Back to Journals » Journal of Pain Research » Volume 16
Effect of a Local Anesthetic Injection Kit on Pain Relief and Postoperative Recovery After Transumbilical Single-Incision Laparoscopic Cholecystectomy
Authors Yang N , Tao QY , Niu JY , Sun H, He Y , Hou YB , Luo H, Zhang Z, Yu JM
Received 14 June 2023
Accepted for publication 7 August 2023
Published 11 August 2023 Volume 2023:16 Pages 2791—2801
DOI https://doi.org/10.2147/JPR.S422454
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jinlei Li
Na Yang,1,* Qing-Yu Tao,1,* Jing-Yi Niu,1 Hao Sun,1 Yan He,1,2 Yong-Bo Hou,1,2 Hong Luo,1 Zhi Zhang,3 Jun-Ma Yu1
1Department of Anesthesiology, the Third Affiliated Hospital of Anhui Medical University (The First People’s Hospital of Hefei), Hefei, Anhui, People’s Republic of China; 2Department of Anesthesiology, Wannan Medical College, Wuhu, Anhui, People’s Republic of China; 3Department of Biophysics and Neurobiology, University of Science and Technology of China, Hefei, Anhui, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zhi Zhang; Jun-Ma Yu, Email [email protected]; [email protected]
Purpose: This study was conducted to explore whether incisional infiltration using a local anesthetic injection kit could better relieve postoperative pain and enhance the quality of recovery compared with ultrasound-guided rectus sheath block (RSB) or conventional local anesthetic infiltration in patients undergoing transumbilical single-incision laparoscopic cholecystectomy (SILC).
Patients and Methods: A total of 60 patients undergoing SILC with American Society of Anesthesiology functional status scores of I-II were randomized into the rectus sheath block group (RSB group), conventional local wound infiltration group (LAI-I group) and incisional infiltration using a local anesthetic injection kit group (LAI-II group). The primary outcomes were the patient-controlled intravenous analgesia (PCIA) demand frequency within 48 hours after the operation and postoperative pain measured by a visual analog scale (VAS) at 2 h, 4 h, 8 h, 24 h, and 48 h after surgery. Secondary outcomes were the total procedure times, cumulative consumption of anesthetic drugs, duration of surgery, duration and awaking time of anesthesia, early recovery indicator and side effects.
Results: The PCIA demand frequency in LAI-II group was significantly lower compared with patients in the RSB and LAI-I group (both P < 0.001). Moreover, the total procedure times in LAI-I and LAI-II group was significantly shorter than that in the RSB group (P < 0.001, respectively), but it was comparable between LAI-I and LAI-II group (P = 0.471). Though lower at 2h and 4h postoperative in LAI-II group, pain scores at each time point had no statistical differences among three groups. There were no significant differences among three groups for other outcomes as well.
Conclusion: The effect of ultrasound-guided RSB and conventional local anesthetic infiltration in SILC patients were found to be similar in terms of relieving postoperative pain and promoting recovery. Incisional infiltration using a local anesthetic injection kit can significantly reduce the demand frequency of PCIA, which serves as a rescue analgesic.
Keywords: laparoscopic cholecystectomy, rectus sheath block, local infiltration analgesia, recovery, pain
Introduction
With changes in people’s lifestyles and eating habits, gallbladder disease is prevalent and multiport laparoscopic cholecystectomy (LC) is becoming more common in surgery; therefore, the further improvement of postoperative pain relief remains a vital issue to consider. Multiport LC has been developed into single-incision laparoscopic cholecystectomy (SILC), which aims to reduce the trauma and pain caused to patients by the procedure, and it has been recognized for its technical feasibility and safety;1–3 its significant cosmetic benefits are even more popular with doctors and patients. However, the outcomes of postoperative pain between SILC and multiport LC are inconsistent,4–6 and many studies have revealed that postoperative pain after SILC is similar to or even worse than that after multiport LC.7
Previous studies indicated that pain at the incision site dominated postoperative pain in multiport LC, and the umbilical incision was associated with the intensity of postoperative pain.8 The location of SILC incision is usually chosen at the umbilicus, and the wound diameter required for SILC is larger than that for multiport LC because it usually occurs through a skin incision at the single-access point.8 Additionally, studies have shown that postoperative incisional pain is directly proportional to the square of the incisional diameter; thus, the intensity of total pain across multiple tiny incisions is always less than that for an incision of the same total length.9 Furthermore, the intraoperative field of view was limited, and the surgeon operated with a large range to meet the demand in SILC, which increased edema and inflammation at the incision site, resulting in significant postoperative pain.7 Thus, patients undergoing SILC tended to have more incisional pain than those undergoing conventional LC.7 Pain prolongs the hospital stay, increases atelectasis and venous thrombosis, delays patients’ return to normal activities, and ultimately decreases patient satisfaction levels,10 so adequate relief of postoperative pain in SILC is essential.
