Back to Journals » Drug Design, Development and Therapy » Volume 17
Effect of Esketamine Gargle on Postoperative Sore Throat in Patients Undergoing Double-Lumen Endobronchial Intubation: A Randomised Controlled Trial
Authors Liang J, Liu J, Qiu Z, Sun G, Xiang P, Hei Z, Li X
Received 11 July 2023
Accepted for publication 9 October 2023
Published 19 October 2023 Volume 2023:17 Pages 3139—3149
DOI https://doi.org/10.2147/DDDT.S430077
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Qiongyu Guo
Jianfen Liang,1,* Jun Liu,1,* Zhuolin Qiu,1 Guoliang Sun,1 Ping Xiang,2 Ziqing Hei,1 Xiang Li1
1Department of Anesthesiology, The Third Affiliated Hospital, Sun Yat-Sen University, Guangzhou, 510630, People’s Republic of China; 2Department of Medical Quality Management, Nanfang Hospital, Southern Medical University, Guangzhou, 510630, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiang Li, Department of Anesthesiology, The Third Affiliated Hospital of Sun Yat-Sen University, Number 600, Tianhe Road, Guangzhou, 510630, Guangdong province, People’s Republic of China, Tel +86-20-85253132, Fax +86-20-85252297, Email [email protected]
Background: Postoperative sore throat is a frequent adverse event after double-lumen endobronchial tube (DLT) intubation. The aim of this study was to investigate whether esketamine gargle has a preventive effect on the incidence of postoperative sore throat in patients undergoing DLT intubation.
Methods: This trial included 140 patients undergoing elective thoracic surgery at the third affiliated hospital of Sun Yat-Sen University. Patients were randomly allocated into the following two groups of 70 patients each: the control group, gargling with saline of 30 mL, and the esketamine group, gargling with an esketamine solution of 30 mL (2 mL/50 mg esketamine in 28 mL saline), 5 min prior to anaesthesia induction. The primary outcome was the incidence of sore throat 24 h after surgery. The main secondary outcomes included the incidence of sore throat and hoarseness at 1 h and 48 h after surgery, as well as the intraoperative haemodynamic responses.
Results: The incidence of sore throat was significantly higher in the control group (47.1%, 33/70 patients) compared with the esketamine group (12.9%, 9/70 patients) at 24 h after surgery (RD, 0.41; 95% confidence interval, 0.26– 0.57; p < 0.001). Furthermore, the incidence of sore throat at 1 h (p = 0.027), 24 h (p = 0.019), and seventh day (p = 0.031) as well as hoarseness at 1 h (p = 0.027), 24 h (p = 0.019), and 48 h (p = 0.031) after surgery were reduced in the esketamine group. Significant differences were seen in the peak levels of systolic blood pressure, mean arterial blood pressure, and heart rate between the groups during the intubation (p < 0.05). Besides, no patient developed an adverse reaction to esketamine.
Conclusion: Preoperative gargling of esketamine can provide an effect against sore throat after DLT intubation without adverse side effects and effectively inhibit sharp elevations in heart rate and blood pressure during double-lumen intubation procedures.
Keywords: esketamine, topical application, postoperative sore throat, double-lumen endobronchial tubes, intubation
Introduction
Postoperative sore throat commonly occurs after tracheal intubation and ranks sixth among adverse outcomes associated with general anaesthesia.1,2 Evidence indicates that a large-sized tracheal tube is a potential risk factor for postoperative sore throat.3,4 Because of their larger external diameters, double-lumen endobronchial tubes, which are widely used in surgeries that require bronchial blockage and one-lung ventilation, may induce postoperative sore throat more often than single-lumen tracheal tubes.3 Postoperative sore throat usually worsens during swallowing and is difficult to treat, even if wound pain is well managed with narcotics.5 Thus, efforts are needed to prevent this complication in patients undergoing double-lumen tube intubation.
Esketamine is a newly developed N-methyl-D-aspartate receptor antagonist. As the dextroisomer of ketamine, esketamine has a greater analgesic effect than racemic ketamine and is approximately two- to four-fold as potent.6 Evidence indicates that ketamine gargle reduces postoperative sore throat after single-lumen tube intubation due to its suppressive effect on N-methyl-D-aspartate receptors and its agonist effects on opioid receptors in the oral and upper respiratory tract mucosa.7 Given this, we hypothesised that esketamine gargle may effectively reduce postoperative sore throat after double-lumen tube intubation. Thus, the current study was conducted to determine whether preoperative administration of esketamine gargle decreases the incidence of postoperative sore throat in patients receiving double-lumen tube intubation for one-lung anaesthesia.
