Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 16
Effect of Chiglitazar and Sitagliptin on Bone Mineral Density and Body Composition in Untreated Patients with Type 2 Diabetes
Authors Wang Y, Zhou Y, Zhou X, Su X, Xu X, Li H , Ma J
Received 9 October 2023
Accepted for publication 14 December 2023
Published 27 December 2023 Volume 2023:16 Pages 4205—4214
DOI https://doi.org/10.2147/DMSO.S439479
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Yuming Wang,1,2,* Yunting Zhou,2,* Xiao Zhou,2,* Xiaofei Su,2,* Xiaohua Xu,2 Huiqin Li,2 Jianhua Ma2
1Department of Geriatrics, Nanjing Drum Tower Hospital, the Affiliated Hospital of Nanjing University Medical School, Nanjing, 210008, People’s Republic of China; 2Department of Endocrinology, Nanjing First Hospital, Nanjing Medical University, Nanjing, 210012, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Huiqin Li; Jianhua Ma, Department of Endocrinology, Nanjing First Hospital, Nanjing Medical University, Nanjing, Jiangsu, People’s Republic of China, Email [email protected]; [email protected]
Aim: To evaluate the changes in bone mineral density (BMD) and body composition in untreated patients with type 2 diabetes mellitus (T2DM) before and after chiglitazar or sitagliptin treatment.
Methods: A total of 81 patients with T2DM were randomly divided to receive chiglitazar or sitagliptin treatment for 24 weeks (54 in the chiglitazar group and 27 in the sitagliptin group). We measured the spine lumbar BMD, hip BMD, fat mass (FM), fat-free mass (FFM), percent body fat (%BF), android FM, gynoid FM and skeleton muscle mass (SMM) using dual-energy X-ray absorptiometry (DEXA) and examined serum adiponectin (ADP) levels at baseline and the end of the study.
Results: There were no significant changes in the BMD of the L2-4, femoral neck, trochanter or total hip as well as in the BMC after 24 weeks of treatment with chiglitazar or sitagliptin. After chiglitazar administration, the FM, gynoid FM and gynoid to total FM ratio were higher, while the android to total FM ratio and the android to gynoid FM ratio (AOI) were significantly lower. Sitagliptin intervention did not result in statistically significant differences in total fat loss, but it did cause significant decreases in %BF and AOI as well as increases in the FFM, gynoid to total FM ratio and SMM. The ADP levels had significantly negative associations with AOI in all eligible patients.
Conclusion: The chiglitazar had no deleterious effects on BMD and resulted in body fat redistribution in untreated patients with T2DM.
Trial Registration: The trial is registered at ClinicalTrials.gov (CT.gov identifier: NCT02173457).
Keywords: type 2 diabetes mellitus, chiglitazar, PPAR, bone mineral density, body composition
Introduction
Type 2 diabetes mellitus (T2DM) is characterized by insulin resistance and β-cell dysfunction, and has been a highly prevalent complex chronic disease worldwide with severe morbidity and mortality.1 Several previous studies have shown T2DM, both in women and men, has a negative impact on bone quality and a twofold increase in the risk of fracture, especially in the hip, proximal humerus and foot.2,3 The exact mechanisms by which T2DM increases bone fragility are uncertain and may be attributable to chronic hyperglycemia, insulin resistance, inflammatory factors and antidiabetic medication use.4–6 In recent years, the potential effect of antidiabetic medications on bone metabolism has attracted increasing attention, indicating that clinicians should focus on the prevention of fracture when selecting antidiabetic medications.
Insulin resistance has been shown to be a basic pathological physiology phenomenon of T2DM, and improvement of insulin resistance is a key target for the treatment of T2DM. Thiazolidinediones (TZDs), which are peroxisome proliferator-activated receptor gamma (PPAR-γ) agonists that directly regulate the expression of genes related to glucose and lipid metabolism to restore glucose and lipid utilization, have been demonstrated to be effective in the treatment of T2DM by improving insulin resistance and glycemic control.7,8 PPAR-γ activation could promote the differentiation of bone marrow mesenchymal stem cells to adipocytes and impair the formation of osteoblasts, thereby leading to osteoporosis.9 There is accumulating evidence that TZDs use is associated with reduced bone mineral density (BMD), accelerated bone loss and increased risk of fracture in patients with diabetes and prediabetes10 as well as in healthy postmenopausal women,11 even for short-term use.12
There are three PPAR subtypes (PPAR-γ, PPAR-α and PPAR-δ), which have distinct biological activities in bone. PPAR-α is highly expressed in brown fat and liver followed by kidneys, heart and skeletal muscles.13 Previous studies have found that fenofibrate, a PPAR-α agonist, increases the BMD of the femur and improves bone microstructure.14 PPAR-δ is mainly expressed in skeletal muscle, and no PPAR-δ agonist has been granted marketing authorization in clinical practice at present.15 However, experimental studies have confirmed that PPAR-δ has a positive effect on bone formation and BMD.16,17 Therefore, improvement of bone loss caused by TZDs therapy may be achieved by developing a PPAR pan agonist, which may have sparing effects on the skeleton. At present, there are no studies evaluating the effect of PPAR pan agonists on BMD.