Multiple studies recommended local infiltration at the trocar insertion sites as the primary option to alleviate multiport LC postoperative pain;11,12 that maneuver was simple to perform and demonstrated adequate pain control in the postoperative period, consistent with the conclusion of Wu et al.13 Conventional local wound infiltration technology requires blind exploration to achieve local infiltration at the first incision. Therefore, full-layer infiltration of local anesthetics at the first trocar site cannot be guaranteed, especially for SILC, to prevent accidental penetration of abdominal organs. For this, we assembled an injection kit from existing materials (Figure 1A and B).
 |
Figure 1 An injection kit composed of a disposable epidural syringe reflecting pressure (5 mL, (A-a and B-a)), a disposable triplet (5 mL, (A-b and B-b)), a disposable puncture needle for anesthesia (AN-SI, 0.7×106, (A-c and B-c)) and another disposable puncture needle for anesthesia (AN-E, 1.2×80, (A-d and B-d)) all from TUORen Medical Equipment Co., Henan, China (A and B). When the air bubble of the syringe (A-b and B-b) was shrunk during the kit was advancing, the puncture needle for anesthesia (AN-SI, 0.7×106, (A-c and B-c)) was withdrew. Then the ropivacaine mixed with dexmedetomidine was injected through the disposable puncture needle (A-d and B-d). The operation process and the flow diagram are as (C-A and D-A and C-b and D-b) of Figure 1, respectively. |
Ultrasound-guided rectus sheath block (RSB) provides effective analgesia for the umbilicus incision in SILC patients,8 but there is insufficient evidence to prove that RSB is more effective in relieving postoperative pain than local incision infiltration after laparoscopic surgery.14–16 Therefore, the study aimed to compare the effects of RSB, conventional local incision infiltration and incisional infiltration using a local anesthetic injection kit on postoperative pain relief and the impact on postoperative recovery in patients undergoing SILC.
Materials and Methods
Patients and Study Design
It was a prospective, double-blinded, randomized study at the Third Affiliated Hospital of Anhui Medical University (The First People’s Hospital of Hefei). We registered the trial prospectively in the Chinese Clinical Trial Registry (ChiCTR2100046964, principal investigator: Jun-Ma Yu; registration: https://www.chictr.org.cn/showproj.aspx?proj=127692; date of registration: June 5, 2021) and was approved by the ethics committee of the current hospital (No. 2021-007-01). With written informed consent by all study patients undergoing elective SILC, and the current research was conducted in accordance with the Declaration of Helsinki.
Male or female patients aged 18–64 years were included in the current research. The exclusion criteria included patients with a body mass index (BMI) ≥30 kg/m2, abnormal liver and kidney function, previous upper abdominal surgery, acute inflammation, pregnancy, inability to obey instructions, long-term use of opioids or benzodiazepines or those who are allergic to the drugs used in this study. Furthermore, patients with a drainage catheter or those patients converted to an open procedure were also excluded.
