Back to Journals » International Journal of Women's Health » Volume 14
Effect of Aerobic Exercises in Improving Premenstrual Symptoms Among Healthy Women: A Systematic Review of Randomized Controlled Trials
Authors Ravichandran H , Janakiraman B
Received 28 April 2022
Accepted for publication 7 July 2022
Published 16 August 2022 Volume 2022:14 Pages 1105—1114
DOI https://doi.org/10.2147/IJWH.S371193
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Elie Al-Chaer
Hariharasudhan Ravichandran, Balamurugan Janakiraman
Department of Physiotherapy, School of Medicine, College of Health Sciences and Ayder Comprehensive Specialized Hospital, Mekelle University, Mekelle, Tigray, Ethiopia
Correspondence: Hariharasudhan Ravichandran, Department of Physiotherapy, School of Medicine, College of Health Sciences and Ayder Comprehensive Specialized Hospital, Mekelle University, Mekelle, Tigray, Ethiopia, Email [email protected]
Background: Premenstrual symptoms in women of reproductive age are associated with substantial distress and functional impairments. A healthy lifestyle is the first step to manage premenstrual symptoms. Recreational physical activities have been recommended as an alternative to medical management in easing premenstrual symptoms.
Objective: The objective of this systematic review is to analyze the effects of aerobic exercises in improving premenstrual symptoms among healthy women.
Methods: Randomized controlled trials (RCTs) published from inception to February 2022, were searched using keywords in electronic databases such as, SCOPUS, PubMed, PEDro, Cochrane and web of science. RCTs published in English, comparing the effects of aerobic exercise with other interventions or controls were included. PEDro scale and Cochrane collaboration tool for risk of bias was used to assess the methodological quality of included trials. Data from the included study and the participant’s characteristics, interventions, outcome and results were extracted.
Results: Five RCTs with 492 participants were included in this systematic review. Methodological quality assessed by PEDro (4.8/10) and Cochrane collaboration tool for risk of bias were moderate. Allocation concealment, blinding of participants and outcome assessors were the most common bias in all included studies. Walking, swimming and running were the common aerobic exercises performed in the RCTs. Aerobic exercise is effective in improving physical physiological symptoms among women with premenstrual syndrome (PMS).
Conclusion: Aerobic exercises are effective in improving premenstrual symptoms. This review provides moderate evidence for improving hematological parameters during PMS. Further RCTs with long term follow up and quality of life would consolidate our findings.
Keywords: premenstrual syndrome, premenstrual tension, aerobic exercise, endurance training, PMS
Introduction
Women of all ages experience unique health needs, and health systems around the world are failing in addressing them. At present the global efforts to improve women’s health largely focuses on reproductive health.1 During the period of fertility, women are mostly concerned about the premenstrual symptoms that occur few days before menstruation. Premenstrual syndrome (PMS) encompasses clinically significant somatic and psychological manifestations during the luteal phase of menstrual cycle.2 The exact mechanism causing PMS still remains unclear. However, it is proposed that PMS is associated with abnormal sensitivity and excessive response to normal hormonal changes.3 PMS occurs one week prior to the onset of menstruation in women of reproductive ages, declines with the onset of menstruation, and presents a set of physical and psychological symptoms, and the severity of which vary from one women to another.4 Various researchers have found that diverse body systems such as cardiovascular, central nervous system, endocrine, and female reproductive system are associated with the symptoms of PMS, related to emotional, physical, cognitive, and behavioral aspects.5,6 Epidemiological data shows that the symptoms are often mild in 75% of the women, but 3–8% reported to experience moderate to severe symptoms that are associated with substantial distress and functional impairment.7 Premenstrual symptoms often interferes with occupational productivity, quality of life, interpersonal relationships and daily living activities and also it is considered as an limiting factor for female adolescents and young women aspiring to achieve developmental goals. Studies have also shown that adolescents with PMS are in poor health8 and in some women it is so bad that they are unable to cope up with their daily lifestyle with PMS.9
The morbidity associated with PMS is considerable and often poorly managed. The aims of interventions prescribed for PMS helps to reduce symptoms and adverse effects, thereby promoting quality of life in women with PMS. Management of PMS includes, exercises, diet modifications, stress management, cognitive behavioral therapy, and drugs (based on the symptoms). According to the American College of Obstetricians and Gynecologists (ACOG) practice bulletin,10 non-pharmacologic therapy is recommended as foremost treatment for all females with PMS. Physical activities are considered as a beneficial alternative to drugs in the management of premenstrual symptoms and it has been associated with improving wellness during the episodes of PMS.8,11 Physical activities could potentially improve hormonal profile, reproductive function, menstrual cyclicity, ovulation and fertility among women of all ages.12 Evidences in the literature has reported improvement in hematological parameters and reduction the levels of prolactin, estradiol, and progesterone with exercises during PMS13 and few other studies12 support exercises to improve self-esteem, depression and anxiety. Few authors analyzed the effect of exercise during PMS and reported their findings inconsistently. Few cross sectional studies14 and RCTs suggest that exercises are effective in reducing global premenstrual distress symptoms and in contrast other studies15,16 reported higher levels of premenstrual symptoms among exercising women compared to those who exercise less or not at all.
