Back to Journals » International Journal of Nanomedicine » Volume 19
Effect of C7-3-Peptide-Loaded Chitosan Nanoparticles Against Multi-Drug-Resistant Neisseria gonorrhoeae
Authors Albdrawy AI , Aleanizy FS , Eltayb EK, Aldossari AA , Alanazi MM, Alfaraj R , Eltahir E , Albasri HM, Alanazi JS, Alqahtani FY
Received 21 November 2023
Accepted for publication 9 January 2024
Published 19 January 2024 Volume 2024:19 Pages 609—631
DOI https://doi.org/10.2147/IJN.S445737
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor R.D.K. Misra
Asma Ismail Albdrawy,1 Fadilah Sfouq Aleanizy,1 Esraa Kamal Eltayb,1 Abdullah A Aldossari,2 Mohammed M Alanazi,2 Rihaf Alfaraj,1 Eram Eltahir,1 Hibah M Albasri,3 Jouri S Alanazi,4 Fulwah Yahya Alqahtani1
1Department of Pharmaceutics, College of Pharmacy, King Saud University, Riyadh, Saudi Arabia; 2Department of Pharmacology and Toxicology, College of Pharmacy, King Saud University, Riyadh, Saudi Arabia; 3Department of Biology, College of Science, Taibah University, Madinah, Saudi Arabia; 4Pharmaceutical Care Department, National Guard Health Affairs, Riyadh, Saudi Arabia
Correspondence: Fulwah Yahya Alqahtani, Department of Pharmaceutics, College of Pharmacy, King Saud University, PO Box 22452, Riyadh, 11495, Saudi Arabia, Tel +966-11-8051478 ; +966503416440, Email [email protected]
Introduction: The emergence of Neisseria gonorrhoeae-resistant strains represents one of the most urgent global threats. In this regard, C7-3 peptide is one of the anti-virulence therapies that has demonstrated promising anti-gonococcal activity. Accordingly, this research aimed to formulate C7-3 peptide and its derivatives in chitosan nanoparticles.
Methods: The peptide loaded chitosan nanoparticles were prepared using ion gelation method, and their physicochemical characteristics were investigated. The anti-gonococcal and antibiofilm activity of prepared NPs was assessed, and their cytotoxicity in human ovarian cells was evaluated.
Results: All prepared NPs were optimized for the smallest particle size of 136.9 to 168.3 nm. The EE% of C7-3, C7-3m1, and C7-3m2 CNPs reached 90.2, 92.5, and 91.8%, respectively. An in vitro release study demonstrated a continuous sustained-release pattern of C7-3 peptide from NPs. The SDS-PAGE assay confirmed the integrity of C7-3 peptide after the fabrication process. When comparing each peptide alone, the generated NPs demonstrated higher anti-gonococcal and anti-biofilm effectiveness against standard and resistant bacterial strains under anaerobic conditions. The cytotoxicity experiments revealed the cytocompatibility of NPs in HeLa cell lines. Given the advantages of enhanced anti-gonococcal activity of the C7-3 peptide and its derivatives when loaded with CNPs, as well as the antimicrobial properties of chitosan NPs, the reported NPs have great potential in the treatment of gonococcal infection.
Keywords: N. gonorrhoeae, chitosan, nanoparticles, C7-3 peptide, biofilm
Introduction
Neisseria gonorrhoeae is an obligate human pathogen and a Gram-negative diplococcus. It is recognized as one of the most prevalent sexually transmitted infections (STIs). According to the World Health Organization (WHO), N. gonorrhoeae causes 87–106 million cases annually worldwide.1–4 It is associated with an increased risk of infertility and neonatal health diseases. N. gonorrhoeae commonly infects the mucosal surfaces of the genitals, conjunctiva, anorectal area, and pharynx.1 It can enter the blood circulation directly due to unintentional exposure, causing fever and serious adverse effects on the knees (septic arthritis).1 However, N. gonorrhoeae can be a contributing factor in raising the potential of HIV acquisition and transmission.1 In the mid-1990s, the discovery of antimicrobials with different modes of action was an outstanding breakthrough in medicine that has treated millions of infected people.5 In the last few decades, there has been a surge of interest in the existence of antimicrobial-resistant (AMR) bacteria. According to the WHO, AMR bacteria are one of the top ten global public health challenges facing humanity. This has resulted in millions of people being infected and thousands of deaths globally.6
In this regard, N. gonorrhoeae strains have shown an alarming potential for resistance acquisition against antimicrobials and human defenses. The genetic mechanisms and mutations have been associated with AMR N. gonorrhea.7 Due to the emergence of N. gonorrhoeae antibiotic resistance, dual therapy has been recommended over single therapy.8 Accordingly, the WHO has recently classified it as a “superbug”.1,3 The spread of highly antibiotic-resistant strains is associated with high risk of morbidity, mortality, and economic burden worldwide. Consequently, there have been global concerns over several years that ordinary infections have taken a dramatic turn toward becoming nearly untreatable.3,9 From this perspective, anti-virulence therapies (AVTs) have been developed as novel strategies that selectively target virulence mechanisms that are necessary for microbial pathogenicity. AVTs combat current AMR isolates while also circumventing the pathways leading to the development of AMR bacteria. Anti-virulence therapy offers numerous approaches to impair bacterial progression and infection cascade.4
In this aspect, C7-3 peptide and its derivatives are among the AVTs. C7-3 peptide was granted a patent to act as an inhibitor of AniA (NGO1276). AniA is a copper-containing nitrite reductase enzyme also known as NirK. This enzyme plays a critical role in N. gonorrhoeae anaerobic growth and biofilm formation. A new study has shown that anaerobic respiration is a crucial stage and represents approximately 10% of the genomic material of N. gonorrhoeae. This provides a range of survival advantages to bacteria, including enhanced resistance to antibiotics and human defense pathways.10,11 Thus, gonococci may be able to survive in the cervical environment because of biofilm formation. The biofilm may represent a special adaptation of the gonococcus, enabling it to cause persistent chronic infection.11 In a previous study, AniA was found in all samples taken from patients infected with N. gonorrhoeae. Antiserum against AniA cross-reacted with all tested samples from the sera of patients infected using Western blot analysis. Therefore, this suggested that AniA was expressed in all isolates.12
A new study carried out by Sikora et al13 was aimed at discovering the most effective AniA inhibitor. Sikora et al13 examined peptide libraries using the phage display approach followed by an enzyme-linked immunosorbent assay for targeting AniA. The researchers also used a combination of computational molecular docking, biolayer interferometry, homologous recombination, and determination of the minimum inhibitory concentration (MIC50). All these experiments resulted in confirming that the C7-3 peptide was the most promising AniA inhibitor. This study also proposes that AniA inhibition has therapeutic potential in reducing N. gonorrhea availability at the genitals, characterized by their oxygen-limited conditions. The findings of this study also suggest that AniA inhibition may augment the existing antimicrobials’ capacity to eradicate the microbe.13