Randomization and Blinding
All patients scheduled for elective SILC were divided into three groups (n = 20 for each group) randomly. Group assignments were sealed in sequentially numbered opaque envelopes using a computer-generated random sequence, which were opened on the morning of surgery. The group assignments were blinded to all study patients and the staff responsible for the data collection. We ensured that every patient learned and understood how to assess pain using the visual analog scale (VAS) and the methods of using the patient-controlled intravenous analgesia (PCIA) system during the preoperative interview. Bilateral RSB under ultrasound-guided was implemented by experienced anesthesiologists according to a previous method17 30 minutes before anesthesia induction18 in group RSB, which consisted of 20 mL local anesthetic solution (0.5% ropivacaine mixed with 1 μg/kg dexmedetomidine) as in the previous study.19 The LAI-I group had 0.5% ropivacaine mixed with 1 μg/kg dexmedetomidine injected through the incision sites in a total volume of 14 mL by a 20 mL syringe.20 The LAI- II group had 0.5% ropivacaine mixed with 1 μg/kg dexmedetomidine injected through the incision sites in a total volume of 14 mL by a local anesthetic injection kit. All devices and procedure in the LAI-II group are as Figure 1. All injections were performed before the skin incision.
Anesthesia
After admission to the operating room, all patients had intravenous access opened and routine monitors, including electrocardiogram (ECG), heart rate (HR), noninvasive blood pressure (NIBP), and oxygen saturation (SpO2). All patients were given injection with butorphanol 0.01 mg/kg intravenously. Then, the induction of general anesthesia was processed with 1 μg/kg remifentanil and 1.5–2.5 mg/kg propofol, followed by 0.2 mg/kg cisatracurium to facilitate tracheal intubation. Next, all patients were provided with mechanical ventilation. The infusions of propofol (3–12 mg·kg−1·h−1) and remifentanil (0.1–0.3 μg·kg−1·min−1) continued. The Narcotrend Index was maintained between 40 and 60 during the procedure. The CO2 partial pressures of end-expiratory were maintained at 35 to 45 mmHg. The intra-abdominal pressure of the CO2 pneumoperitoneum was maintained at 12 mmHg during laparoscopy. At the end of surgery, CO2 was excreted by manual compression of the abdomen through the open trocars. All patients were transferred to the post-anesthesia care unit (PACU) to extubate tracheal through a standardized protocol after surgery and a PCIA composed of 10 mg butorphanol with a total volume of 100 mL was used as rescue analgesia within 48 hours. A rescue analgesia dose of 2.5 mL was administered each time with an interval of 15 minutes, without any background infusion.
Description of Incisional Infiltration Using a Local Anesthetic Injection Kit
Before the procedure, the kit is assembled as follows: a disposable epidural syringe (5 mL, Figure 1A-a and B-a) whose balloon is inflated to bring negative pressure and is connected to a closed disposable triplet (Figure 1A-b and B-b). And the triplet is connected to the tail of the inner needle. The inner needle (AN-SI, 0.7×106, Figure 1A-c and B-c) is inside the outer needle (AN-E, 1.2×80, Figure 1A-d and B-d), and the inner needle is about 2mm longer than the outer needle. After visual observation shows that both inner and outer needles are inserted behind the subcutaneous area, open the triplet, slowly insert the kit until the air in the balloon drop sharply (Figure 1C-a-D-a and C-b-D-b). It indicates that the inner needle punctures the peritoneum, and this moment the outer needle just reaches the peritoneum. Then stop inserting the kit, keep the position still, remove kit components except the outer needle, after that the injection of local anesthetics from peritoneum to subcutaneous is mediated by outer needle.
Data Collection
Before the operation, data on age, weight, height, BMI, sex, ASA score, and type of gallbladder disease of eligible study subjects were collected. The primary outcomes were the PCIA demand frequency within 48 hours after the operation and postoperative pain measured by VAS at 2 h, 4 h, 8 h, 24 h, and 48 h after surgery. The analgesic effect was evaluated with the PCIA demand frequency and VAS scores. The secondary outcomes were evaluated as follows. The total procedure times, the cumulative consumption of anesthetic drugs (propofol, remifentanil or cisatracurium), the duration of surgery and anesthesia were recorded. Moreover, awaking time of anesthesia, time to unassisted walking, waking up at the night of surgery due to pain and the occurrence of postoperative nausea and vomiting (PONV) were recorded after surgery. The Global Satisfaction Score (GSS) was assessed within 48 hours to determine patients’ satisfaction with pain control after the operation using a 4-point scale (1 to 4 representing “poor”, “fair”, “good”, or “very good”, respectively). Patients undergoing SILC were routinely hospitalized for up to 48 hours after surgery in our hospital. Bleeding related to the analgesic operation was observed for all patients in the three groups after the laparoscopic lens entered the abdominal cavity.