Methods
Ethics
This prospective, double-blind, single-centre, randomised controlled trial was approved by the Research Ethics Committee of the third affiliated hospital of Sun Yat-Sen University and registered in the Chinese Clinical Trial Registry (ChiCTR2100044061). Written informed consent was obtained from all participants. In this report, we do not contain any personal information that could lead to the identification of the patient(s). The work has been reported in line with the Consolidated Standards of Reporting Trials (CONSORT) Guidelines,8 and protocols were conducted in accordance with the tenets of the Declaration of Helsinki.
Patients
This clinical trial was conducted between November 2021 and November 2022. We enrolled patients who were hospitalised at the third affiliated hospital of Sun Yat-Sen University for elective thoracic surgery with double-lumen tube intubation for one-lung ventilation. The age range of eligible participants was 18–70 years old, and the ASA physical status classification was limited to grades I–III. Those who had a Mallampati score of IV, pre-existing sore throat or hoarseness, recent or recurrent respiratory tract infection, tracheostomy tube, predicted postoperative aspiration, known or predicted difficult airway, or contraindications to esketamine were excluded. Patients who had a Cormack-Lehane grade III or IV laryngoscopic view, required orogastric or nasogastric tubes during mask ventilation, or required postoperative mechanical ventilation were also excluded.
Randomisation
Participants were divided into two groups (control group or esketamine group) with a 1:1 ratio, using random lists generated by SPSS statistical software (Version 20.0, IBM Corp., NY, USA) sealed in opaque, sequentially numbered envelopes. Considering the potential adverse events from esketamine, the group assignment of each patient was revealed to the participating anaesthesiologist who had no knowledge about the purpose of the data analysis. Data collection was conducted by an independent research coordinator who had no knowledge of patient assignments.
Intervention
There was no premedication for the participants. Intraoperative monitoring included non-invasive and invasive blood pressure measurement, electrocardiography, pulse oximetry, and measurement of the Narcotrend Index (MT MonitorTechnik GmbH & Co., KG, Germany). Five minutes before anaesthesia induction, 50 mg esketamine (25 mg.l−1, Hengrui Medicine, Jiangsu, China) mixed in 28 mL saline or 30 mL normal saline was poured into a paper cup and administered to patients in the esketamine and control groups, respectively, by a nurse who was blinded to the assigned intervention grouping information. According to the previous study, patients were required to gargle for 30s.9
Anaesthesia management was standardised for all participants. Medications used to induce general anaesthesia included dexamethasone, 5 mg; midazolam, 0.02 mg.kg−1; propofol, 1.5–2.0 mg.kg−1; sufentanil, 0.5 µg.kg−1; and cisatracurium, 0.2 mg.kg−1. Muscle relaxation was assessed every 10s using a “train of four” (neuromuscular transmission) monitor (Mindray Bio-Medical Electronics, China). The double-lumen tube intubation was performed using a Mallinckrodt tracheal tube (Covidien, MA, USA) with video laryngoscopy (TD-C-IV-3, UE Medical Corporation, Zhejiang, China) by an independent anaesthesiologist with over ten years of experience in double-lumen tube intubation who was unaware of the patient’s group assignment. Tube size was selected based on the preoperative computed tomography-measured bronchial width of each patient, as described in a previous study.10 Using fibreoptic bronchoscopy, the position of the double-lumen tube was adjusted immediately after insertion, and the patient was turned to the lateral position.
Anaesthesia was maintained through micropump infusion of remifentanil (0.12–0.2 µg.kg−1 per min) plus cisatracurium (1–2 µg.kg−1 per min), with inhalation of 2–4 vol% sevoflurane in an oxygen-medical air mixture, to achieve adequate anaesthetic depth (Narcotrend stages C, D, E0, and E1). Concurrently, patients were monitored for changes in blood pressure outside ±20% from baseline. In this study, we determined the baseline blood pressure according to the report by Bijker JB et al.11 They defined the baseline blood pressure as the average of the blood pressure measured at the preoperative evaluation clinic (measured on either arm with the patient in the sitting position) and all available blood pressure measurements in the operating room before the administration of induction medication.