In addition, intra-abdominal fat accumulation is highly related to insulin resistance, and insulin resistance can be improved by reducing intra-abdominal fat depot via diet or exercise.18,19 TZDs promote the redistribution of body fat, which may contribute to insulin sensitization.20 The levels of adiponectin (ADP), which has a protective effect against the development of insulin resistance, are significantly higher in subjects with peripheral obesity than in those with central obesity.21
Chiglitazar, a novel configuration-restricted non-TZD PPAR pan agonist, has been designed for the treatment of T2DM due to its moderate and balanced activation properties in all three PPAR subtypes, with EC 50 values of 1.1 μmol/L, 0.08 μmol/L, and 1.7 μmol/L for PPARa, PPARc, and PPARd, respectively.22 Therefore, chiglitazar showed a greater inhibitory effect than the TZD drugs on PPAR-γ phosphorylation induced by inflammatory insults, which is one of the key point for PPAR-γ dysfunction and results in insulin resistance.23 Previous in vitro and in vivo studies have shown that chiglitazar improves insulin sensitivity and regulates glucose and lipid metabolism as well as facilitates fatty acid oxidation.22,23 In addition, PPAR pan agonists might also help to alleviate or counteract the side effects associated with PPAR-γ activation. For instance, weight gain or bone fractures observed in TZD drugs might be offset by the activity of PPAR-α or PPAR-δ, which can increase lipid catabolism and stimulate osteoblast activity in bone.24 Clinical studies have also confirmed the effectiveness and safety of chiglitazar in the treatment of T2DM, even in elderly individuals.25,26 However, the effect of chiglitazar, a novel pan PPAR pan-agonist, on BMD and body fat composition in diabetic patients is still unclear.
In the present study, we analyzed BMD and body fat composition using dual-energy X-ray absorptiometry (DEXA), which is the standard diagnostic method of assessing the extent of osteoporosis, and we measured serum ADP levels in T2DM patients before and after 24 weeks of treatment with chiglitazar, aiming to evaluate the effect of chiglitazar on BMD and body fat composition.
Materials and Methods
Study Design and Participants
The present randomized, double-blind study was conducted in Nanjing First Hospital (ClinicalTrials.gov identifier: NCT02173457) from December 2014 to May 2016 which was approved by the Ethics Committee of Nanjing First Hospital. The study procedures were conducted in accordance with the 1964 Helsinki Declaration. Written informed consent was provided by 136 patients before screening. General information, such as age, gender, duration of T2DM of the enrolled patients was collected by a dedicated person. A total of 84 untreated patients with T2DM were rigorously screened according to the inclusion and exclusion criteria27 and were randomized (in a 2:1 ratio) to receive oral chiglitazar or sitagliptin monotherapy for 24 weeks and 81 patients finished the study. The flowchart was shown in Figure 1.
 |
Figure 1 Study flowchart. |
Serum Profile Measurements
The blood samples of all study patients were subjected to measurements of hemoglobin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatinine (Cr), total cholesterol (TC), triglycerides (TGs), HbA1c and adiponectin (ADP) before and after treatment. HbA1c was evaluated by high-performance liquid chromatography assay (Bio–Rad Laboratories, Inc., CA, USA). The serum levels of ADP were determined using an enzyme-linked immunosorbent assay kit (Elabscience Biotechnology Co. Ltd., Wuhan, China).
BMD and Body Composition Measurements
BMD at the lumbar spine2–4 (L2–4), femoral neck, trochanter and total hip were measured through whole-body scans using DEXA (GE-lunar Prodigy, Madison, WI, USA) by a single experienced technician. Instrument quality control was performed by daily calibration using a standard phantom. The coefficient of variation for the phantom scans was 0.3%. Body weight (BW) was measured with only light clothing, and height was measured without shoes. DEXA was used to measure total fat mass (FM), percent body fat (%BF), fat-free mass (FFM), android FM, gynoid FM, bone mineral content (BMC) and skeletal muscle mass (SMM). Fat-free mass index (FFMI) was calculated by dividing FFM (in kilograms) by height (in meters) squared. The inferior boundary of the android region was cut at the pelvis with the upper boundary 96 mm superior to the lower part of this region. The lateral boundaries of this region were the arm cuts. For the gynoid region, the upper boundary was defined by the superior part of the trochanter major. The lower part of this region was 96 mm inferior to the upper boundary. The lateral part of this region was the outer leg cuts. Eventually, 76 cases underwent BMD and body composition measurements before and after treatment (50 in the chiglitazar group and 26 in the sitagliptin group).