Existing reviews in the literature recommending exercises during PMS are performed with inclusion of all physical exercises such as yoga, pilates, strength and conditioning. Despite the fact that exercises are beneficial in PMS, there is a debate remains unclear regarding the type and dose of therapeutic exercises that is recommended for females with PMS. There is no specific reviews are available to identify the impact of aerobic exercise among females with PMS. Therefore the aim of this systematic review is to identify whether aerobic exercise is beneficial in improving premenstrual symptoms in women of reproductive age.
Methods
Two authors were involved in this systematic review has been conducted according to the guidelines of Preferred Reporting Items for Systematic reviews and Meta-analysis (PRISMA).17
Eligibility criteria for this review are set, according to the Patient Intervention Comparison Outcome model:18
- Population: Women of reproductive age with regular menstrual cycle and diagnosed with PMS according to diagnostic criteria for PMS mentioned by ACOG guidelines for women’s health care.
- One of the following affective symptom (angry outbursts, anxiety, confusion, depression, irritability, social withdrawal) and somatic symptoms (abdominal bloating, breast tenderness, headache, joint or muscle pain, weight gain) during the 5 days before menses in each of the three previous menstrual cycle and relief from these symptoms within 4 days of the onset of menses, without recurrence until at 13 of the cycle and must be present in the absence of any pharmacologic therapy, hormone ingestion, or drug or alcohol use.19
- Intervention: Aerobic exercises performed in moderate intensity
- Comparison: Any other interventions or control
- Outcome: Somatic and affective symptoms
Additionally randomized controlled trials (RCTs) published in English language were included. Quasi-experimental studies or cross-sectional studies and RCTs with female athletes were excluded.
Databases such as SCOPUS, PubMed, PEDro, Cochrane and web of science were searched for articles published up to 29 February 2022 using specific keywords. Keywords such as aerobic exercise or endurance exercise or swimming or walking or jogging or running or physical activity or physical exercise or exercise and PMS or premenstrual symptom or premenstrual tension and RCT or comparative study were used (Table 1). After the initial search, both the authors were involved together to identify the duplicate articles. Authors (BJ and HR) independently screened titles and abstracts and retrieved full texts for relevant articles. Full texts have been screened for inclusion criteria. Eligible RCTs are identified and included in this review. Discrepancies during the study search strategy process was resolved through discussion with an expert (performed double checking of the search strategy process).
 |
Table 1 Search Strategy |
Two authors (BJ and HR) independently assessed the methodological quality of the included studies using PEDro scale.20 The minimum possible score is 0 and the maximum possible score is 10. Based on the score, the quality of the study was classified as excellent (9–10 points), good quality (6–8 points), fair quality (4–5 points) and poor quality (less than 4 points). Additionally, Cochrane collaboration tool for risk of bias was assessed using Review manager 5 software. Disagreements in PEDro assessment and Risk of bias were resolved by discussion with an expert who was not concerned with the included studies. (Reviews the PEDro assessment and risk of bias performed by the authors individually and a consensus is achieved through discussion with the expert’s opinion).
Data extraction from the included RCTs was performed independently by the authors (HRS and HR). Study characteristics (Authors name, year, country), population characteristics (sample size, mean age), intervention (frequency, intensity, type, duration) and outcome were extracted from included studies.