The tidal volume was set at 4–6 mL.kg−1 during the one-lung ventilation period, and other ventilator parameters were adjusted to the peak inspiratory pressure of < 35 cmH2O and end tidal CO2 of < 45 mmHg.12 We utilised the ideal body weight (IBW) to calculate tidal volume. As suggested by Appelbaum N and Clarke J in their editorial, the IBW was determined using the body mass index method, specifically calculated as IBW = 22×H2, where H represents the patient’s height in meters.13
At the end of surgery, all patients were intravenously injected with tropisetron (5 mg) and neostigmine (0.05 mg.kg−1) plus atropine (0.02 mg.kg−1) to antagonise the residual neuromuscular block. To alleviate postoperative pain, the thoracic surgeon performed wound infiltration with 0.375% ropivacaine. Moreover, intravenous patient-controlled analgesia was induced using a combination of sufentanil (150 µg) and tropisetron (15 mg) in 150 mL 0.9% sodium chloride administered at a continuous infusion rate of 2.0 mL.h−1, with a single bolus dose of 1 mL and a lockout time of 15 min. Tramadol (50 mg) was administered for rescue analgesia if the postoperative visual analogue pain scale score of the patient was > 4.
Data Collection
Demographic data of each patient, including age, sex, height, and weight, were recorded. An anaesthesiologist blind to group assignment rated the Mallampati grade of each patient prior to surgery. The anaesthesiologist who performed the tracheal intubation assessed the Cormack and Lehane grading and rated the subjective sensation grade (none, mild, moderate, or severe) of the advancement resistance of the double-lumen tube through the glottis. Intubation time was defined using the definition of Park et al,14 which is the duration from the insertion of the laryngoscope blade to the achievement of an end-tidal CO2 value ≥ 30 mmHg. Double-lumen tube positioning time was defined as the duration of bronchoscopy to verify the correctness of the position of the double-lumen tube. We recorded the number of intubation attempts and position adjustments per participant.
Haemodynamic variables, including systolic blood pressure, mean arterial blood pressure, and heart rate, were recorded at baseline (T1), 1 min after gargling (T2), immediately before tracheal intubation (T3), and at 2 (T4) and 5 min (T5) after intubation. Additionally, the peak values of systolic blood pressure, mean arterial blood pressure, and heart rate during intubation were noted.
At 1 h, 24 h, 48 h, and the seventh day after surgery, a staff member blind to group assignment evaluated the level of sore throat and hoarseness in each patient. If the patient is discharged within seven days after surgery, we conduct follow-up and assessment through the telephone annually. Sore throat was measured and categorised as described previously: none (no sore throat [Grade I]); mild (discomfort with deglutition [Grade II]); moderate (constant discomfort that increases with swallowing [Grade III]); and severe (discomfort that interferes with eating and requires rescue medication [Grade IV]).14,15 A grade ≥ II was regarded as unacceptable. In accordance with a previous study,15 we used a numerical rating scale, ranging from 0 (no sore throat) to 10 (worst throat discomfort imaginable), to measure the severity of sore throat. Hoarseness was evaluated on a grade I–IV scale as follows: none (no hoarseness [Grade I]); mild (minimal change in voice character that the patient complained of only when examined [Grade II]); moderate (moderate change in voice character that the patient complained of [Grade III]); or severe (severe deterioration in voice character observed by the examiner [Grade IV]).14 A grade ≥ II was regarded as an occurrence of hoarseness. Postoperative wound pain was measured at rest and during coughing on the first, second, and seventh postoperative days using an 11-point numeric rating scale. According to the previous report,16 we also recorded the occurrence of adverse reactions to esketamine, including hallucinations, confusion, delirium, impaired vision, agitation, nausea or vomiting, and discomfort in the oral cavity.
As postoperative sore throat is most commonly reported within the first 24 h after double-lumen tube insertion,14,15 we selected the incidence of sore throat 24 h after surgery as the primary outcome. Secondary outcomes included the incidence of sore throat 1 h and 48 h after surgery; severity of sore throat and incidence of hoarseness 1 h, 24 h, and 48 h after surgery; intraoperative haemodynamic responses; and postoperative wound pain scores.
Sample Size Calculation
In a previous study,17 postoperative sore throat occurred in 44% of patients within the first 24 h of double-lumen tube intubation. If a 50% reduction in the incidence of postoperative sore throat is regarded as clinically significant,15 then a sample size of 68 participants per group would be needed to achieve a power of 0.8 and a risk of 0.05 for type-I errors in two-tailed statistical analyses. Considering the potential for loss of follow-up or consent withdrawals, a total of 70 patients were included in each group for this trial.