Statistical Analysis
In our pilot study, patients in LAI-II group possessed lower PCIA demand frequency than other two groups (n = 10 in each group, PCIA demand frequency: 13.1±3.2, 12.5±3.3 and 8.8±3.0, respectively). To achieve a power of 95% and an α-error of 5%, 17 patients needed in each group as calculated using PASS15.0 software. After supplementing the 20% missed visit rate, 20 patients were included in each group.
Data were analyzed using SPSS statistical software version 20.0 (IBM). The normality of continuous data was confirmed through a Shapiro‒Wilk test. Quantitative variables are expressed as the mean and standard deviation (SD), nonnormally distributed data were represented as the median and interquartile range (IQR), and categorical variables are presented as numbers (n/%). Normally distributed date of quantitative variables were analyzed using one-way analyses of variance (ANOVA) followed by the Bonferroni post hoc test, and nonnormal distributed date of quantitative variables were analyzed using the Kruskal−Wallis rank-sum test. Categorical variables were assessed using χ2 or Fisher’s exact test. The P value was adjusted according to Bonferroni method and fixed at 0.017 for pairwise comparison. P value <0.05 was considered to indicate significance.
The PCIA demand frequency, cumulative consumption of anesthetic drugs, duration of surgery, duration of anesthesia, and awaking time of anesthesia were analyzed using ANOVA with Bonferroni correction. The total procedure times, time to unassisted walking and GSS were analyzed using the Kruskal−Wallis rank-sum test. The incidence of waking up and PONV among the three groups was analyzed using the χ2 test or Fisher’s exact test. A linear mixed model followed by pairwise comparisons with Bonferroni correction was conducted to evaluate changes of VAS scores over time among the three groups.21
Results
A total of 60 patients were recruited, and fifty-nine patients were finally enrolled and randomized. A 62-year-old female patient with loose subcutaneous tissue in group LAI-II was excluded because the balloon shrank before the tip of the internal needle breaking the peritoneum, which resulted in analgesia procedure failure (Figure 2). Patients’ characteristics and distribution of types of gallbladder disease are summarized in Table 1. These baseline data were similar for the three groups.
 |
Table 1 Demographic Characteristics of Patients Undergoing Single-Incision Laparoscopic Cholecystectomy |
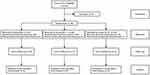 |
Figure 2 CONSORT diagram of study. |
Meanwhile, there were no significant differences in the duration of surgery and anesthesia, awaking time of anesthesia, consumption of intraoperative general anesthetics, time to unassisted walking, or number of waking up at the night of surgery due to pain. The total number of cases of PONV was similar in each group. The total procedure times in group LAI-I and group LAI-II were significantly shorter than that in group RSB (both P < 0.001), but it was similar between groups LAI-I and LAI-II. Compared with group RSB and group LAI-I, group LAI-II had a lower PCIA demand frequency (P < 0.001, respectively). The patients of three groups had similar GSS points (Table 2). No bleeding related to the preoperative analgesic operation was observed for any patients in any groups after the laparoscopic lens entered the abdominal cavity (data not shown).
 |
Table 2 Primary and Secondary Outcomes |
There were no differences in VAS scores among the three groups at 2 h, 4 h, 8 h, 24 h, and 48 h after surgery. However, the VAS scores in the LAI-II group were seemingly lower than those in the other two groups at T1-2, but with no significant difference. Compared with the RSB group, both the LAI-I and LAI-II groups had lower VAS scores at T3 seemingly, with no significant difference either. After T3, VAS scores in all groups tended to decline consistently (Figure 3).
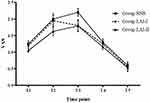 |
Figure 3 Average VAS scores in three groups for various time points of follow-up. |
Discussion
This prospective randomized study evaluated the efficacy of three analgesia methods, including RSB, LAI-I and LAI-II, to reduce the rescue analgesic requirements, save anesthesia procedure times and enhance the recovery in SILC patients, and all cases achieved satisfactory results. The results indicated that PCIA demand frequency in group LAI-II patients was significantly lower than that in the other two groups, and the total procedure times in group LAI-I and group LAI-II were significantly shorter than that in group RSB. The VAS scores in the LAI-II group were lower than those in the other two groups at T1-2, but there were no significant differences. This is the first study to evaluate the treatment of postoperative pain among RSB, conventional local incision infiltration and incisional infiltration using a local anesthetic injection kit in SILC patients. Our trial demonstrates that the requirement for rescue analgesia in the local anesthetic injection kit group was lowest among the three groups for SILC patients. However, the effect of analgesia was equivalent between RSB and conventional local incision infiltration in SILC.