C7-3 peptide may have numerous drawbacks in terms of being administered in vivo, owing to its high instability, either chemically or physically. Peptides can easily undergo several alterations such as denaturation, aggregation, oxidation, and hydrolysis, which could affect their therapeutic activity. Another limitation of peptides is their susceptibility to enzymatic degradation when exposed to peptidases, which are abundantly expressed in the whole body.14
Accordingly, nanotechnology including polymeric nanoparticles (PNPs) has shown significant potential to combat antimicrobial resistance. In PNPs, encapsulation within NPs made up of natural polymers such as chitosan (Cs) is commonly used. Naturally derived chitosan is a linear polymer consisting of N-acetyl-D-glucosamine and β-(1,4)-linked D-glucosamine linked by glycosidic bonds obtained as a result of the alkaline deacetylation of chitin. It is gaining attention because of its advantages such as its biocompatibility, biodegradability, increased permeability, and mucoadhesive properties. Through in vitro and in vivo studies, chitosan has shown neither an inflammatory response nor hepatorenal toxicity. Hence, it has been stated by the US Food and Drug Administration as being generally regarded as safe (GRAS).15 Recent studies have demonstrated that chitosan nanoparticles (CNPs) exhibited anti-gonococcal activity. A CNP formulation was prepared via the ion gelation method and their effect against N. gonorrhoeae-resistant strains was investigated using the broth dilution method. The results revealed a significant reduction in bacterial viability and adherence to host cells, suggesting CNPs act as an anti-gonococcal drug carrier.2 Different hypotheses have been proposed to explain chitosan’s antibacterial action; however, the precise mechanism of action remains unclear. Among these hypotheses is membrane alteration, in which positively charged chitosan interacts with negatively charged bacterial surfaces such as lipopolysaccharides (LPS). This interaction has resulted in the leakage of intracellular components and cell death.16 Chitosan nanoencapsulation of peptides could protect them from alterations caused by acidic pH and enzymatic degradation. It could also provide controlled drug release with a prolonged drug retention time.17 Confirmed evidence from another study proves CNPs capability to overcome the limitations of the vulnerable peptide structure and unfavorable stability. The chitosan-loaded peptide could withstand a variety of temperature and pH conditions. Furthermore, cell lines risk evaluation justified that CNPs didn’t induce toxicity. It can also be concluded that the nature of CNPs stabilizes the peptide structure and activity.16
In conclusion, this study aims to formulate C7-3-peptide-loaded chitosan nanoparticles (CNPs) and investigate their antimicrobial activity against multi-drug-resistant N. gonorrhoeae isolates.
Materials and Methods
Materials
Low-molecular-weight chitosan (LMW, 50–190 kDa; degree of deacetylation, DD = 75–85%), tripolyphosphate (TPP), and acetic acid were purchased from Sigma-Aldrich (St Louis, USA). Gonococcus medium (GC) agar, supplemented with 1% IsoVitaleX and gonococcus broth medium (GCP) was purchased from (Difco). All of the other solvents and chemicals were of analytical grade. Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), and antibiotic–antimycotic solution was purchased from Gibco (NY, USA). Synthetic water-soluble peptides C7-3 (H-ACNYCRLNLWGGGS-NH2), C7-3m1 (H-ACNYSRLNLWGGGS-NH2), and C7-3m2 (H-ACSY CRLNLWGGGS-NH2) were purchased from Pepmic Co., Ltd (Suzhou, China). A control strain of N. gonorrhoeae, NCTC 12,700, and the NCTC 14,208 N. gonorrhoeae reference strain were purchased from Public Health England (PHE). Bio-Rad Protein Assay Dye Reagent Concentrate, Bio-Rad Protein Assay Kit I, and Bio-Rad Protein Assay Kit II were purchased from Bio-Rad (USA). HeLa (human cervical cancer epithelial cells) was purchased from the American Type Culture Collection (ATCC CCL-2). Methylthiazolyldiphenyl-tetrazolium Bromide (MTT) was purchased from Sigma-Aldrich (Saint Louis, USA).
Methods
Optimization of CNPs
Chitosan NPs were generated using the ionic gelation method, as described previously.2,18 A total of 1 mg/mL of LMW chitosan solution were dissolved in a solution of 1% v/v acetic acid. The pH of the chitosan solution was adjusted to pH 5 by 0.2 M NaOH solution. A solution of 2 mL of (1 mg/mL) TPP was added, dropwise, to 5 mL of chitosan solution under continuous magnetic stirring at 800 rpm for 90 minutes and the nanoparticles were synthesized spontaneously. Afterwards, the formulation was centrifuged at 14,000 g for 45 min at 4°C, then reconstituted in ultrapure DNase/RNase-free water and stored at 4°C.
The effects of different factors on CNPs were investigated by measuring nanoparticle size, zeta potential, and polydispersity index (PDI). The tested parameters were chitosan/TPP ratio, pH, and stirring time. The Cs/TPP formulations were formed following the addition of 2 mL of (1 mg/mL) TPP to different concentrations of chitosan solution under magnetic stirring, resulting in Cs/TPP volume ratios of 1:1, 1.5:1, 2:1, 2.5:1, 3:1, 3.5:1, and 4:1, respectively. The effect of different Cs/TPP volume ratios on the physicochemical characteristics of nanoparticles was measured. Different pH values of chitosan solution (3, 4, 5, and 6) were also optimized, as well as different stirring times (30, 60, 90, and 120 minutes).
Loading of C7-3 Peptide and Its Derivatives in CNPs
C7-3 peptide was encapsulated within chitosan NPs using the ionic gelation method following the standardized parameters evaluated in this study. Briefly, a concentration of (1 mg/mL) LMW chitosan with a molecular weight (190 kDa) was prepared and dissolved using 1% v/v acetic acid. The chitosan solution was adjusted to pH 5 using 0.2 M NaOH solution. The peptide (4.8 mM) was dissolved in the chitosan solution for drug encapsulation. Then, 2 mL of (1 mg/mL) TPP solution was added dropwise to 5 mL of the drug–chitosan solution under continuous magnetic stirring at 800 rpm for 30 minutes, and the nanoparticles were spontaneously generated. The nanoparticles were then obtained by centrifugation at 14,000 g for 45 min at 4°C. The same process was followed for the encapsulation of C7-3m1 and C7-3m2 peptides into CNPs.
Characterization of Particle Size (PS), Polydispersity Index (PDI), and Zeta Potential (ζ Potential)
A Zetasizer Nano ZS90 (Malvern Instruments Ltd, UK) was used to measure the zeta potential, polydispersity index (PDI), and particle size (PS) of the formed nanoparticles.
Determination of Encapsulation Efficiency (EE%)
The encapsulation efficiency (EE%) of the peptide-loaded CNPs formulation was measured using an indirect method. Peptide-loaded CNP formulations were centrifuged for 45 minutes at 14,000 g, and the amount of unencapsulated peptide in the supernatant was assessed using HPLC.