Statistical Analysis
All statistical analyses were conducted with SPSS software (version 20.0; IBM Corp., Armonk, NY, USA). The continuous data were presented as mean ± standard deviation (SD) and compared using independent sample t-tests. The scores of sore throat and postoperative pain were indicated as median (interquartile [range]) and analysed using Mann–Whitney tests, and the median difference (MD) with associated 95% confidence interval (CI) was calculated by Hodges-Lehmann estimation method. The repeated measured data, such as haemodynamic variables, were expressed as mean ± SD and analysed through repeated-measures analysis of variance followed by post-hoc Bonferroni correction. Categorical data were compared using the chi-square or Fisher’s exact tests, as appropriate, and are described as numbers (%). The rate difference (RD) with associated 95% CI for categorical data was calculated through the Wilson method. Statistical significance was set at p < 0.05. Based on the previous studies,18,19 missing data pertaining to the incidence and severity of postoperative sore throat and hoarseness were addressed using multiple imputation by chained equations (MICE) with five iterations.
Results
After screening 149 individuals, we excluded nine patients (six with recent sore throat and three with Mallampati scores of IV) from participation. Thus, 140 eligible patients were included (Figure 1). The baseline variables are shown in Table 1. As per the study conducted by de Boeret al,20 we did not perform the statistical comparison for the baseline data. Furthermore, there were no between-group differences in surgical characteristics and factors related to tracheal intubation (Table 2). Besides, there were no episodes of esketamine-related adverse effects in either group, including hallucinations, confusion, delirium, impaired vision, agitation, nausea or vomiting, and discomfort in the oral cavity.
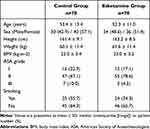 |
Table 1 Baseline Variables for Patients |
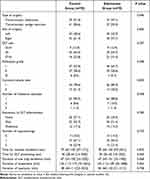 |
Table 2 Surgical Characteristics and Factors Related to Tracheal Intubation |
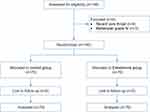 |
Figure 1 Study flow diagram. |
There were no missing outcomes related to the incidence and severity of postoperative sore throat and hoarseness. As shown in Table 3, compared with the control group, the esketamine group had a lower incidence of sore throat at 1 h (RD, 0.41; 95% CI, 0.26–0.57; p < 0.001), 24 h (RD, 0.34; 95% CI, 0.20–0.48; p < 0.001), 48 h (RD, 0.24; 95% CI, 0.12–0.36; p = 0.001), and 7 days (RD, 0.10; 95% CI, 0.02–0.18; p = 0.033) after surgery. Moreover, the VAS scores of sore throat were significantly decreased at the four postoperative time points (1 h postoperatively: MD, −2.00; 95% CI, −2.00– −1.00; p < 0.001; 24 h postoperatively: MD, 0.00; 95% CI, −1.00–0.00; p < 0.001; 48 h postoperatively: MD, 0.00; 95% CI, 0.00–0.00; p < 0.001; 7 days postoperatively: MD, 0.00; 95% CI, 0.00–0.00; p = 0.029). The incidence of hoarseness at 1 h (RD, 0.19; 95% CI, 0.04–0.34; p = 0.027), 24 h (RD, 0.17; 95% CI, 0.04–0.30; p = 0.019), and 48 h (RD, 0.11; 95% CI, 0.02–0.21; p = 0.031) after surgery was reduced in the esketamine group. However, there was no between-group difference in the incidence of hoarseness on the seventh postoperative day (Table 3). In addition, the postoperative visual analogue wound pain scores at rest and during coughing were comparable between the two groups (Table 4).
 |
Table 3 Incidence and Severity of Postoperative Sore Throat and Hoarseness |
 |
Table 4 Postoperative Visual Analogue Wound Pain Score |
There were no differences between the two groups in haemodynamic variables at baseline, 1 min after gargling, or immediately before tracheal intubation. However, relative to the control group, the esketamine group showed lower systolic blood pressure 2 min after intubation (p = 0.014) as well as lower mean arterial blood pressure and heart rate 2 min (mean arterial blood pressure; HR, p = 0.001) and 5 min (mean arterial blood pressure, p = 0.046; HR, p = 0.034) after intubation. In addition, the peak values of systolic blood pressure, mean arterial blood pressure, and heart rate recorded in the esketamine group were lower than those in the control group (systolic blood pressure, p = 0.004; mean arterial blood pressure, p = 0.002; heart rate, p = 0.001). Changes in haemodynamic variables at specific timepoints are shown in Figure 2.