Statistical Analysis
Data were analyzed with the SPSS Statistics package version 21. Descriptive statistics were used to examine the baseline distribution of data, which are presented as the mean ± SEM. The Kolmogorov–Smirnov test was used to assess the distribution of data. The differences within groups were compared by paired t-test. The changes from baseline to the second test performed at the end of the study were examined by analysis of covariance model, which were adjusted for age, sex, BMI and baseline levels. Bivariate correlations were determined using partial Pearson correlation analysis. P values were two-tailed with a significance level of 5%.
Results
Baseline Characteristics
A total of 81 patients completed the 24-week treatment, and the baseline characteristics of these patients are shown in Table 1. No significant differences in age, sex, BMI, FBG or HbA1c were observed between the two groups at baseline. The mean age of all participants was 53.79 years, and men accounted for 65.4% of the patients. The mean HbA1c was 8.29%.
 |
Table 1 Baseline Characteristics of Study Patients |
Response of BMD to Treatment
No fractures occurred in any enrolled patient during the entire study period. Table 2 shows the change in BMD from baseline to week 24 and the differences between the two groups. The BMD of the L2-4, femoral neck, trochanter and total hip showed no significant decrease at the end of the study in the chiglitazar group. Moreover, sitagliptin group seems to have an extremely small increase in the BMD of the L2-4 and trochanter (1.147 ± 0.030 vs 1.153 ± 0.031 and 0.762 ± 0.028 vs 0.764 ± 0.029, respectively), but there were no significant differences. After adjusting for age, sex, BMI and baseline levels, the changes in BMD did not differ between the two groups.
 |
Table 2 Comparison of BMD Within Groups Before and After 24-Weeks Treatment and the Changes from Baseline to Week 24 Between Two Groups. Data Shown as Mean ± SEM |
Response of Body Fat Distribution and ADP Levels to Treatment
Chiglitazar treatment induced a body fat mass gain of 1.289 ± 0.353 kg with a significant increase in gynoid fat mass from baseline to week 24 (3.048 ± 0.22 vs 3.349 ± 0.245, P < 0.001) but not android fat mass. Consistently, chiglitazar significantly reduced the android-to-total FM ratio after treatment, and this reduction was accompanied by an elevated gynoid-to-total FM ratio (Table 3). In the sitagliptin group, the average weight gain was not attributed to an increase in FM but instead to FFM, which was accompanied by a decrease in % BF, predominantly in the SMM (0.580 kg, P < 0.05) (Table 3 and Table 4). The total fat mass after sitagliptin treatment was lower in both the android and gynoid regions, but the difference from baseline was not statistically significant (decrease by 0.088 kg in the android region, P = 0.052; decrease by 0.008 kg in the gynoid region, P = 0.79) (Table 3 and Table 4). In general, both chiglitazar and sitagliptin resulted in a decrease in the android-to-gynoid FM ratio (AOI), but patients administered chiglitazar had a greater decrease in AOI over the treatment period than those administered sitagliptin (−0.067 ± 0.012 vs −0.025 ± 0.012, P < 0.05) (Table 4). In addition, there was no obvious change in BMC from baseline to week 24 within or between the two groups, which was consistent with the BMD results. Both chiglitazar and sitagliptin generally resulted in an increase in ADP levels (Table 3), and patients administered chiglitazar showed a greater increase in ADP levels over the treatment period than those administered sitagliptin (11.850±1.094 vs 1.974±0.381, P < 0.001)(Table 4).
 |
Table 3 Comparison of Body Composition and ADP Levels Within Groups Before and After 24-Weeks Treatment. Data Shown as Mean ± SEM |
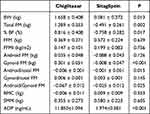 |
Table 4 Changes of Body Composition and ADP Levels from Baseline to Week 24 Between Two Groups. Data Shown as Mean ± SEM |
Relationship Between Change in AOI and Change in ADP
At the end of the study, the mean level of serum ADP increased after the intervention, while AOI significantly decreased (Figure 2a, b). Changes in serum ADP were positively correlated with changes in BMI (r = 0.324, P < 0.05) and FM (r = 0.317, P < 0.01). Moreover, BMI and FM both increased during the study (0.41 ± 0.12, P < 0.05 vs 0.6 ± 0.27, P < 0.05, respectively). After controlling for changes in BMI and FM, partial Pearson correlation analysis showed that changes in ADP were negatively correlated with those in AOI (r = −0.405, P < 0.001) (Figure 2c).
