providing crucial information for labeling recommendations for TMF dosing.
Conclusion
In conclusion, our results demonstrate that TMF tablets are well-tolerated in healthy volunteers. Consumption of TMF tablets with food delayed Tmax and increased exposure compared with administration after fasting. The modest changes observed are not considered clinically relevant, thus TMF can be taken with or without food.
Data Sharing Statement
The data contained in this article will be shared upon reasonable request to the corresponding author.
Ethics Statement
The study protocol was approved by the independent Ethical Committee of The First Affiliated Hospital, Zhejiang University School of Medicine. Before undergoing any study procedures, all subjects were informed of the study’s nature, objectives, procedures, possible risks, and requirements.
Acknowledgments
We thank all staff members of the Research Center for Clinical Pharmacy for their hard work and the volunteers who participated in the study. We also would like to thank Shanghai Frontage Lab Bio-technology Co., Ltd. for analyzing biological samples and Shanghai Bojia Medical Technology Co., Ltd. for the statistical data analysis. The graphical abstract was drawn by Figdraw (https://www.figdraw.com/#/).
Funding
The study was funded by Jiangsu Hansoh Pharmaceutical Group Co., Ltd. (Jiangsu, China).
Disclosure
All authors confirm that there are no conflicts of interest in this work.
References
1. Terrault NA, Lok ASF, McMahon BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67(4):1560–1599. doi:10.1002/hep.29800
2. European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines for the management of hepatitis B virus infection. J Hepatol. 2017;67(2):370–398. doi:10.1016/j.jhep.2017.03.021
3. World Health Organization. Guidelines for the prevention, care and treatment of persons with chronic hepatitis B infection; 2015. Available from: https://www.who.int/publications/i/item/guide-care-treat-persons-diagnosed-chronic-hepatitis-c-978-92-4-154905-9.
4. Tong MJ, Pan CQ, Han SB, et al. An expert consensus for the management of chronic hepatitis B in Asian Americans. Aliment Pharmacol Ther. 2018;47(8):1181–1200. doi:10.1111/apt.14577
5. Cathcart AL, Chan HL, Bhardwaj N, et al. No resistance to tenofovir alafenamide detected through 96 weeks of treatment in patients with chronic hepatitis B infection. Antimicrob Agents Chemother. 2018;62(10):e01064–18. doi:10.1128/AAC.01064-18
6. Buti M, Gane E, Seto WK, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of patients with HBeAg-negative chronic hepatitis B virus infection: a randomised, double-blind, Phase 3, non-inferiority trial. Lancet Gastroenterol Hepatol. 2016;1(3):196–206. doi:10.1016/S2468-1253(16)30107-8
7. Chan HL, Fung S, Seto WK, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of HBeAg-positive chronic hepatitis B virus infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol Hepatol. 2016;1(3):185–195. doi:10.1016/S2468-1253(16)30024-3
8. Agarwal K, Fung SK, Nguyen TT, et al. Twenty-eight day safety, antiviral activity, and pharmacokinetics of tenofovir alafenamide for treatment of chronic hepatitis B infection. J Hepatol. 2015;62(3):533–540. doi:10.1016/j.jhep.2014.10.035
9. Babusis D, Phan TK, Lee WA, et al. Mechanism for effective lymphoid cell and tissue loading following oral administration of nucleotide prodrug GS-7340. Mol Pharm. 2013;10(2):459–466. doi:10.1021/mp3002045
10. Woodward CL, Hall AM, Williams IG, et al. Tenofovir-associated renal and bone toxicity. HIV Med. 2009;10(8):482–487. doi:10.1111/j.1468-1293.2009.00716.x
11. Bam RA, Yant SR, Cihlar T. Tenofovir alafenamide is not a substrate for renal organic anion transporters (OATs) and does not exhibit OAT-dependent cytotoxicity. Antivir Ther. 2014;19(7):687–692. doi:10.3851/IMP2770
12. Hong X, Cai Z, Zhou F, et al. Improved pharmacokinetics of tenofovir ester prodrugs strengthened the inhibition of HBV replication and the rebalance of hepatocellular metabolism in preclinical models. Front Pharmacol. 2022;13:932934. doi:10.3389/fphar.2022.932934
13. Koziolek M, Alcaro S, Augustijns P, et al. The mechanisms of pharmacokinetic food-drug interactions -A perspective from the UNGAP group. Eur J Pharm Sci. 2019;134:31–59. doi:10.1016/j.ejps.2019.04.003
14. Crauwels HM, Baugh B, Van Landuyt E, et al. Bioequivalence of the once-daily single-tablet regimen of Darunavir, Cobicistat, Emtricitabine, and Tenofovir Alafenamide compared to combined intake of the separate agents and the effect of food on bioavailability. Clin Pharmacol Drug Dev. 2019;8(4):480–491. doi:10.1002/cpdd.628
15. China National Medical Products Administration. Guideline for good clinical practice; 2003. Available from: https://www.nmpa.gov.cn/xxgk/fgwj/bmgzh/20030806010101443.html.
16. China National Medical Products Administration. Technical Guidelines for Clinical Pharmacokinetic Studies of Chemical Drugs; 2007. Available from: https://www.cde.org.cn/zdyz/domesticinfopage?zdyzIdCODE=4247ffceca57f2b47aa0f67a3fdc7c43.
17. US Food and Drug Administration. Guidance for Industry: assessing the Effects of Food on Drugs in INDs and NDAs-Clinical Pharmacology Considerations; 2019. Available from: https://www.regulations.gov/document/FDA-2018-D-4368-0016.
18. The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). Topic E 6 (R2): guideline for Good Clinical Practice; 2016. Available from: https://database.ich.org/sites/default/files/E6_R2_Addendum.pdf.
19. World Medical Association. WMA Declaration of Helsinki-Ethical Principles for Medical Research Involving Human Subjects; 2013. Available from: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/.
20. Liu Z, Jin Q, Zhang Y, et al. Randomised clinical trial: 48 weeks of treatment with tenofovir amibufenamide versus tenofovir disoproxil fumarate for patients with chronic hepatitis B. Aliment Pharmacol Ther. 2021;54(9):1134–1149. doi:10.1111/apt.16611
21. Zhang H, Hu Y, Wu M, et al. Randomised clinical trial: safety, efficacy and pharmacokinetics of HS-10234 versus tenofovir for the treatment of chronic hepatitis B infection. Aliment Pharmacol Ther. 2021;53(2):243–252. doi:10.1111/apt.16196
22. Lu C, Jia Y, Chen L, et al. Pharmacokinetics and food interaction of a novel prodrug of tenofovir, tenofovir dipivoxil fumarate, in healthy volunteers. J Clin Pharm Ther. 2013;38(2):136–140. doi:10.1111/jcpt.12023
23. Yasuji T, Kondo H, Sako K. The effect of food on the oral bioavailability of drugs: a review of current developments and pharmaceutical technologies for pharmacokinetic control. Ther Deliv. 2012;3(1):81–90. doi:10.4155/tde.11.142
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