Discussion
The results of this study indicate that esketamine gargle effectively reduced the incidence of postoperative sore throat in patients undergoing double-lumen tube intubation, without adverse reactions related to esketamine. Furthermore, the preventive effect of esketamine against postoperative sore throat persisted for at least seven days after surgery. Moreover, esketamine gargle had an impact on acute haemodynamic responses, including transient hypertension and tachycardia, during the intubation procedure itself.
In this study, the incidence of sore throat in the control group on the first postoperative day was 47.1%, which is comparable to that in previous studies.21–23 The size of double-lumen tubes is directly associated with the rate of occurrence of laryngeal complications. The outer diameters of commonly used double-lumen tubes range from 12 to 14 mm, which is considerably larger than the outer diameters of single-lumen tubes, which range from 9.5 to 10.7 mm. Moreover, the frequent manipulation and repositioning needed with double-lumen tubes to achieve optimal bronchial blockage and one-lung ventilation leads to friction between the tube and airway, which may induce airway damage.24
Gargling is a recently proposed route of drug administration for the treatment of postoperative sore throat.9,25 It is quick and simple to perform, commonly requiring less than one minute, and can be done by almost all individuals. Recent meta-analyses have demonstrated that prophylactic utilisation of a set of pharmacologic agents, including corticosteroids, glycyrrhiza, ketamine, and magnesium, yields varying degrees of alleviation of postoperative sore throat.2,26–29 Although there are effective therapeutic agents to treat postoperative sore throat after single-lumen tracheal tube intubation, the optimal agent for postoperative sore throat after double-lumen tube intubation has been unclear.30 The present study is the first to suggest that preoperative gargling with esketamine significantly diminishes the incidence and severity of postoperative sore throat in patients after double-lumen tube intubation. Moreover, regarding safety and patient tolerance, other literature has reported that intravenous administration of esketamine can result in unwanted outcomes, such as psychomimetic effects,31 vivid dreaming,32 and increased blood pressure.16 In the present study, no participant experienced side effects related to esketamine gargle. To explain this outcome, it is worth noting that preclinical studies have suggested that topically applied ketamine is not detectable in the plasma.33,34 Thus, the clinical safety and systemic effects of esketamine gargling are yet to be determined by further studies.
Current research suggests that esketamine has various effects on receptors and channels.16 We propose that the efficacy of esketamine gargle in relieving postoperative throat soreness can be attributed to the following mechanisms: Firstly, as an antagonist on N-methyl-D-aspartate (NMDA) receptors, esketamine could suppress the activation of downstream signalling pathways by inhibiting NMDA receptors and exerting analgesic effects.6 Thus, the analgesic effects of esketamine against postoperative sore throat might be attributed to inhibiting NMDA receptors in the oral and upper respiratory tract mucosa. Secondly, postoperative sore throat is believed to result from an inflammatory process that damages the pharyngeal and tracheal mucosa.26 Studies have shown that ketamine, for example, attenuates inflammation by inhibiting the activity of the nuclear factor κB pathway.35 Therefore, we speculate that the effectiveness of esketamine gargle is partly due to its anti-inflammatory effects. Thirdly, it is now recognised that ketamine can act as a local anaesthetic by blocking voltage-gated sodium channels.16 Since the mechanism of action of esketamine is similar to that of ketamine, we hypothesise that esketamine gargle may alleviate postoperative throat soreness by inhibiting cation channels and exerting a local anaesthetic effect. Further investigations are necessary to explore the mechanisms underlying the esketamine effect against throat soreness.
Compared to single-lumen tubes, stimulation of the carina and inner wall of the trachea by double-lumen tubes can be more serious, potentially leading to heightened haemodynamic responses.36 Thus, the cardiovascular reactions in response to double-lumen tube intubation can be quite different from those in response to single-lumen tube intubation. Similar to previous reports,36,37 patients in our control group showed obvious increases in blood pressure and heart rate immediately after intubation compared to patients in the esketamine group. A sharp haemodynamic response to double-lumen tube intubation can dramatically elevate the risk of cardiovascular complications in patients. This is a concern, as those who require endobronchial intubation with double-lumen tubes are usually elderly and commonly have deteriorated physiological reserves or altered autonomic function.37 In addition, elderly patients may have increased sensitivity to opioids and anaesthetic drugs, which can result in abrupt hypotension and bradycardia during induction. Our study suggests that preoperative topical application of esketamine may prevent a heightened cardiovascular response during double-lumen tube intubation.