Discussion
Our study revealed that there were no significant changes in the BMD of the L2-4, femoral neck, trochanter or total hip after 24 weeks of treatment with chiglitazar or sitagliptin. Consistently, the changes in BMC were similar to those in BMD in the two groups. Compared to sitagliptin treatment, chiglitazar treatment significantly increased body weight, body fat and serum ADP levels in patients with T2DM. The increase in body fat mass mainly occurred in the gynoid region compared to the android region in the torso area, which corresponded to a sharp decline in AOI. Sitagliptin intervention did not result in statistically significant differences in total fat loss, but it did result in significantly decreased body fat percentage and increased SMM and FFM, which was consistent with the results of a previous study.28
Currently, it has been shown that the increased risk of fractures in patients with T2DM is associated with a variety of factors, including muscle mass loss, hypoglycemia and chronic complications of diabetes.4,29 It remains controversial whether BMD is decreased in patients with T2DM. A review article has suggested that 13 studies demonstrated decreased BMD in T2DM, especially femoral neck30 and that 8 studies found no change in BMD in T2DM.31 Moreover, Schwartz et al reported that older T2DM women have an increased fracture risk even though their BMD is higher than that of nondiabetic subjects.32 However, BMD is still a critical risk factor for fracture in T2DM because the incidence of fractures is higher in T2DM patients with low BMD.33
The effect of hypoglycemic drugs on bone metabolism has gradually attracted attention. Several studies have reported that these antidiabetic drugs, including metformin, GLP-1RAs and dipeptidyl peptidase-4 (DPP-4) inhibitors, have a positive or neutral effect on bone health.34 However, the present study found that sitagliptin, a dipeptidyl peptidase-4 (DPP-4) inhibitor, has no significant effects on BMD of the L2-4, trochanter, femoral neck and total hip. There is accumulating evidence that TZDs, such as rosiglitazone and pioglitazone, which directly activate PPAR-γ, lead to bone loss,35,36 and bone loss has been demonstrated even with a short use of TZDs. A randomized controlled trial has shown that the BMD of the femoral neck and lumbar spine decreases only after 16 weeks of treatment with pioglitazone in polycystic ovary syndrome subjects.12 Similar research has been conducted with rosiglitazone in the treatment of diabetes with consistent results.11
PPAR-γ regulates the lineage commitment of mesenchymal stem cells (MSCs), which can differentiate into osteoblasts, chondrocytes and adipocytes, and it controls the expression of multiple cytokines, such as receptor activator of nuclear factor-κB ligand (RANKL), which is the key regulator of osteoclastogenesis.37 Pharmacological induction of PPAR-γ is crucial in osteoclasts, leading to an increased risk of fracture of TZDs. However, the other two isoforms of PPAR, namely, PPAR-α and PPAR-δ, have divergent effects on bone metabolism from PPAR-γ. A clinical study has found that fenofibrate, a PPAR-α agonist, increases the BMD of the femur and improves bone microstructure.14 In vivo and in vitro experiments have shown that PPAR-δ has a positive effect on osteoblast differentiation.16,38 Moreover, the PPAR-α/δ dual agonists, linoleic acid and bezafibrate, upregulate osteoblast differentiation and increase cortical bone area and periosteal formation, but these agonists have no significant effect on osteoclasts, suggesting that PPAR-α/δ is a potential therapeutic target for osteoporosis.39 Therefore, dual or pan PPAR agonists may be a better option in the treatment of diabetes, exerting therapeutic benefits beyond the limits of increased fracture risk.
In the present study, there were no significant changes in the BMD of the lumbar spine and hip after treatment with chiglitazar, a newly identified PPAR pan agonist, and the changes in BMD with chiglitazar were similar to those with sitagliptin. BMD may be affected by increased body fat, which alters the distances between bones and X-ray when measured using DEXA. There may be another possibility that the change in BMD is due to body fat gain accompanying chiglitazar intervention. We also examined BMC changes, which are less likely to be affected, and the result was consistent with BMD. However, further studies are required to elucidate the exact mechanism through which chiglitazar exerts its effects on bone metabolism.