HPLC Assay for EE% Measurements
Pure peptide was quantified using the HPLC method as a preliminary experiment using the manual produced by the peptide’s manufacturer. The HPLC system (Waters e2695 separation modules, Waters, Milford, USA) is provided with a wavelength detector (Waters 2489 dual-wavelength detector). Briefly, the peptide was dissolved in filtrated deionized water. Then, 35 μL of the filtrate sample was injected into an Interstil ODS-4 HPLC column (4.6 * 250mm * 5μm; GL SCIENCES INC., USA). The experiment was optimized at a flow rate of 1 mL/min using both solvent B (0.1% trifluoroacetic acid (TFA) in 100% acetonitrile) and solvent A (0.1% TFA in 100% H2O). Eluted peptides were monitored at 220 nm, and significant peaks were observed in consecutive and reproducible runs. The peptide was serially diluted at concentrations of 0.125, 0.0625, 0.0312, 0.0156, 0.0078, and 0.0039 mM/mL to determine the calibration curve for each peptide. All experiments were carried out at room temperature. The encapsulation efficiency was calculated by determining the amount of free peptide recovered in the supernatant.
The equation used to calculate encapsulation efficiency is as follows:
Characterization of Nanoparticles Morphology
The morphology of empty CNPs (ECNPs) and peptide-loaded CNPs was imagined with the measurement of particle diameter by a transmission electron microscope (TEM). A total of 5ul of sample was dropped on a 300 mesh formvar carbon-supported grid (TedPella, Inc.) and incubated for 3 min. Then, the grid was dried using filter paper. Afterwards, the sample was stained for contrast with UranyLess solution (EMS) for 1 min. The grid was dried again by filter paper. TEM images were then collected on a JEOL-JEM 1400 electron microscope operating at 120 kV through a MegaView G2 camera (Olympus) equipped with iTEM Olympus Soft Imaging Solutions GmbH 5.2 imaging software.
In vitro Release Study of C7-3 Peptide and Its Derivatives from the CNP Formulation
Peptide-loaded CNPs were resuspended in 1 mL PBS buffer (pH=7.4) and shaken in a water bath (200 rpm) at 37°C, as described previously.18 A predetermined amount (300 µg/mL) of formed nanoparticles was re-dispersed in 1 mL fresh phosphate buffer saline (PBS) at pH 7. At predetermined time intervals, samples were centrifuged at 14,000 g, at 4°C for 30 minutes. Then, 1 mL of the supernatant was collected for analysis and replaced with 1 mL of fresh PBS medium. The amount of C7-3 released from the CNPs was measured using the Bradford protein assay and HPLC.
Storage Stability
Loaded peptide nanoparticles were stored for 2 months at 4°C, –20°C, and ± 25°C, and their physicochemical characteristics including PS, PDI, and zeta potential were evaluated.
Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE)
Peptide Integrity Assessment in Formulated CNPs
SDS-polyacrylamide gel electrophoresis was used to assess the integrity of the peptide encapsulated in CNPs after the release study. The first, second, and third days’ release samples were prepared for loading on 12% separating and 4% stacking polyacrylamide gel. Each sample was dissolved in the sample buffer and placed onto the gel. As a control, a 4.8 mM amount of native peptide was used. For 3–4 hours, the gel was run at a constant voltage of 60 V in Tris glycine running buffer. The gel was then stained for 1 hour with Coomassie Brilliant Blue dye before being de-stained to show the clear bands. The integrity of C7-3, C7-3m1, and C7-3m2 peptide-loaded CNPs was demonstrated.
Antibacterial Activity
Susceptibility Test of N. gonorrhoeae Strains
Disc diffusion tests on GC media were carried out in accordance with CLSI M02-A12 (2019).19 Overnight cultures of gonococcal strains (standard strain and WHO Q) were resuspended in GCBL medium to 0.5 McFarland for each strain before plating confluently into GC agar with a sterile cotton swab, as described previously.20 After drying for 10 minutes, azithromycin 15 g and ceftriaxone 30 g discs from Oxoid (England) were added to the plates and incubated overnight at 37°C and 5% CO2. For the determination of zone diameter breakpoints for ceftriaxone and azithromycin, the CLSI M100 (2021) recommendations were used.21
Determination of the Minimum Inhibitory Concentration (MIC) of C7-3 and Its Derivatives Encapsulated with CNPs
The effect of loaded CNPs on NCTC 12,700 and NCTC 14,208 N. gonorrhoeae isolates was studied in this study. For 18–22 hours, the bacteria were grown overnight on GCB agar at 37°C in 5% CO2. The bacteria were then resuspended in gonococcal base liquid medium (GCBL) and supplemented in 1:100 and 1:1,000 ratios with 0.042% sodium bicarbonate and Kellogg’s supplement, respectively. The bacterial suspension was shaken for 3 hours at 250 rpm at 37°C. The bacterial cell suspensions were all diluted to McFarland 0.5 (1–2108 CFU/mL). The colony forming units (CFU) method was used to determine the MIC50 (minimum inhibitory concentration that inhibited 50% of the tested microorganisms) of the formed nanoparticles in GCB broth supplemented with 2 mM sodium nitrite under anaerobic conditions, as described previously.14 In this study, all three synthetic peptides, C7-3, C7-3m1, and C7-3m2, were serially diluted and studied. The generated bacterial suspension was treated anaerobically with the ECNPs, peptide-loaded CNPs, and peptides for 18 hours at 37°C. The following day, cultures were serially diluted and spotted onto GCB to count the CFUs. Experiments were carried out in triplicate.
Biofilm Formation Assay
The effect of C7-3-peptide-loaded CNPs and its derivatives on gonococcal formation of biofilms was studied using NCTC 12,700 and NCTC 14,208 N. gonorrhoeae isolates as controls. Crystal violet (CV) staining was used to measure the formation of biofilms. The bacteria were grown in a supplemented GCBL on the first day and incubated at 37°C with shaking at 250 rpm. The bacterial suspension was adjusted to 0.5 on the McFarland standard after 3 hours. A total of 100 µL of bacterial broth suspension was introduced to 100 µL of serially diluted pure peptide, peptide-loaded CNPs, and ECNPs on a flat-bottom polystyrene 96-well micro-titer plate. The plate was incubated overnight at 37°C with 150 rpm shaking. After 24 hours, the medium was withdrawn and the plates were gently washed with 0.9% NaCl, air-dried for 1 hour, and stained for 15 minutes with 0.1% (v/v) crystal violet. Three washes with 0.9% NaCl were performed on the unbound stain. The bound crystal violet was then solubilized by adding 200 µL of ethanol–acetone (80:20 v/v) to each well. The absorbance of adhering pigmented bacteria was measured using the Microtiter Plate Reader (BioTek Instruments, VT, USA) at a wavelength of 590 nm. The experiment was carried out three times.