Result
PRISMA diagram in Figure 1 illustrates the study search process. Initial search of databases resulted in 338 articles. After removing duplicates and screening abstracts and titles, 13 articles with full texts were retrieved. Five articles published in Persian languages and 3 quasi experimental design studies were removed. Totally, 5 RCTs20–25 were included in this systematic review. Performing a meta-analysis was not appropriate because of the heterogeneity in outcomes used among included RCTs.
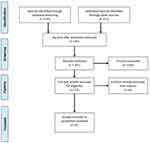 |
Figure 1 PRISMA flow diagram illustrating selection process. |
In this review the methodological quality of the studies included was 4.8 out of 10 in PEDro scale, indicating fair or moderate in quality. Allocation concealment practice was not mentioned in most of the studies and blinding of participants, therapists and assessor were not performed in the included studies. Additionally, further follow up assessment was not performed in any of the RCTs included in this review. All the individual RCTs scored between 4 and 5 in PEDro scale, and hence classified as moderate in methodological quality (Table 2). Cohen’s Kappa statistics for inter-rater reliability was 0.82.
 |
Table 2 PEDro Scale Score of Included Studies |
Risk of bias assessed with Cochrane collaboration tool was moderate among the included articles. High risk was demonstrated for allocation concealment and blinding procedures (Figure 2). Randomization method was not mentioned in two of the included RCTs (Figure 3). All the included RCTs demonstrated moderate risk of bias. Kappa statistics for inter-rater reliability was 0.84.
 |
Figure 2 Risk of bias graph. |
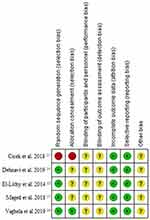 |
Figure 3 Risk of bias of included studies. |
Five RCTs included in this review involved a total of 492 participants. The study characteristics were presented in Table 3. All these RCTs are performed in Asia and Africa. Sample size of participants in these RCTs varied between 30 and 220. The mean age of women included in the RCTs ranges between 17.80 and 28.35 representing the reproductive age group. RCTs compared aerobic exercises with yoga or vitamin supplements or with control groups. Aerobic activities such as walking, swimming and running were performed in moderate training intensity, for about 30 minutes per session, 3 to 5 sessions per week. The mean duration of aerobic exercise provided in all RCTs was 10.4 weeks. During the course of the study 19 participants from the intervention group and 16 from the control group were excluded in two RCTs for not following the study protocol and hence 457 were the actual participants who completed the trials.
 |
Table 3 Study Characteristics |
Regarding physical outcome measures, aerobic exercise interventions resulted in significant improvement of physical symptoms such as headache (P = 0.001), nausea, constipation diarrhea (P = 0.01), abdominal bloating (P = 0.002), flushing (P = 0.04), increase in appetite (P = 0.008), hyperhydration (P=0.001), menstrual cramps (P=0.001) and backache (P=0.002). In one study23 aerobic exercises demonstrated significant effects in reducing psychological symptoms such as anxiety (P=0.001), craving (P =0.002), depression (P= 0.001). Another study22 using hematological parameter as outcome has reported that there is significant improvement in hemoglobin, hematocrit, red cell and platelet counts following 12 weeks of aerobic exercise. Contradictory to these findings, two RCTs21,24 have reported non-significant effects in physical psychological symptoms of aerobic exercise compared to yoga exercises and control group. None of the studies reported on adverse effects during or after the interventions.
Discussion
This systematic review demonstrates the effects of aerobic exercise for women with premenstrual symptoms. Based on the findings of this review, 30 min of aerobic exercise, 3 to 5 times a week could be effective in reducing physical and psychological symptoms of PMS among women. Statistical results of studies included demonstrate that aerobic exercises are more effective in improving physiological than psychological symptoms for women with PMS. Meta-analysis is not performed in this review, as there are limited RCT’s available in the literature. Qualitative findings of this review is based on the moderate level of methodological quality of included RCTs.