DEXA has been a reliable technique to evaluate body composition, and it accurately quantifies regional adiposity using the region of interest (ROI) program.40 The DEXA results are in fair agreement with CT measurements in different populations.41 It is well known that insulin resistance is linked to obesity, but insulin resistance is more closely associated with body fat distribution than with total fat mass.42 Available data indicate that chiglitazar remarkably improves glycemic control and insulin sensitivity.43 Correspondingly, the present study suggested that the non-TZD insulin sensitizer, chiglitazar, caused a favorable body fat redistribution despite treatment-related weight and total fat gain, as characterized by an increase in gynoid region and decrease in AOI, which is a simple but effective indicator to evaluate body fat distribution associated with an increased risk of insulin resistance.44 Sitagliptin primarily reduces the percentage of body fat and increases FFM and SMM, which may have a positive effect on the improvement of pancreatic islet function in T2DM.45 Xiaohua Fu conducted a clinical trial, which suggested that AOI is negatively associated with total BMD.46 Therefore, a decrease in AOI with chiglitazar may prevent the decline in BMD to a certain extent. Moreover, gynoid fat mass is considered to protect against cardiovascular disease (CVD),21 and the AOI measured by DEXA is positively associated with multiple cardiovascular risk factors, including hypertension, IGT and hypertriglyceridemia.47 Therefore, chiglitazar may be beneficial for CVD, and we are conducting a Phase III clinical trial to evaluate the effect of chiglitazar on CVD. Intra-abdominal visceral adipocytes are different from subcutaneous fat, which may lead to dysmetabolism and release greater amounts of free fatty acids (FFAs) into the splanchnic circulation.48 Gynoid adipocytes effectively deposit free fatty acids and increase the secretion of adiponectin (ADP), resulting in an improvement in insulin sensitivity.49 Consistent with the decrease in AOI and increase in gynoid fat mass, the levels of ADP were elevated after treatment, and the changes in ADP were significantly negatively correlated with AOI. Adiponectin is a 30 kDa multimeric protein secreted mainly by white adipose tissue, whose reduction plays a central role in obesity-related diseases, including insulin resistance/type 2 diabetes and cardiovascular disease. There is evidence that serum levels of adiponectin decrease with obesity and are positively associated with insulin sensitivity.50 And chronic over expression of adiponectin leads to massive increase in subcutaneous fat, and it protects against diet induced insulin resistance.51
In addition, previous studies have indicated that edema, and congestive heart failure are associated the thiazolidinedione (TZD) class of PPAR-γ agonists (rosiglitazone and pioglitazone), most likely contributed to PPAR-γ activation (on-target effect) and drug structure-related property (off-target effect).52 Therefore, a dual or triple combination would result in conditions favorable for treating metabolic syndrome and type 2 diabetes, while the negative effects, such as increased adiposity caused by PPAR-γ leading to weight gain, would be negated by the increasing fat oxidation promoted by PPAR-α and PPAR-δ.24 In this present study, modest weight gain occurred in most patients with chiglitazar treatment, but no obvious edema or heart failure was reported in this study. However, patients with heart failure were excluded from the study, and the risk of adverse effects of chiglitazar in this populations remains unclear.
The present study had several potential limitations. First, the sample size of this study was small. Second, we did not measure bone metabolic markers in the present study. However, the biochemical markers of bone formation and resorption are unstable in different stages of hypoglycemic drug treatment, and there is inconsistency among different markers.53 Finally, the present 24-week study was relatively short, and a long-term clinical study should be conducted in the near future with an increased sample size and prolonged follow-up time.
In conclusion, the present study demonstrated that chiglitazar and sitagliptin treatments do not have deleterious effects on BMD but that they result in body fat redistribution in untreated patients with T2DM. Chiglitazar selectively increases gynoid fat mass and may have significant implications for improving insulin sensitivity and preventing treatment-related osteoporosis independent of fat mass gain.
Acknowledgments
This trial is registered at ClinicalTrials.gov (CT.gov identifier: NCT02173457). We thank the members of Endocrinology department of Nanjing First hospital for their support.