The current study has several limitations. Firstly, since ketamine has essentially been withdrawn from the Chinese market and gradually replaced by esketamine,38,39 there was no ketamine-treated group included in this study. Secondly, the effect size for sample calculation was chosen as a 50% reduction, which is considered relatively large and idealistic. Additionally, we plan to conduct future studies with smaller effect sizes and larger sample sizes to explore the clinical significance of esketamine in preventing postoperative sore throat. Finally, it is worth noting that previous studies have proposed the effectiveness of prophylactic IV dexamethasone at a dose of 0.2 mg/kg in preventing postoperative sore throat.40,41 Although a relatively low average dose of dexamethasone (control group: 0.083 mg/kg, esketamine group: 0.081 mg/kg) was used before anaesthesia induction in our study, it might still be important to consider the potential synergistic effect of dexamethasone in preventing postoperative sore throat.
Conclusion
This study offers novel insights into the use of topical esketamine for preventing postoperative sore throat in patients undergoing double-lumen intubation. Preoperative gargling of esketamine significantly ameliorates postoperative sore throat up to the seventh postoperative day without adverse side effects. Moreover, there is evidence suggesting that gargling with esketamine may help mitigate the rapid increase in heart rate and blood pressure that can occur during double-lumen intubation procedures.
Data Sharing Statement
All data generated or analyzed during this study were included in the published article. Further inquiries about the datasets can be directed to the corresponding author on reasonable request. Any information we share will be deidentified.
Ethical Statement
This trial was approved by the research ethics committee of the third affiliated hospital of Sun Yat-Sen university and registered in the Chinese Clinical Trial Registry (ChiCTR2100044061). Written informed consent was obtained from all patients.
Acknowledgments
The authors thank all the participants enrolled into in this study and the researchers involved in the study design, recruitment of patients, treatment, and data collection.
Funding
This work was supported by the Basic and Applied Basic Research Foundation of Guangdong Province (2021A1515220081), the Science and Technology Program of Guangzhou (202201020446) and the Precision Medicine Foundation of Guangdong Province Hospital Association (2020-10).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Jenkins K, Grady D, Wong J, Correa R, Armanious S, Chung F. Post-operative recovery: day surgery patients’ preferences. Br J Anaesth. 2001;86(2):272–274. doi:10.1093/bja/86.2.272
2. Kuriyama A, Nakanishi M, Kamei J, Sun R, Ninomiya K, Hino M. Topical application of ketamine to prevent postoperative sore throat in adults: a systematic review and meta-analysis. Acta Anaesthesiol Scand. 2020;64(5):579–591. doi:10.1111/aas.13553
3. El-Boghdadly K, Bailey CR, Wiles MD. Postoperative sore throat: a systematic review. Anaesthesia. 2016;71(6):706–717. doi:10.1111/anae.13438
4. Ali S, Khan A, Ashfaq AD. Comparison of two different sizes of endotracheal tracheal tube for postoperative sore throat in breast cancer patients undergoing surgeries. Cureus. 2021;13(1):e12896. doi:10.7759/cureus.12896
5. Park SY, Kim SH, Lee SJ, et al. Application of triamcinolone acetonide paste to the endotracheal tube reduces postoperative sore throat: a randomized controlled trial. Can J Anaesth. 2011;58(5):436–442. doi:10.1007/s12630-011-9478-6
6. Li X, Xiang P, Liang J, Deng Y, Du J. Global trends and hotspots in esketamine research: a bibliometric analysis of past and estimation of future trends. Drug Des Devel Ther. 2022;16:1131–1142. doi:10.2147/DDDT.S356284
7. Quibell R, Prommer EE, Mihalyo M, Twycross R, Wilcock AK. Ketamine*. J Pain Symptom Manage. 2011;41(3):640–649. (). doi:10.1016/j.jpainsymman.2011.01.001
8. Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340(mar23 1):c332. doi:10.1136/bmj.c332
9. Kheirabadi D, Ardekani MS, Honarmand A, Safavi MR, Salmasi E. Comparison prophylactic effects of gargling different doses of ketamine on attenuating postoperative sore throat: a single-blind randomized controlled trial. Int J Prev Med. 2021;12:62. doi:10.4103/ijpvm.IJPVM_147_19
10. Suvvari P, Kumar B, Singhal M, Singh H. Comparison between computerized tomography-guided bronchial width measurement versus conventional method for selection of adequate double lumen tube size. Ann Card Anaesth. 2019;22(4):358–364. doi:10.4103/aca.ACA_117_18
11. Bijker JB, Persoon S, Peelen LM, et al. Intraoperative hypotension and perioperative ischemic stroke after general surgery: a nested case-control study. Anesthesiology. 2012;116(3):658–664. doi:10.1097/ALN.0b013e3182472320
12. Park JW, Jo JH, Park JH, et al. Comparison of conventional and fibreoptic-guided advance of left-sided double-lumen tube during endobronchial intubation: a randomised controlled trial. Eur J Anaesthesiol. 2020;37(6):466–473. doi:10.1097/EJA.0000000000001216
13. Appelbaum N, Clarke J. Ideal body weight calculations: fit for purpose in modern anaesthesia? Eur J Anaesthesiol. 2021;38(12):1211–1214. doi:10.1097/EJA.0000000000001515
14. Park JJ, Huh H, Yoon SZ, et al. Two-handed jaw thrust decreases postoperative sore throat in patients undergoing double-lumen endobronchial intubation: a randomised study. Eur J Anaesthesiol. 2020;37(2):105–112. doi:10.1097/EJA.0000000000001149
15. Seo JH, Cho CW, Hong DM, Jeon Y, Bahk JH. The effects of thermal softening of double-lumen endobronchial tubes on postoperative sore throat, hoarseness and vocal cord injuries: a prospective double-blind randomized trial. Br J Anaesth. 2016;116(2):282–288. doi:10.1093/bja/aev414
16. Zhang -X-X, Zhang N-X, Liu D-X, Ding J, Zhang Y-N, Zhu Z-Q. Research advances in the clinical application of esketamine. Ibrain. 2022;8(1):55–67. doi:10.1002/ibra.12019
17. Knoll H, Ziegeler S, Schreiber JU, et al. Airway injuries after one-lung ventilation: a comparison between double-lumen tube and endobronchial blocker: a randomized, prospective, controlled trial. Anesthesiology. 2006;105(3):471–477. doi:10.1097/00000542-200609000-00009
18. Jiang Z, Liu S, Wang L, et al. Effects of 30% vs. 60% inspired oxygen fraction during mechanical ventilation on postoperative atelectasis: a randomised controlled trial. BMC Anesthesiol. 2023;23(1):265. doi:10.1186/s12871-023-02226-6
19. Wang W, He Q, Wang M, et al. Associations of fentanyl, sufentanil, and Remifentanil with length of stay and mortality among mechanically ventilated patients: a registry-based cohort study. Front Pharmacol. 2022;13:858531. doi:10.3389/fphar.2022.858531
20. de Boer MR, Waterlander WE, Kuijper LD, Steenhuis IH, Twisk JW. Testing for baseline differences in randomized controlled trials: an unhealthy research behavior that is hard to eradicate. Int J Behav Nutr Phys Act. 2015;12:4. doi:10.1186/s12966-015-0162-z
21. Higgins PP, Chung F, Mezei G. Postoperative sore throat after ambulatory surgery. Br J Anaesth. 2002;88(4):582–584. doi:10.1093/bja/88.4.582
22. Borazan H, Kececioglu A, Okesli S, Otelcioglu S. Oral magnesium lozenge reduces postoperative sore throat: a randomized, prospective, placebo-controlled study. Anesthesiology. 2012;117(3):512–518. doi:10.1097/ALN.0b013e3182639d5f
23. Chang J-E, Kim H, Han S-H, Lee J-M, Ji S, Hwang J-Y. Effect of endotracheal tube cuff shape on postoperative sore throat after endotracheal intubation. Anesth Analg. 2017;125(4):1240–1245. doi:10.1213/ANE.0000000000001933
24. Jeon J, Lee K, Ahn G, Lee J, Hwang W. Comparison of postoperative sore throat and hoarseness between two types of double-lumen endobronchial tubes: a randomized controlled trial. J Cardiothorac Vasc Anesth. 2015;29(1):121–125. doi:10.1053/j.jvca.2014.05.028