All the 5 RCTs reporting the effects of aerobic exercise among females with PMS varied in their outcome findings. Three RCTs stated that aerobic exercise is effective in improving physiological symptoms, psychological well-being and hematological parameters in PMS. The findings of this systematic review is found to be similar to the findings of a Yilmaz-Akyuz et al,26 reporting reduction in PMS related symptoms in female students following diet modifications and participation aerobic exercise program. Unlikely, their review analysis included any type of physical activities such as strength training, relaxation and aerobic activities performed in non-randomized trials and preliminary reports. Moreover, their review has not included any high level of evidence such as RCTs to interpret their findings.
Aerobic exercises performed in the included studies were not effective in relieving all the physical symptoms of PMS. However, these exercises are effective for headache, nausea, bowel disturbances, abdominal bloating, flushing, appetite, hyper-hydration, menstrual cramps and backache that occur during PMS. Generally physical symptoms of PMS appear with ovulation and disappear during suppression of ovarian cycle. In females with PMS, the physical and psychological symptoms are often associated with fluctuations in the level of estrogen, progesterone and serotonin levels. Reports suggest that there exists an imbalance between the ovarian hormones estrogen and progesterone.27 Estrogen levels are found to be increased during the premenstrual phase, with insufficient levels of progesterone to counteract it.28 Probably, estrogen levels could be reduced by performing aerobic exercises. To agree with this, a study by Razzak et al29 reported that prescription of 150 minutes of moderate to vigorous aerobic exercise per week for 16 weeks resulted in significant changes in estrogen metabolism. Findings from Wint et al and Hackney et al support the fact that aerobic exercise decreases estrogen hormone level. The possible mechanism could be due to the potential of aerobic exercise to increase sex hormone binding globulin, a blood borne protein that binds the circulating free estrogen in blood.
Previous studies30,31 reported that, beta-endorphin levels are diminished during the premenstrual phase and contributes to symptoms such as headache, menstrual cramps, backache, anxiety, craving and depression. In this review it is demonstrated that aerobic exercise is effective for premenstrual pain and psychological symptoms. It is reported that there is decrease in plasma beta endorphin which is associated with PMS symptoms.14 Several studies suggest that aerobic exercise of moderate intensity has demonstrate increase in circulating beta endorphins.32 This could be the possible mechanism that is responsible for reduction in pain related symptoms among females with PMS.
Physical and psychological stress during PMS is associated with altered cortisol awakening response.33 Cortisol plays a major role in completing healthy stress-response circuit in PMS. Performing aerobic exercise at an intensity of at least 60% of maximum capacity of oxygen uptake leads to elevated levels of cortisol for at least 2 hours after exercise. Thus cortisol inhibits hypothalamus and pituitary glands and restores homeostasis for healthy stress response.34 Similarly in this review, aerobic exercise is performed at moderate intensity in all included studies, which is attributed to significant effects of aerobic exercise in reduction of stress related symptoms such as anger, mood swing, anxiety, sadness, depression, craving, fatigue and so on.
Contradictorily, two studies21,24 included in this review found non-significant effects of aerobic exercise in PMS. In one of this study, the intervention duration is 4 weeks and the menstrual cyclicity analyzed could be inadequate. In another study, we assume that the cyclical nature of PMS could have affected their study findings.
Strength of This Review
To the best of our knowledge, this is the first systematic review conducted to analyze the effects of aerobic exercise by including the evidence from RCTs. This systematic review on aerobic exercise adds evidence on non-pharmacological management for PMS. We have found no unpublished trials or thesis works related to this review.
Limitations
Few RCTs identified through google scholar, published in non-indexed domestic journals and suspicious to be predatory were excluded. We pre-determined our criteria to include articles published only in English as a language which could have limited our search to include articles published in other languages without English version. Common flaws of reviews such as limited number of RCTs in aerobic exercise could have limited our findings of this review. Long term effects or retention effects of aerobic exercise in PMS could not be determined as because, none of the included RCTs performed follow up analysis. Variance in outcomes among the included RCTs restricted to conduct meta-analysis on effects of aerobic exercise.
Conclusions
In conclusion, it is stated that performing 30 minutes of aerobic exercise, 3–5 sessions per week for about 10 weeks, has potential benefits in improving premenstrual symptoms of women in reproductive age. It is also suggested that women with PMS could benefit from aerobic type of physical activity in managing their physical and psychological symptoms. Moreover, further studies are required to consolidate our findings and to demonstrate the retention effects of aerobic exercise.