SILC uses the incision at umbilicus has been widely applied in clinical practice to allow easy access to and closure of the peritoneal cavity, as well as easy conversion to standard laparoscopy.1 Currently, many researchers have raised concerns about whether single-access approach LC is associated with reduced postoperative pain compared with multiport LC. It was reported that the inflammation was similar in SILC and conventional LC, as reflected by the lack of significant differences in assessed pain and the rescue analgesic dose.22 Patients undergoing SILC had a trend toward increased incisional pain, which was possibly due to stress exerted on the tissue by the surgical instruments and laparoscope during the surgery.7 Additionally, the tension of a single incision is greater than the total tension of multiple small incisions of the same length, and the amount of tension corresponds to pain intensity.9 Therefore, postoperative pain after SILC continues to affect the early recovery of patients, and reliable analgesic methods are worthy of adoption in the clinic.
Local incision infiltration is often used as an analgesic method during surgery because it has a simple procedure and remarkable analgesic effect, with low requirements for facilities. Local anesthetics can directly block the activity of pain receptors; among them, ropivacaine has been recommended for postoperative pain management in patients undergoing laparoscopic surgery.23 Numerous studies have recorded the efficacy of dexmedetomidine added to ropivacaine;24,25 moreover, the inflammatory reaction caused by external stimulation was relieved by local injection of dexmedetomidine, as it reduced the local production of inflammatory factors.26 Yu et al13 also recommended local incision infiltration as a routine option for conventional LC postoperative analgesia, which was consistent with Barazanchia’s conclusion.11 In another study, they found that local infiltration of the mixture of ropivacaine and dexmedetomidine at the trocar sites could effectively relieve postoperative pain, shorten unassisted walking time and improve sleep quality without increasing side effects.20 The reason for choosing the mixture of ropivacaine and dexmedetomidine in a total volume of 14 mL for patients in groups LAI-I and LAI-II was explained as follows. Before designing this trial, we routinely performed conventional umbilical incisional infiltration with 20 mL 0.5% ropivacaine mixed with 1 μg/kg dexmedetomidine in SILC patients, and no obvious pain was reflected in the follow-up. However, surgeons complained that since the umbilicus was thin, a large amount of local anesthetic fluid accumulated around the umbilicus and influenced their incision operation. Therefore, we chose the present volume.
In 1996, Alexander et al conducted a randomized trial to explore the influence of direct injection of local anesthetic on postoperative pain after four-port LC in relation to port sites arrived the parietal peritoneum, and the conclusion is the local anesthetic arriving at the parietal peritoneum had better control of immediate postoperative pain than standard subcutaneous tissue injection.27 The effect of different depths of anesthetic infiltration on postoperative pain relief was explored in another study in patients undergoing multiport LC,28 and the results were consistent with the outcomes of the above study, showing that deep local anesthetic infiltration significantly alleviated the acute postoperative pain and reduced the consumption of analgesic drugs after surgery; however, subcutaneous infiltration alone did not work compared with the no-infiltration group. It is noteworthy that surgery in those two studies was all standard multiport LC, and local anesthetic infiltration was performed postoperatively in Wikran Suragul’s design.28 Studies have reported that the establishment of central sensitization and incisional and inflammatory injuries can be prevented before pain stimulus by preemptive analgesia.29,30 Moreover, preemptive analgesia was proven to be more effective for postoperative pain relief than the same management performed postoperatively, which contains regional anesthesia and local wound infiltration.31,32 Although results about the implement timing of local anesthetic infiltration were inconsistent,28 it was suggested that incisional local anesthetic infiltration at the beginning or at the end of the operation depended on preference.33 We believe that skin incision and edema at the incision site caused by surgical operation would influence the diffusion of local anesthetics for postoperative incisional infiltration. On the one hand, it has been proven that the local anesthetics are potent inhibitors of inflammation-induced edema formation under different conditions,34 and the migration of leukocytes and subsequent release of tissue-toxic agents was inhibited by the local wound infiltration.35 On the other hand, preoperative local infiltration could also be combined with postoperative local anesthetic infiltration to compensate for local anesthetics for incision sites not covered by pre-incision infiltration; thus, we chose preoperative local incision infiltration at the trocar side. Moreover, it is difficult to determine the depth of anesthetic infiltration to reach the parietal peritoneum accurately for preventing accidental injury of the abdominal organs or direct injection of local anesthetic into the abdominal cavity. Based on the reason above, we hypothesized that there would be a greater impact of incisional local anesthetic infiltration depth on SILC. Therefore, we designed a local anesthetic injection kit product that obtains accurate identification of needle tips reaching the peritoneum based on variation in component shape (the air bubble of the syringe; Figure 1B-a and D-a), thus ensuring deep local anesthetic infiltration (Figure 1C and D).