Cytotoxicity Studies
Evaluation of the Effect of Prepared ECNPs and Peptide-Loaded CNPs in Terms of the Cytotoxicity on Cell Lines
Cell Culture
Human cervical cancer (HeLa) cells were taken from the American Type Culture Collection (ATCC) and cultivated in DMEM containing 10% FBS and 1.0% penicillin–streptomycin solution. Cells were cultured in flasks at 37°C and 5% CO2 under standard conditions. Cells were kept at 37°C in a humidified incubator with 5% CO2. When the cells were confluent, they were passaged into T-75 flasks by trypsinization (300µL). All proposed tests were carried out on cells ranging in passage from 3 to 15.
MTT Assay
The MTT assay was utilized to assess the cytotoxicity of the ECNPs, and C7-3, C7-3m1, and C7-3m2 CNPs on HeLa (human cervical cancer) cells. Cells were seeded into 96-well plates overnight in DMEM supplemented with 10% FBS and 1% antibiotic antimycotic solution, until 70% to 90% confluency was achieved. ECNPs and peptide-loaded CNPs were added to cells cultivated in 96-well plates at doses ranging from 2.4 to 0.075 mM/mL and incubated for 24 and 48 hours. Following incubation, 10µL of MTT (5 mg/mL PBS) reagent was added to each well and incubated for 30 minutes, or until a purple precipitate was visible. Then, at room temperature, 100µL of detergent reagent (DMSO) was added to each well while shaking for 5 minutes. A SpectraMax microplate reader was used to measure absorbance at 570 nm.
Statistical Analysis
Using Prism 8 (GraphPad), the unpaired two-tailed Student’s t-test was utilized to analyze differences between the experimental groups. When appropriate, one-way ANOVA was employed, followed by the Tukey’s post hoc comparison test. The mean SE of at least three experiments (n = 3) was used to calculate the results. A p-value <0.05 was considered to be significant.
Results
This study intended to produce CNPs capable of encapsulating, protecting, and delivering the C7-3 peptide and its derivatives as a potential therapy for gonococcal infections. It aimed to generate nanoparticles with the smallest PS, a homogeneous formulation with a PDI lower than 0.35, and a generally positive surface charge. As a result, the effect of the chitosan (Cs) pH and its ratio to TPP on the size and charge of produced nanoparticles was examined, optimized, and loaded with C7-3 peptide and its derivatives. Following that, the best formula was subjected to physicochemical characterization, antibacterial activity, and cytotoxicity tests.
Optimization of CNPs
Influence of the Chitosan/TPP Ratio (w/w)
The effect of various weight ratios of Cs to TPP solutions, ranging from 4:1 to 1:1, on the PS, PDI, and ZP of produced NPs was studied. The particle size of the formed CNPs rose as the Cs/TPP ratio increased, as illustrated in Table 1. The zeta potential value increased as the Cs/TPP ratio increased. The zeta potential declined almost linearly from 32 mV to 14 mV when the chitosan to TPP ratio decreased (Table 1). Except for the 2:1 and 1:1 ratios of Cs to TPP, where the PDI was 0.75 and 0.60, the PDI of the formulations ranged from 0.32 to 0.43.
 |
Table 1 The Effect of Cs/TPP Ratio on CNP PZ, PDI, and ZP |
Influence of CS pH
The effect of the chitosan solution pH on the CNP formulation was examined. Four CS solutions were adjusted to pH values of 3, 4, 5, and 6 with 0.2M NaOH or HCL before being crosslinked with TPP to generate CNPs. As is demonstrated in Figure 1, the particle size and PDI were lowest when CNPs were synthesized at pH 5 and highest when CNPs were generated at pH 6. The surface charge of NPs decreased as the pH of Cs increased. The CNPs formed from the Cs solution at pH 5 had the smallest PS, optimal PDI and ZP; hence, pH 5 was chosen as the ideal condition for further formulations.
The Effect of Stirring Time
This experiment was performed to study the effect of stirring time on the properties of CNPs. When the stirring duration was increased from 30 min to 120 min, the mean particle size increased gradually from 136.2 nm to 257.3 nm, clearly indicating the impact of stirring time (Figure 2). In addition, the PDI of the resulting NPs gradually increased from 0.178 to 0.35 over the extended stirring period (Figure 2). Furthermore, the results demonstrated no significant change in the zeta potential value by increasing the stirring time. As revealed by the results, the stirring duration of 30 min was the best condition for producing CNPs with smaller particle sizes, optimal zeta potential, and the lowest size distribution (0.178).
Preparation and Characterization of C7-3 CNPs and Its Derivatives
CNPs were easily produced using an ionic gelation process with cationic Cs and an anionic crosslinker TPP, as previously reported.19 In this investigation, the formulation parameters (Cs/TPP ratio, Cs solution pH, and stirring time) were previously optimized to be 2.5, 5, and 30 min, respectively, and were used to encapsulate C7-3 in CNPs. Physical characterization results showed that peptide loading increased the average diameter of the NPs, with diameters of 136 ± 0.74 and 167 ± 1.5 nm obtained for blank and C7-3 CNPs, respectively (Table 2, Figure S1). In addition, the PS of C7-3m1 and C7-3m2 peptide-loaded CNPs were 156± 1.7 and 157 ± 0.5 nm, respectively, which also showed an enlargement in the PZ of NPs following the encapsulation process. The PDI of CNPs formulations was 0.18, with a slight rise in loaded formulations ranging from 0.21 to 0.23, indicating homogeneity. Due to the cationic nature of Cs, both CNPs and peptide-loaded NPs had positive zeta potential values. The peptide loading reduced the zeta potential value (Table 2, Figure S2). The calibration curves of peptides were generated using HPLC and Bradford assay and used to determine the EE% (Figure S3). The EE% for C7-3, C7-3m1, and C7-3m2 CNPs were found to be 90.2%, 92.5%, and 91.8%, respectively (Table 2).
 |
Table 2 Main Characteristics of Empty CNPs and Peptide-Loaded CNPs |
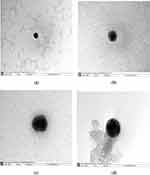
Transmission Electron Microscopy (TEM)
Transmission electron microscopy was used to visualize all ECNPs and peptide-loaded formulations. As is shown in Figure 3, all loaded and empty CNPs showed spherical shape and smooth surface.
In vitro Release of C7-3 CNPs and Its Derivatives
The release pattern of the C7-3 peptide and its derivatives from NPs was examined in PBS at pH 7.4 for a duration of twelve days. The peptide-CS system demonstrated a progressive linear release pattern in all three peptides after the first equilibration time (lag time), as illustrated in Figure 4. The cumulative release percentage of C7-3, C7-3m1, and C7-3m2 peptides in PBS buffer (pH=7.4) was 7.83, 7.9, and 9.8%, respectively, over the first 24 hours. Every day for the next 11 days, a constant sustained-release percentage of 6–7% was recorded for all three peptides encapsulated in NPs. However, after 12 days of release evaluation, C7-3, C7-3m1, and C7-3m2 peptides released 80–90% of the encapsulated peptides.