Acknowledgment
We sincerely thank the College of Medicine and Health Sciences, of the Mekelle University for providing us the support, encouragement and for the online library access, and library services during this review process. We also thank Alvas Education Foundation, Alvas Health Center and Alvas College of Physiotherapy, Dakshina Karnataka, India for their support in expert opinions.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Peters SAE, Woodward M, Jha V, Kennedy S, Norton R. Women’s Health: a new global agenda. BMJ Glob Health. 2016;1(3):e000080. doi:10.1136/bmjgh-2016-000080
2. Uzuncakmak T, Alkaya SA. Effect of aromatherapy on coping with premenstrual syndrome: a randomized controlled trial. Complement Ther Med. 2018;36:63–67. doi:10.1016/j.ctim.2017.11.022
3. Gudipally PR, Sharma GK. Premenstrual syndrome 2021. StatPearls Publishing; 2022: 32809533.
4. Labots_vogelesang MS, Teunissen DAM, Kranenburg V, Lagro-Janssen ALM. Views of Dutch general practitioners about premenstrual symptoms: a qualitative interview study. Eur J Gen Pract. 2021;27(1):19–26. doi:10.1080/13814788.2021.1889505
5. Gao M, Gao D, Sun H, Cheng X, An L, Qiao M. Trends in research related to premenstrual syndrome and premenstrual dysphoric disorder from 1945 to 2018: a bibliometric analysis. Front Public Health. 2021;21(9):596128. doi:10.3389/fpubh.2021.596128
6. Wu M, Liang Y, Wang Q, Zhao Y, Zhou R. Emotion dysregulation of women with premenstrual syndrome. Sci Rep. 2016;6(1):38501. doi:10.1038/srep38501
7. Abu Alwafa R, Badrasawi M, Haj Hamad R. Prevalence of premenstrual syndrome and its association with psychosocial and lifestyle variables: a cross sectional study from Palestine. BMC Womens Health. 2021;21(1):233. doi:10.1186/s12905-021-01374-6
8. Mostafa R, Sabzevary MT, Dehnavi ZM. Factors associated with premenstrual syndrome in female high school students. J Edu Health Promot. 2018;3(7):64.
9. Yoshimi K, Shiina M, Takeda T. Lifestyle factors associated with premenstrual syndrome: a cross-sectional study of Japanese high school students. J Pediatr Adolesc Gynecol. 2019;32(6):590–595. doi:10.1016/j.jpag.2019.09.001
10. Hofmeister S, Bodden S. Premenstrual syndrome and premenstrual dysphoric disorder. Am Fam Physician. 2016;94(3):236–240.
11. Bhuvaneswari K, Rabindran P, Bharadwaj B. Prevalence of premenstrual syndrome and its impact on quality of life among selected college students in Puducherry. Natl Med J India. 2019;32(1):17–19. doi:10.4103/0970-258X.272109
12. Orio F, Muscogiuri G, Ascione A, et al. Effects of physical exercise on the female reproductive system. Minerva Endocrinol. 2013;38(3):305–319. PMID: 24126551.
13. Farrokh-Eslamlou H, Oshnouei S, Heshmatian B, Akbari E. Premenstrual syndrome and quality of life in Iranian medical students. Sex Reprod Healthc. 2015;6(1):23–27. doi:10.1016/j.srhc.2014.06.009
14. Kroll-Desrosiers AR, Ronnenberg AG, Zagarins SE, Houghton SC, Takashima-Uebelhoer BB, Bertone-Johnson ER. Recreational physical activity and premenstrual syndrome in young adult women: a cross sectional study. PLoS One. 2017;12(1):e0169728. PMID: 28081191. doi:10.1371/journal.pone.0169728
15. Deuster P, Adera T, South-Paul J. biological, social and behavioral factors associated with premenstrual syndrome. Arch Fam Med. 1999;8(2):122–128. doi:10.1001/archfami.8.2.122
16. Rasheed P, Al-Sowielem L. Prevalence and predictors of premenstrual syndrome among college-aged women in Saudi Arabia. Ann Saudi Med. 2003;23(6):381–387. doi:10.5144/0256-4947.2003.381
17. Moher D, Shamseer L, Clarke M, et al.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. doi:10.1186/2046-4053-4-1
18. Eriksen MB, Frandsen TF. The impact of patient, intervention, comparison, outcome (PICO) as a search strategy tool on literature search quality: a systematic review. J Med Libr Assoc. 2018;106(4):420–431. doi:10.5195/jmla.2018.345