25. Singh NP, Makkar JK, Cappellani RB, Sinha A, Lakshminarasimhachar A, Singh PM. Efficacy of topical agents for prevention of postoperative sore throat after single lumen tracheal intubation: a Bayesian network meta-analysis. Can J Anaesth. 2020;67(11):1624–1642. doi:10.1007/s12630-020-01792-4
26. Kuriyama A, Maeda H. Topical application of licorice for prevention of postoperative sore throat in adults: a systematic review and meta-analysis. J Clin Anesth. 2019;54:25–32. doi:10.1016/j.jclinane.2018.10.025
27. Kuriyama A, Maeda H, Sun R. Topical application of magnesium to prevent intubation-related sore throat in adult surgical patients: a systematic review and meta-analysis. Can J Anaesth. 2019;66(9):1082–1094. doi:10.1007/s12630-019-01396-7
28. Kuriyama A, Maeda H, Sun R, Aga M. Topical application of corticosteroids to tracheal tubes to prevent postoperative sore throat in adults undergoing tracheal intubation: a systematic review and meta-analysis. Anaesthesia. 2018;73(12):1546–1556. doi:10.1111/anae.14273
29. Kuriyama A, Maeda H, Sun R. Aerosolized corticosteroids to prevent postoperative sore throat in adults: a systematic review and meta-analysis. Acta Anaesthesiol Scand. 2019;63(3):282–291. doi:10.1111/aas.13275
30. Ruetzler K, Fleck M, Nabecker S, et al. A randomized, double-blind comparison of licorice versus sugar-water gargle for prevention of postoperative sore throat and postextubation coughing. Anesth Analg. 2013;117(3):614–621. doi:10.1213/ANE.0b013e318299a650
31. Chen Y, Chen J, Wang Q, et al. Safety and tolerability of esketamine in propofol based sedation for endoscopic variceal ligation with or without injection sclerotherapy: randomized controlled trial. Dig Endosc. 2023. doi:10.1111/den.14539
32. Ping LI, Wensheng HE, Liang C, Lingling GUO. Application of esketamine in painless colonoscopy and its effects on dreams and mood. J Clin Med. 2022;26(6):86–89.
33. Lynch ME, Clark AJ, Sawynok J, Sullivan MJ. Topical 2% amitriptyline and 1% ketamine in neuropathic pain syndromes: a randomized, double-blind, placebo-controlled trial. Anesthesiology. 2005;103(1):140–146. doi:10.1097/00000542-200507000-00021
34. Finch PM, Knudsen L, Drummond PD. Reduction of allodynia in patients with complex regional pain syndrome: a double-blind placebo-controlled trial of topical ketamine. Pain. 2009;146(1–2):18–25. doi:10.1016/j.pain.2009.05.017
35. Wang T, Weng H, Zhou H, et al. Esketamine alleviates postoperative depression-like behavior through anti-inflammatory actions in mouse prefrontal cortex. J Affect Disord. 2022;307:97–107. doi:10.1016/j.jad.2022.03.072
36. Yoo KY, Jeong CW, Kim WM, et al. Cardiovascular and arousal responses to single-lumen endotracheal and double-lumen endobronchial intubation in the normotensive and hypertensive elderly. Korean J Anesthesiol. 2011;60(2):90–97. doi:10.4097/kjae.2011.60.2.90
37. Choi B-H, Lee Y-C. Effective Bolus Dose of Sufentanil to Attenuate Cardiovascular Responses in Laryngoscopic Double-Lumen Endobronchial Intubation. Anesth Pain Med. 2016;6(2):e33640. doi:10.5812/aapm.33640
38. Fu D, Wang D, Li W, Han Y, Jia J. Pretreatment with Low-Dose Esketamine for Reduction of Propofol Injection Pain: a Randomized Controlled Trial. Pain Res Manag. 2022;2022:4289905. doi:10.1155/2022/4289905
39. Tan M, Zhang C, Zeng W, Chen M, Huang Z, Huang D. Determining the effective dose of esketamine for mitigating pain during propofol injection by Dixon’s up-and-down method: a double-blind, prospective clinical study of drug dose response. BMC Anesthesiol. 2022;22(1):368. doi:10.1186/s12871-022-01914-z
40. Park S-H, Han S-H, Do S-H, Kim J-W, Rhee K-Y, Kim J-H. Prophylactic dexamethasone decreases the incidence of sore throat and hoarseness after tracheal extubation with a double-lumen endobronchial tube. Anesth Analg. 2008;107(6):1814–1818. doi:10.1213/ane.0b013e318185d093
41. Kuriyama A, Maeda H. Preoperative intravenous dexamethasone prevents tracheal intubation-related sore throat in adult surgical patients: a systematic review and meta-. Can J Anaesth. 2019;66(5):562–575. doi:10.1007/s12630-018-01288-2
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