Funding
This study was supported by the National Key R&D Program of China (No. 2018YFC1314103), the Chinese National and Provincial Major Project for New Drug Innovation (Provincial: 2011A080501010; National: 2008ZX09101-002, 2013ZX09401301) and Jiangsu Provincial Double Innovation Doctor Program (to Yt.Z, No.JSSCBS20211546).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Napoli N, Chandran M, Pierroz DD, Abrahamsen B, Schwartz AV, Ferrari SL. Mechanisms of diabetes mellitus-induced bone fragility. Nat Rev Endocrinol. 2017;13(4):208–219. doi:10.1038/nrendo.2016.153
2. Yamamoto M, Yamaguchi T, Yamauchi M, Kaji H, Sugimoto T. Diabetic patients have an increased risk of vertebral fractures independent of BMD or diabetic complications. J Bone Miner Res. 2009;24(4):702–709. doi:10.1359/jbmr.081207
3. Anagnostis P, Gkekas NK, Achilla C, et al. Type 2 diabetes mellitus is associated with increased risk of sarcopenia: a systematic review and meta-analysis. Calcif Tissue Int. 2020;107(5):453–463. doi:10.1007/s00223-020-00742-y
4. Dede AD, Tournis S, Dontas I, Trovas G. Type 2 diabetes mellitus and fracture risk. Metabolism. 2014;63(12):1480–1490. doi:10.1016/j.metabol.2014.09.002
5. Koromani F, Ghatan S, van Hoek M, et al. Type 2 diabetes mellitus and vertebral fracture risk. Curr Osteoporos Rep. 2021;19(1):50–57. doi:10.1007/s11914-020-00646-8
6. Khosla S, Samakkarnthai P, Monroe DG, Farr JN. Update on the pathogenesis and treatment of skeletal fragility in type 2 diabetes mellitus. Nat Rev Endocrinol. 2021;17(11):685–697. doi:10.1038/s41574-021-00555-5
7. Brunzell JD. A comparison of lipid and glycemic effects of pioglitazone and rosiglitazone in patients with type 2 diabetes and dyslipidemia: response to Goldberg et al. Diabetes Care. 2005;28(12):2984–5; author reply 2985–6. doi:10.2337/diacare.28.12.2984
8. Liu CH, Lee TH, Lin YS, Sung PS, Wei YC, Li YR. Pioglitazone and PPAR-γ modulating treatment in hypertensive and type 2 diabetic patients after ischemic stroke: a national cohort study. Cardiovasc Diabetol. 2020;19(1):2. doi:10.1186/s12933-019-0979-x
9. Wan Y. PPARγ in bone homeostasis. Trends Endocrinol Metab. 2010;21(12):722–728. doi:10.1016/j.tem.2010.08.006
10. Yaturu S, Bryant B, Jain SK. Thiazolidinedione treatment decreases bone mineral density in type 2 diabetic men. Diabetes Care. 2007;30(6):1574–1576. doi:10.2337/dc06-2606
11. Grey A, Bolland M, Gamble G, et al. The peroxisome proliferator-activated receptor-gamma agonist rosiglitazone decreases bone formation and bone mineral density in healthy postmenopausal women: a randomized, controlled trial. J Clin Endocrinol Metab. 2007;92(4):1305–1310. doi:10.1210/jc.2006-2646
12. Glintborg D, Andersen M, Hagen C, Heickendorff L, Hermann AP. Association of pioglitazone treatment with decreased bone mineral density in obese premenopausal patients with polycystic ovary syndrome: a randomized, placebo-controlled trial. J Clin Endocrinol Metab. 2008;93(5):1696–1701. doi:10.1210/jc.2007-2249
13. Kersten S, Desvergne B, Wahli W. Roles of PPARs in health and disease. Nature. 2000;405(6785):421–424. doi:10.1038/35013000
14. Mosti MP, Ericsson M, Erben RG, Schüler C, Syversen U, Stunes AK. The PPARα Agonist fenofibrate improves the musculoskeletal effects of exercise in ovariectomized rats. Endocrinology. 2016;157(10):3924–3934. doi:10.1210/en.2016-1114
15. Maltarollo VG, Kronenberger T, Windshugel B, Wrenger C, Trossini G, Honorio KM. Advances and challenges in drug design of PPARδ ligands. Curr Drug Targets. 2018;19(2):144–154. doi:10.2174/1389450118666170414113159
16. Scholtysek C, Katzenbeisser J, Fu H, et al. PPARβ/δ governs Wnt signaling and bone turnover. Nat Med. 2013;19(5):608–613. doi:10.1038/nm.3146
17. Mosti MP, Stunes AK, Ericsson M, et al. Effects of the peroxisome proliferator-activated receptor (PPAR)-δ agonist GW501516 on bone and muscle in ovariectomized rats. Endocrinology. 2014;155(6):2178–2189. doi:10.1210/en.2013-1166