19. American College of Obstetricians and Gynecologists. Guidelines for Women’s Health Care: A Resource Manual.
20. de Morton NA. The PEDro scale is a valid measure of the methodological quality of clinical trials: a demographic study. Aust J Physiother. 2009;55(2):129–133. doi:10.1016/S0004-9514(09)70043-1
21. Cicek G. The effect of regular aerobic exercises on premenstrual syndrome in sedentary women. Balt J Health Phys Act. 2018;10(2):34–42. doi:10.29359/BJHPA.10.2.04
22. El-Lithy A, El-Mazny A, Sabbour A, El-Deeb A. effect of aerobic exercise on premenstrual symptoms, haematological and hormonal parameters in young women. J Obstet Gynaecol. 2014;35(4):389–392. doi:10.3109/01443615.2014.960823
23. Maged AM, Abbassy AH, Sakr HRS, et al. Effect of swimming exercise on premenstrual syndrome. Arch Gynecol Obstet. 2018;297(4):951–959. doi:10.1007/s00404-018-4664-1
24. Vaghela N, Mishra D, Sheth M, Dani VB. To compare the effects of aerobic exercise and yoga on premenstrual syndrome. J Edu Health Promot. 2019;8:199.
25. Dehnavi ZM, Jafarnejad F, Goghary SS. The effect of 8 weeks aerobic exercise on severity of physical symptoms of premenstrual syndrome: a clinical trial study. BMC Womens Health. 2018;18(1):80. doi:10.1186/s12905-018-0565-5
26. Yilmaz-Akyuz E, Aydin-Kartal A. the effect of diet and aerobic exercise on Premenstrual Syndrome: randomized controlled trial. Rev Nutr. 2019;32:e18024. doi:10.1590/1678-9865201932e180246
27. Charkoudian N, Joyner MJ. Physiologic considerations for exercise performance in women. Clin Chest Med. 2004;25(2):247–255. doi:10.1016/j.ccm.2004.01.001
28. Noviyanti NI, Gusriani R, Mappaware NA, Ahmad M. The effect of estrogen hormone on premenstrual syndrome (PMS) occurrences in teenage girls at Pesantren Darul Arqam Makassar. Gac Sanit. 2021;35(2):S571–S575. doi:10.1016/j.gaceta.2021.10.103
29. Razzak ZA, Khan AA, Farooqui SI. Effect of aerobic and anaerobic exercise on estrogen level, fat mass, and muscle mass among postmenopausal osteoporotic females. Int J Health Sci. 2019;13(4):10–16.
30. Straneva PA, Maixner W, Light KC, Pedersen CA, Costello NL, Girdler SS. Menstrual cycle, beta-endorphins, and pain sensitivity in premenstrual dysphoric disorder. Health Psychol. 2002;21(4):358–367. doi:10.1037/0278-6133.21.4.358
31. Bertone-Johnson ER, Ronnenberg AG, Houghton SC, et al. Association of inflammation markers with menstrual symptom severity and premenstrual syndrome in young women. Hum Reprod. 2014;29(9):1987–1994. doi:10.1093/humrep/deu170
32. Pilozzi A, Carro C, Huang X. Roles of β - endorphin in stress, behavior, neuroinflammation, and brain energy metabolism. Int J Mol Sci. 2021;22(1):338. doi:10.3390/ijms22010338
33. Heijnen S, Hommel B, Kibele A, Colzato LA. Neuromodulation of aerobic exercise – a review. Front Psychol. 2015;6:1890. doi:10.3389/fpsyg.2015.01890
34. Hou L, Huang Y, Zhou R. Premenstrual syndrome is associated with altered cortisol awakening response. Stress. 2019;22(6):640–646. doi:10.1080/10253890.2019.1608943
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