Evaluation of Peptide Integrity in the Formulated NPs
To assess the integrity of peptides loaded in CNPs, released samples from each peptide were placed on SDS-PAGE gel, with the native peptide as a control. Figure 5 shows that each peptide from the in vitro release samples was generally intact. Furthermore, pure peptide and its release samples with a molecular weight of less than 10 kDa moved similarly in the gel illustrations. The peptide structure was neither altered or deteriorated in the bands. The presence of intact and stable bands demonstrated that the integrity of the encapsulated peptide was not compromised by the production technique or release conditions. The findings revealed that the peptide-loaded nanoparticle preserved its structural integrity.
Storage Stability
To assess the storage stability of both ECNPs and peptide-loaded formulations, collected samples were stored at three distinct temperatures (4°C, −30°C, and −80°C). At predefined intervals (0, 30, and 60 days), particle size, PDI, and surface charge were assessed. Figure 6 depicts the effects of varied storage conditions (temperature and time) on the stability of nanoparticles over a two-month period. Particle size, PDI, and zeta potential of the formulation stored at 4°C were stable for up to 60 days (Figure 6). The particle size of all formulations stored at 4°C remained within the target ranges of 130–170 nm and 0.18–0.27, respectively. The particle size and PDI of ECNPs and loaded CNPs were substantially changed after storage at –30 and –80°C. The particle size and PDI of CNPs increased with increasing temperature and storage duration due to aggregation. When compared to the fresh formulation, the zeta potential data showed no significant change at the various temperatures over 30 or 60 days.
 |
Figure 6 NPs storage stability. Notes: (A) PZ, (B) PDI, and (C) ZP of the formulation after two months of storage at 4°C, –30°C, and –80°C. The measurements indicated the mean SD, n=3. |
Anti-Gonococcal Activity of ECNPs and Peptide-Loaded CNPs
Disc Diffusion Susceptibility Assay
The standard strain (NCTC 12700) was found to be sensitive to ceftriaxone and azithromycin, with zones of inhibition (ZoI) of 52 mm and 30 mm, respectively (Table 3, Figure 7). The WHO Q was resistant to ceftriaxone and azithromycin, as expected, with ZoI diameters of 26 and 0 mm, respectively. According to the CLSI criteria, ZoI with diameters of more than 35 mm suggest susceptibility of N. gonorrhoeae to ceftriaxone, while ZoI had diameters of 26 mm (non-susceptible) and 27 mm (susceptible).
 |
Table 3 Zones of Inhibition Diameters (in Mm) for Standard Strain and WHO Q, Differentiating Susceptible and Non-Susceptible Isolates |
Measurement of the MIC50 of ECNPs and Peptide-Loaded CNPs Using the Killing Assay
Figure 8 illustrates that C7-3 pure peptide and its derivatives reduced gonococcal viability in a dose-dependent manner. C7-3m2 native peptide had the most powerful anti-gonococcal activity against the gonococcal standard strain, followed by C7-3 and C7-3m1, with MIC50 values of 0.3, 0.6, and 1.7 mM/mL, respectively (Table 4). The effect of peptide-loaded CNPs against the standard strain was then investigated. It is clear that encapsulating C7-3 and its derivatives within CNPs resulted in improved anti-gonococcal activity and efficacy (Figure 9). The MIC50 values of the C7-3-, C7-3m1-, and C7-3m2-loaded CNPs were 0.19, 0.26, and 0.099 mM/mL, respectively, which is significantly lower than the MIC50 values of each peptide alone (p 0.05).
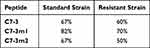 |
Table 4 The Percentage of MIC50 Reduction for Peptides After Encapsulation Within CNP Formulation |
 |
Figure 8 Continued. |
Then, the antimicrobial effect of C7-3 and its derivatives on the WHO Q, a clinical isolate resistant to azithromycin and ceftriaxone, was studied. As demonstrated in Figure 10, peptide treatment resulted in a considerable dose-dependent reduction in bacterial growth. Bacterial cell survival was reduced by 50% at concentrations of 1.1, 1.1, and 0.6 mM/mL of C7-3, C7-3m1, and C7-3m2, respectively. When the WHO Q was treated with CNPs loaded with peptides, the MIC50 value for the C7-3, C7-3m1, and C7-3m2 CNPs was 0.45, 0.34, and 0.3 mM/mL, respectively (Table 4). These findings suggest that encapsulating C7-3 and its derivatives into CNPs improves their anti-gonococcal action. In particular, the C7-3m2 peptide and its loaded formulation exhibited the highest anti-gonococcal efficacy with the lowest MIC50 against both the standard and WHO Q strains of N. gonorrhoeae (Table 4). Figure 11 further demonstrates the proportion of gonococcal growth inhibition of the WHO Q strain in response to peptides alone and peptide-loaded CNPs. Bacterial growth was significantly reduced in response to CNPs containing peptides as compared to bacteria treated with peptides alone.
 |
Figure 10 Continued. |
CNPs at 0.5 mg/mL reduced gonococcal growth by 92% compared to the standard strain with a MIC50 of 0.1 mg/mL. To suppress the growth of the WHO Q N.-gonorrhoeae-resistant strain by 50% (Figures 8 and 10), a higher concentration of CNPs equal to 0.2 mg/mL was required.
Table 5 compares the percentage reduction in MIC50 for peptides when encapsulated into CNPs. Briefly, after loading within CNPs, the anti-gonococcal activity of C7-3, C7-3m1, and C7-3m2 peptides was raised by 67%, 67%, and 82%, respectively, for the standard strain. When placed within CNPs, C7-3, C7-3m1, and C7-3m2 peptides demonstrated a 60%, 70%, and 50% greater antibacterial activity, respectively. The drop in MIC50 was greater in the standard strain of N. gonorrhea than in the resistant strain, as expected. CNPs synergism, on the other hand, was observed in terms of a significant synergism of peptide inhibitory effect (Table 5).
 |
Table 5 MIC50 of Peptides and Peptides-Loaded NPs Against Gonococcal Standard Strain and WHO Q Isolate |
Biofilm Formation of N. gonorrhoeae in Response to C7-3 CNPs and Its Derivatives
Considering the role of AniA in the biofilm formation of N. gonorrhoeae and the inhibitory effect of C7-3 and its derivatives on gonococcal AniA, this study also aimed to evaluate the potential biofilm inhibitory effect of peptide-loaded CNPs and ECNPs on gonococcal isolates used in this study.13 C7-3m2 and its loaded NPs were shown to be more effective than peptide in decreasing biofilm development in both gonococcal strains. Furthermore, the anti-biofilm action of peptides and their NPs was dose-dependent, with low concentrations required in all cases to achieve comparable inhibition. To clarify, 0.018 mM/mL of C7-3m2 peptide and C7-3m2 CNPs inhibited 63.4 and 73.4% of standard-strain biofilms, respectively. In the presence of C7-3 and C7-3 CNPs at a 0.018 mM/mL concentration, the inhibition of the biofilm was 52.18 and 65.47%, respectively. Furthermore, C7-3m1 and C7-3m1 NPs at 0.018 mM/mL inhibited standard gonococcal biofilm development by 45.4 and 55.2%, respectively. In this assay, 0.018 mM/mL of C7-3m2 peptide and its loaded NPs were more effective against the biofilm formed by resistant strains, inhibiting its production by 56.4 and 68.8%, respectively. The C7-3 peptide and C7-3 CNPs then reduced resistant-strain biofilm development by 38.3 and 46.7%, respectively. Furthermore, C7-3m1 peptide and loaded CNPs inhibited biofilm development by 35.9 and 42.88%, respectively. When a higher dosage (0.3 mM/mL) of C7-3, C7-3m1, and C7-3m2 pure peptides were incubated with the standard strain, biofilm inhibition was 96.2, 82.7, and 97.32%, respectively. Moreover, the biofilm inhibitory activity of CNPs was investigated. The results demonstrated that ECNPs interfere with the pathogen’s biofilm production in both strains. Figure 12 shows that 0.015, 0.007, and 0.003 mg/mL of ECNPs reduced biofilm formation by 52, 42, and 32%, respectively, for the reference strain. At the same concentrations, ECNPs reduced biofilm formation in resistant strains by 46, 37, and 12%, respectively.