18. Reichkendler MH, Auerbach P, Rosenkilde M, et al. Exercise training favors increased insulin-stimulated glucose uptake in skeletal muscle in contrast to adipose tissue: a randomized study using FDG PET imaging. Am J Physiol Endocrinol Metab. 2013;305(4):E496–506. doi:10.1152/ajpendo.00128.2013
19. Cowan TE, Brennan AM, Stotz PJ, Clarke J, Lamarche B, Ross R. Separate effects of exercise amount and intensity on adipose tissue and skeletal muscle mass in adults with abdominal obesity. Obesity. 2018;26(11):1696–1703. doi:10.1002/oby.22304
20. Akazawa S, Sun F, Ito M, Kawasaki E, Eguchi K. Efficacy of troglitazone on body fat distribution in type 2 diabetes. Diabetes Care. 2000;23(8):1067–1071. doi:10.2337/diacare.23.8.1067
21. Tankó LB, Bagger YZ, Alexandersen P, Larsen PJ, Christiansen C. Peripheral adiposity exhibits an independent dominant antiatherogenic effect in elderly women. Circulation. 2003;107(12):1626–1631. doi:10.1161/01.CIR.0000057974.74060.68
22. He BK, Ning ZQ, Li ZB, et al. In vitro and in vivo characterizations of Chiglitazar, a newly identified PPAR pan-agonist. PPAR Res. 2012;2012:546548. doi:10.1155/2012/546548
23. Pan DS, Wang W, Liu NS, et al. Chiglitazar preferentially regulates gene expression via configuration-restricted binding and phosphorylation inhibition of PPARγ. PPAR Res. 2017;2017:4313561. doi:10.1155/2017/4313561
24. Gross B, Pawlak M, Lefebvre P, Staels B. PPARs in obesity-induced T2DM, dyslipidaemia and NAFLD. Nat Rev Endocrinol. 2017;13(1):36–49. doi:10.1038/nrendo.2016.135
25. Li X, Yu J, Wu M, et al. Pharmacokinetics and Safety of Chiglitazar, a peroxisome proliferator-activated receptor pan-agonist, in patients < 65 and ≥ 65 years with type 2 diabetes. Clin Pharmacol Drug Dev. 2021;10(7):789–796. doi:10.1002/cpdd.893
26. Xu HR, Zhang JW, Chen WL, Ning ZQ, Li XN. Pharmacokinetics, safety and tolerability of chiglitazar, A Novel Peroxisome Proliferator-Activated Receptor (PPAR) pan-agonist, in healthy Chinese volunteers: a phase i study. Clin Drug Investig. 2019;39(6):553–563. doi:10.1007/s40261-019-00779-4
27. Wang Y, Li H, Gao H, et al. Effect of chiglitazar and sitagliptin on glucose variations, insulin resistance and inflammatory-related biomarkers in untreated patients with type 2 diabetes. Diabet Res Clin Pract. 2022;183:109171. doi:10.1016/j.diabres.2021.109171
28. Kato H, Nagai Y, Ohta A, et al. Effect of sitagliptin on intrahepatic lipid content and body fat in patients with type 2 diabetes. Diabet Res Clin Pract. 2015;109(1):199–205. doi:10.1016/j.diabres.2015.04.008
29. Vestergaard P, Rejnmark L, Mosekilde L. Diabetes and its complications and their relationship with risk of fractures in type 1 and 2 diabetes. Calcif Tissue Int. 2009;84(1):45–55. doi:10.1007/s00223-008-9195-5
30. Lee HS, Yoon JS, Park KJ, Lim JS, Hwang JS. The relationship between bone mineral density and type 2 diabetes in obese children and adolescents at the time of initial diagnosis. Horm Metab Res. 2019;51(1):42–46. doi:10.1055/a-0755-2799
31. Abdulameer SA, Sulaiman SA, Hassali MA, Subramaniam K, Sahib MN. Osteoporosis and type 2 diabetes mellitus: what do we know, and what we can do. Patient Prefer Adherence. 2012;6:435–448. doi:10.2147/PPA.S32745
32. Schwartz AV, Sellmeyer DE, Ensrud KE, et al. Older women with diabetes have an increased risk of fracture: a prospective study. J Clin Endocrinol Metab. 2001;86(1):32–38. doi:10.1210/jcem.86.1.7139
33. Strotmeyer ES, Cauley JA, Schwartz AV, et al. Nontraumatic fracture risk with diabetes mellitus and impaired fasting glucose in older white and black adults: the health, aging, and body composition study. Arch Intern Med. 2005;165(14):1612–1617. doi:10.1001/archinte.165.14.1612
34. Gilbert MP, Pratley RE. The impact of diabetes and diabetes medications on bone health. Endocr Rev. 2015;36(2):194–213. doi:10.1210/er.2012-1042
35. Kanazawa I, Yamaguchi T, Yano S, et al. Baseline atherosclerosis parameter could assess the risk of bone loss during pioglitazone treatment in type 2 diabetes mellitus. Osteoporos Int. 2010;21(12):2013–2018. doi:10.1007/s00198-009-1161-1