The encapsulation procedure of the three peptides demonstrated enhanced efficacy by using a lower concentration of loaded peptide to display almost the same inhibitory impact as higher concentrations of the same peptide alone (Figure 12). The data also demonstrated that the WHO Q strain produced more biofilm than the normal strain. C7-3 CNPs, for example, inhibited 65% of the standard-strain biofilm and 46% of the resistant-strain biofilm at 0.018 mM/mL.
Cytocompatibility Study
Cells were treated with 2.2, 1.1, 0.6, 0.3, 0.15, and 0.0075 mM/mL of C7-3 CNPs for 24 hours, and the percentage of cell viability was 87.7, 90.7, 92.75, 94.6, 98.4, and 101.2%, respectively. Cell viability for C7-3m1 CNPs was 84.7, 92.9, 98.9, 90.8, 98.6 and 96.5% at the same concentration range, whereas cell viability for C7-3m2 CNPs was 84.4, 95.06, 95.4, 102.17, 102.3, and 102.6%. Our MTT results showed that 24 hours of treatment with all types of particles did not significantly reduce the viability of HeLa cells in different NPs types, with the exception of 2.2 mM/mL of C7-3m2, which significantly reduced the viability of HeLa cells. Furthermore, nanoparticle treatment for 48 h did not affect HeLa cell viability with all forms of treatment, except treatment with C7-3 CNPs and C7-3m2 CNPs at 2.2 mM/mL, where HeLa cell viability decreased significantly (Figure 13).
Additionally, the cytocompatibility of CNPs (0.5, 0.25, 0.125, 0.06, 0.03, 0.015, and 0.0078 mg/mL) was evaluated, as shown in Figure 12. The outcomes showed no significant reduction in the percentage of HeLa cell viability at concentrations ranging from 0.5 mg/mL to 0.0078 mg/mL at either time point.
Discussion
Anti-virulence therapy has been a potential goal for the treatment-resistant gonococcal infection, and several studies have investigated its part in controlling the emergence of resistant N. gonorrhoeae strains.4 With the growing prevalence of antibiotic resistance, the search for new approaches over traditional antibiotics has become a major goal of health research; however, in this study, we investigated C7-3 peptide in terms of the properties that interfere with in vivo administration, and previously examined it for its anti-gonococcal actions. Previous research has demonstrated the effect of C7-3 peptide and its derivatives on FA1090 gonococcal strain. After screening various peptides, C7-3 peptide was identified as a potential target to block the AniA enzyme, a pivotal enzyme for gonococcal anaerobic survival.13 However, numerous natural biocompatible and biodegradable polymers have attracted increasing attention as matrix structures for the formulation of antimicrobial peptide carriers in recent years. Cs looks to be one of the most attractive polymers for use in the development of nanostructured antimicrobial systems.18
C7-3 peptide and its derivatives were encapsulated into CNPs in this study, and the resultant nano-system was optimized and characterized. Pre-specified parameters were examined in order to qualify a formulation with the lowest PS and a considerable zeta potential value. The pH of Cs, its ratio to TPP, and formulation stirring time were investigated as crucial process parameters that greatly influence the size and charge of generated NPs. The pH of Cs solutions has been found to have a significant effect on PS and ZP. To generate the smallest PS, a slightly acidic solution with a pH of 5 was reasonably chosen.
The degree of protonation of chitosan is primarily affected by the pH of the solution. When the Cs pH decreases below 6.5, amino groups in the Cs structure are protonated into positive ions, enhancing chitosan interaction with negatively charged TPP to form stable nanoparticles.22 As a result of our findings, reducing the pH of Cs solution is associated with a highly positive protonated surface (zeta potential). This was found to be nearly 30 mV, resulting in stable particles that are less susceptible to aggregation.23 It was challenging to create nanoparticles with uniform particle size distributions when the pH was not 5. When the pH was 5, however, a homogeneous clear formulation with the lowest PDI value was produced, as shown in Figure 1. Overall, the Cs solution was kept at a pH of 5 throughout the experiment to achieve the smallest and most stable NPs.
The Cs to TPP weight ratio was also optimized. Table 1 exhibited higher particle size as the Cs/TPP ratio increased. Reduced Cs content reduces the solution viscosity, increasing solubility and improving the electrostatic interaction of Cs with TPP, resulting in a smaller particle size. In comparison, mass ratios ranging from 2:1 to 1:1 produced an opaque turbid formulation with a bigger particle size in an opaque solution. In this study, the most stable Cs/TPP mass ratio for generating CNPs was 2.5:1 because it had the smallest size diameter, a homogeneous distribution of NPs, and a suitable zeta potential value.
We examined the effect of stirring time on the physicochemical properties of CNPs further. According to the findings, increasing the stirring duration from 30 to 120 minutes increased the particle size and PDI. Consequently, 30 minutes of stirring time was selected to fabricate the smallest particles.
All generated nanoparticles, as expected, had a positive surface charge and a nanoscale diameter, and a high encapsulation efficiency was achieved (above 90%). TEM images of ECNPs and peptide-loaded CNPs allowed the visualization of smooth spherical nano-scaled particles.
As is shown in Figure 4, the first day release of the three peptides had a greater release percentage ranging from 8% to 10%. Then, over a period of 11 days, all peptides were released at a rate of approximately 6% per day. In accordance with earlier studies, the C7-3 peptide and its derivatives were gradually released from NPs in a continuous sustained-release manner, and the release kinetics were comparable to that observed in our study.18,24 The kinetics of antimicrobial agent release via delivery devices is an important concern. Quick-releasing kinetics may deliver a relatively high initial dose, causing cytotoxicity and/or a short duration of action. Slow-releasing kinetics, on the other hand, may not ensure the required therapeutic level of the antimicrobial drug, increasing the risk of resistant cell adaptation. As a result, an “ideal” antibacterial delivery system should have a long duration of drug release, while remaining within the therapeutic effective dose zone.18,25,26
SDS-PAGE assay confirmed the integrity of the C7-3 peptide and its derivatives after CNP loading. These findings are consistent with prior research in which the SDS-PAGE test was utilized to guarantee the integrity of the bFGF peptide, which was maintained during CNPs synthesis and in vitro release assessment.27
The stability assessment in our study suggested the storage of the prepared NPs at 4°C for further investigation. There was no significant difference in particle size, PDI, or zeta potential of CNPs when stored at 4°C for different time intervals (Figure 5). On the other hand, holding NPs at −30°C and −80°C resulted in a significant increase in particle size as the storage intervals increased. Despite the fact that storage at −30°C and −80°C kept the zeta potential value constant, the particle size and PDI of NPs rose dramatically as storage intervals increased, possibly due to NP agglomeration. A previous study demonstrated that storing of CNPs at −30°C causes a significant increase in PS and a fluctuation in PDI and ZP values.28
Following the CLSI guidelines, a susceptibility evaluation was used in our study to distinguish between the susceptible standard strain and the resistant strain (Table 3). Encapsulation of the C7-3 peptide and its derivatives within CNPs resulted in an increase in antibacterial activity with a significant decrease in CFU quantity in our study. The results showed that the MIC50 was reduced by 67, 82, and 67% for the standard strain and by 60, 70, and 50% for the resistant strain. The enhanced anti-gonococcal activity of the C7-3 peptide appears to be based on the interaction of chitosan with microbial membranes. Furthermore, CNPs may transfer the encapsulated peptide directly to the bacterium, avoiding peptide inactivation. The electrostatic contact of CNPs with the bacterial membrane may allow the peptide to penetrate more quickly to the bacterial cellular components, where it can subsequently exhibit antimicrobial activity by inhibiting AniA enzyme, which is essential for gonococcal anaerobic survival and biofilm formation.
Improving the antimicrobial activity of peptides using encapsulation within CNPs has been reported by other researchers.18,29–31 Piras et al revealed that incorporating LLPIVGNLLKSLL-amide (called temporin B (TB)) peptide into CNPs increased the bactericidal activity of TB against Staphylococcus epidermidis strains and decreased their cytotoxicity.18 Another study showed loading RBRBR peptide in CNPs resulted in an improvement in the antimicrobial activity against S. aureus and reduced the cytotoxicity and hemolysis effect.29 CNPs loaded with protamine, which is a natural cationic antimicrobial peptide, exert enhanced antibacterial properties against E. coli.32 Furthermore, higher antibacterial and antifungal activity against A. baumannii and C. albicans was observed when Octominin peptide was loaded into CNPs, as revealed by time–kill kinetic and microbial viability assays.30
C7-3 peptide and its derivatives, as previously stated, inhibit the AniA enzyme, which is directly associated with pathogenic anaerobic growth and biofilm production.13 As a result, blocking anaerobic respiration reduced the pathogen’s ability to conduct the biofilm development process. The difference between peptide alone and peptide-loaded CNPs was evaluated in our study to determine whether the encapsulation process of peptides in NPs impacts their influence on pathogen biofilm inhibition. When compared with the control biofilm generation, both peptides alone in all derivatives and the loaded formulations reduced biofilm formation. In our investigation, we demonstrated that ECNPs can minimize biofilm formation in both standard and resistant strains. Peptide-loaded CNPs were shown to have a higher inhibitory percentage against both gonococcal strains than peptide alone. As chitosan has previously been found to disrupt biofilm formation and maturation, such enhancement in peptide-loaded CNPs in terms of biofilm inhibition in standard and resistant strains could be driven by CNPs.33,34
The cytotoxicity of ECNPs and peptide-loaded CNPs on HeLa cells was examined in this study for 24 and 48 hours. Importantly, after 24 hours, all ECNPs and three peptide-loaded CNPs did not impair cell viability in HeLa cells at doses of up to 2.2 mM/mL, with the exception of C7-3m2 CNPs, which significantly reduced HeLa cell viability at 2.2 mM/mL. Furthermore, after 48 hours, all NPs had no effect on HeLa cell viability except for 2.2 mM/mL C7-3 CNPs and C7-3m2 CNPs, which significantly lowered HeLa cell viability (Figure 12). It is noteworthy that all produced NPs were cytocompatible at their MIC50, revealing their cytocompatibility at these doses. CNPs cytotoxicity in HELA cells has recently been examined. Only high dosages of CNPs (5 and 2.5 mg/mL) were shown to be cytotoxic. CNP cytocompatibility was demonstrated as ECNPs generated from 1 mg/mL Cs solution had no significant effect on HeLa cell viability. The findings of this study regarding the non-cytotoxic effect of ECNPs generated from Cs concentrations of 1 mg/mL were consistent with the findings of a previous study.2
Conclusion
Antibiotic-resistant bacteria, including Neisseria gonorrhoeae, necessitate the development of new strategies, for example, the use of anti-virulence peptides such as C7-3 peptide and its derivatives, for combating infection without developing resistance. In this investigation, cytocompatible CNPs were utilized for encapsulating C7-3, C7-3m1, and C7-3m2 peptides. All developed formulations were optimized in terms of particle size, PDI, surface charge, EE %, and continuous, sustained, in vitro release. The CNP-loaded peptides enhanced the anti-gonococcal activity and anti-virulence effect of C7-3 peptide against standard and resistant strains. The findings of this study suggest the use of chitosan-based NPs as a potential carrier for antimicrobial C7-3 peptide and its derivatives, improving its antibacterial and anti-virulence properties. The formulated peptide-loaded CNP in this study represents a promising approach to combating gonococcal infections, particularly in the era of increasing antimicrobial resistance.
Acknowledgments
This research project was supported by a grant from the “Research Centre of the Female Scientific and Medical Colleges”, Deanship of Scientific Research, King Saud University.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research project was supported by a grant from the “Research Centre of the Female Scientific and Medical Colleges”, Deanship of Scientific Research, King Saud University.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Jefferson A, Smith A, Fasinu PS, Thompson DK. Sexually transmitted Neisseria gonorrhoeae infections-update on drug treatment and vaccine development. Medicines. 2021;8(2):11. doi:10.3390/medicines8020011
2. Alqahtani F, Aleanizy F, El Tahir E, et al. Antibacterial activity of chitosan nanoparticles against pathogenic n. gonorrhoea. Int J Nanomed. 2020;15:7877–7887. doi:10.2147/IJN.S272736
3. Suay-Garcia B, Perez-Gracia MT. Future prospects for Neisseria gonorrhoeae treatment. Antibiotics. 2018;7(2):49. doi:10.3390/antibiotics7020049
4. Lim KYL, Mullally CA, Haese EC, et al. Anti-virulence therapeutic approaches for Neisseria gonorrhoeae. Antibiotics. 2021;10(2):103. doi:10.3390/antibiotics10020103
5. Saravanan M, Belete MA, Niguse S, et al. Antimicrobial resistance and antimicrobial nanomaterials: an overview; 2021.
6. Micoli F, Bagnoli F, Rappuoli R, Serruto D. The role of vaccines in combatting antimicrobial resistance. Nat Rev Microbiol. 2021;19(5):287–302. doi:10.1038/s41579-020-00506-3
7. Goire N, Lahra MM, Chen M, et al. Molecular approaches to enhance surveillance of gonococcal antimicrobial resistance. Nat Rev Microbiol. 2014;12(3):223–229. doi:10.1038/nrmicro3217
8. World Health Organization. WHO Guidelines for the Treatment of Neisseria Gonorrhoeae. World Health Organization; 2016.