36. Lecka-Czernik B. Bone loss in diabetes: use of antidiabetic thiazolidinediones and secondary osteoporosis. Curr Osteoporos Rep. 2010;8(4):178–184. doi:10.1007/s11914-010-0027-y
37. Lecka-Czernik B. PPARs in bone: the role in bone cell differentiation and regulation of energy metabolism. Curr Osteoporos Rep. 2010;8(2):84–90. doi:10.1007/s11914-010-0016-1
38. Qian G, Fan W, Ahlemeyer B, Karnati S, Baumgart-Vogt E. Peroxisomes in different skeletal cell types during intramembranous and endochondral ossification and their regulation during osteoblast differentiation by distinct peroxisome proliferator-activated receptors. PLoS One. 2015;10(12):e0143439. doi:10.1371/journal.pone.0143439
39. Still K, Grabowski P, Mackie I, Perry M, Bishop N. The peroxisome proliferator activator receptor alpha/delta agonists linoleic acid and bezafibrate upregulate osteoblast differentiation and induce periosteal bone formation in vivo. Calcif Tissue Int. 2008;83(4):285–292. doi:10.1007/s00223-008-9175-9
40. Kaul S, Rothney MP, Peters DM, et al. Dual-energy X-ray absorptiometry for quantification of visceral fat. Obesity. 2012;20(6):1313–1318. doi:10.1038/oby.2011.393
41. Micklesfield LK, Goedecke JH, Punyanitya M, Wilson KE, Kelly TL. Dual-energy X-ray performs as well as clinical computed tomography for the measurement of visceral fat. Obesity. 2012;20(5):1109–1114. doi:10.1038/oby.2011.367
42. De Pergola G, Tartagni M, d’Angelo F, et al. Abdominal fat accumulation, and not insulin resistance, is associated to oligomenorrhea in non-hyperandrogenic overweight/obese women. J Endocrinol Invest. 2009;32(2):98–101. doi:10.1007/BF03345694
43. Weiping J, Jianhua M, Heng M, et al. Chiglitazar monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes: a randomized, double-blind, Phase 3 trial (CMAS). Sci Bull (Beijing). 2021;66(15):1581–1590.
44. Aucouturier J, Meyer M, Thivel D, Taillardat M, Duché P. Effect of android to gynoid fat ratio on insulin resistance in obese youth. Arch Pediatr Adolesc Med. 2009;163(9):826–831. doi:10.1001/archpediatrics.2009.148
45. Lim S, Kim JH, Yoon JW, et al. Sarcopenic obesity: prevalence and association with metabolic syndrome in the Korean Longitudinal Study on Health and Aging (KLoSHA). Diabetes Care. 2010;33(7):1652–1654. doi:10.2337/dc10-0107
46. Fu X, Ma X, Lu H, He W, Wang Z, Zhu S. Associations of fat mass and fat distribution with bone mineral density in pre- and postmenopausal Chinese women. Osteoporos Int. 2011;22(1):113–119. doi:10.1007/s00198-010-1210-9
47. Wiklund P, Toss F, Weinehall L, et al. Abdominal and gynoid fat mass are associated with cardiovascular risk factors in men and women. J Clin Endocrinol Metab. 2008;93(11):4360–4366. doi:10.1210/jc.2008-0804
48. Hou XG, Moser S, Sarr MG, Thompson GB, Que FG, Jensen MD. Visceral and subcutaneous adipose tissue diacylglycerol acyltransferase activity in humans. Obesity. 2009;17(6):1129–1134. doi:10.1038/oby.2008.659
49. Toss F, Wiklund P, Franks PW, et al. Abdominal and gynoid adiposity and the risk of stroke. Int J Obes Lond. 2011;35(11):1427–1432. doi:10.1038/ijo.2011.9
50. Hotta K, Funahashi T, Arita Y, et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000;20(6):1595–1599. doi:10.1161/01.ATV.20.6.1595
51. Kim JY, van de Wall E, Laplante M, et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest. 2007;117(9):2621–2637. doi:10.1172/JCI31021
52. Soccio RE, Chen ER, Lazar MA. Thiazolidinediones and the promise of insulin sensitization in type 2 diabetes. Cell Metab. 2014;20(4):573–591. doi:10.1016/j.cmet.2014.08.005
53. Watanabe S, Takeuchi Y, Fukumoto S, Fujita H, Nakano T, Fujita T. Decrease in serum leptin by troglitazone is associated with preventing bone loss in type 2 diabetic patients. J Bone Miner Metab. 2003;21(3):166–171. doi:10.1007/s007740300026
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.