9. Nelson RE, Hatfield KM, Wolford H, et al. National estimates of healthcare costs associated with multidrug-resistant bacterial infections among hospitalized patients in the United States. Clin Infect Dis. 2021;72(Supplement_1):S17–S26. doi:10.1093/cid/ciaa1581
10. Isabella VM, Clark VL. Deep sequencing-based analysis of the anaerobic stimulon in Neisseria gonorrhoeae. BMC Genomics. 2011;12(1):51. doi:10.1186/1471-2164-12-51
11. Falsetta ML, Bair TB, Ku SC, et al. Transcriptional profiling identifies the metabolic phenotype of gonococcal biofilms. Infect Immun. 2009;77(9):3522–3532. doi:10.1128/IAI.00036-09
12. Clark VL, Knapp JS, Thompson S, Klimpel KW. Presence of antibodies to the major anaerobically induced gonococcal outer membrane protein in sera from patients with gonococcal infections. Microb Pathog. 1988;5(5):381–390. doi:10.1016/0882-4010(88)90038-1
13. Sikora AE, Mills RH, Weber JV, et al. Peptide inhibitors targeting the Neisseria gonorrhoeae pivotal anaerobic respiration factor AniA. Antimicrob Agents Chemother. 2017;61. doi:10.1128/AAC.00186-17
14. Guzman CA, Feuerstein GZ. Pharmaceutical biotechnology. Curr Opinion Biotechnol. 2004;15(6):503–505. doi:10.1016/j.copbio.2004.10.009
15. Sosnik A. Reversal of multidrug resistance by the inhibition of ATP-binding cassette pumps employing “Generally Recognized As Safe” (GRAS) nanopharmaceuticals: a review. Adv Drug Deliv Rev. 2013;65(13–14):1828–1851. doi:10.1016/j.addr.2013.09.002
16. Yu H, Ma Z, Meng S, et al. A novel nanohybrid antimicrobial based on chitosan nanoparticles and antimicrobial peptide microcin J25 with low toxicity. Carbohydr Polym. 2021;253:117309. doi:10.1016/j.carbpol.2020.117309
17. Wong CY, Al-Salami H, Dass CR. The role of chitosan on oral delivery of peptide-loaded nanoparticle formulation. J Drug Target. 2018;26(7):551–562. doi:10.1080/1061186X.2017.1400552
18. Piras AM, Maisetta G, Sandreschi S, et al. Chitosan nanoparticles loaded with the antimicrobial peptide temporin B exert a long-term antibacterial activity in vitro against clinical isolates of Staphylococcus epidermidis. Front Microbiol. 2015;6:372. doi:10.3389/fmicb.2015.00372
19. Melvin M, Weinstein P. Performance Standards for Antimicrobial Disk Susceptibility Tests. Vol. 38. CLSI; 2018:92.
20. Spence JM, Wright L, Clark VL. Laboratory maintenance of Neisseria gonorrhoeae. Curr Protocols Microbiol. 2008;8(1). doi:10.1002/9780471729259.mc04a01s8
21. Melvin M, Weinstein P. Performance Standards for Antimicrobial Disk Susceptibility Tests. Vol. 35.
22. Mao S, Bakowsky U, Jintapattanakit A, Kissel T. Self-assembled polyelectrolyte nanocomplexes between chitosan derivatives and insulin. J Pharm Sci. 2006;95(5):1035–1048. doi:10.1002/jps.20520
23. Gokce Y, Cengiz B, Yildiz N, Calimli A, Aktas Z. Ultrasonication of chitosan nanoparticle suspension: influence on particle size. Colloids Surf A. 2014;462:75–81. doi:10.1016/j.colsurfa.2014.08.028
24. Yadav P, Yadav AB. Preparation and characterization of BSA as a model protein loaded chitosan nanoparticles for the development of protein-/peptide-based drug delivery system. Future J Pharm Sci. 2021;7:200. doi:10.1186/s43094-021-00345-w
25. Hetrick EM, Schoenfisch MH. Reducing implant-related infections: active release strategies. Chem Soc Rev. 2006;35(9):780–789. doi:10.1039/b515219b
26. Wu P, Grainger DW. Drug/device combinations for local drug therapies and infection prophylaxis. Biomaterials. 2006;27(11):2450–2467. doi:10.1016/j.biomaterials.2005.11.031
27. Cetin M, Aktas Y, Vural I, et al. Preparation and in vitro evaluation of bFGF-loaded chitosan nanoparticles. Drug Deliv. 2007;14(8):525–529. doi:10.1080/10717540701606483
28. Alqahtani FY, Aleanizy FS, El Tahir E, et al. Capsule independent antimicrobial activity induced by nanochitosan against Streptococcus pneumoniae. Polymers. 2021;13(17):2924. doi:10.3390/polym13172924
29. Almaaytah A, Mohammed GK, Abualhaijaa A, Al-Balas Q. Development of novel ultrashort antimicrobial peptide nanoparticles with potent antimicrobial and antibiofilm activities against multidrug-resistant bacteria. Drug Design Dev Ther. 2017;11:3159–3170. doi:10.2147/DDDT.S147450
30. Jayathilaka EHTT, Nikapitiya C, De Zoysa M, Whang I. Antimicrobial peptide octominin-encapsulated chitosan nanoparticles enhanced antifungal and antibacterial activities. Int J Mol Sci. 2022;23(24):15882. doi:10.3390/ijms232415882
31. Primo L, Roque-Borda CA, Carnero Canales CS, et al. Antimicrobial peptides grafted onto the surface of N-acetylcysteine-chitosan nanoparticles can revitalize drugs against clinical isolates of Mycobacterium tuberculosis. Carbohydr Polym. 2024;323:121449. doi:10.1016/j.carbpol.2023.121449
32. Tamara FR, Lin C, Mi FL, Ho YC. Antibacterial effects of chitosan/cationic peptide nanoparticles. Nanomaterials. 2018;8(2):88. doi:10.3390/nano8020088
33. Carlson RP, Taffs R, Davison WM, Stewart PS. Anti-biofilm properties of chitosan-coated surfaces. J Biomater Sci Polym Ed. 2008;19(8):1035–1046. doi:10.1163/156856208784909372
34. Khan F, Pham DTN, Oloketuyi SF, Manivasagan P, Oh J, Kim Y-M. Chitosan and their derivatives: antibiofilm drugs against pathogenic bacteria. Colloids Surf B. 2020;185:110627. doi:10.1016/j.colsurfb.2019.110627
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.