Ultrasound-guided RSB was employed extensively in laparoscopy surgeries with a subxiphoid incision or single-access approach to laparoscopic cholecystectomy with the main incision at the umbilical port.36 It was reported that RSB was effective in postoperative pain control for SILC.8,37 In Dingeman’s study, ultrasonography-guided bilateral RSB group has a significantly higher percentage of patients with a FACES scores of 0 (ie, no pain), lower FACES scores at multiple time points and reduced total use of analgesic medications in the PACU compared with the LAI group after umbilical hernia repair in children, those findings may be generalizable to patients undergoing single-incision laparoscopic surgery for abdominal operations using an umbilical port.14 In addition, Gurnaney’s study demonstrates a superior analgesia effect of ultrasound-guided RSB than local infiltration after umbilical hernia repair.15 Kitamura et al reported that VAS scores and use frequency of analgesic agents were similar within 3 days between conventional local anesthetic infiltration group and RSB group after four-port LC.16 Also in our study, there was no difference in VAS scores at each time point or requirements for rescue analgesic between the RSB and LAI-I groups, which may be influenced by the volume of local anesthetics in the LAI-I group. Because lacking of SILC trials comparing analgesia effect between RSB and local incisional infiltration, our outcome values cannot be contrasted with others completely. Future trials are needed to verify whether a larger dosage of anesthetic will influence the results between the RSB and LAI-I groups and to explore the optimal dose of local anesthetic for injection kits in SILC patients.
It is worth noting that the VAS scores were seemingly lower in group LAI-II than in the other two groups at 2 h and 4 h after surgery, but with no significant difference. We guessed that lower VAS scores at 2 h and 4 h after surgery in group LAI-II compared with group LAI-I might be influenced by depth of infiltration like the two studies.27,28 Moreover, the PCIA demand frequency in group LAI-II patients was significantly lower than that in the other two groups. The reason may be that (1) surgical incisions can damage the nerve-rich abdominal fascia and peritoneum, (2) the postoperative pain was influenced obviously by the peritoneum and deep muscle layers injured by incisions of abdominal wall, and (3) abdominal wall pain may sensitize the visceral pain pathways of the spinal dorsal horn neurons.38,39 Local anesthetics injected at deep muscle layers and the peritoneum can lock nociceptive pain input from peripheral nerve fibers and have intrinsic anti-inflammatory properties, as they modulate the local and systemic release of inflammatory mediators, thereby inhibiting central sensitization.40 Our product guarantees local anesthetic infiltration of deep muscle layers and the peritoneum. Thus, early postoperative pain in group LAI-II was well managed in our study. These results also predict the advantage of a local anesthetic injection kit for reducing the local anesthetic dosage without affecting the analgesic effect when applied in SILC incisional infiltration. Unfortunately, we did not observe a difference in late postoperative pain between the groups. As demonstrated in the study, local incisional infiltration was less time-consuming and easily performed, especially in LC (relatively “fast” operations).12 Total anesthesia procedure times in group LAI-I and LAI-II were significantly shorter than group RSB. VAS scores were no more than 3 among three groups at any time points, showing that enough pain control for every patient, so GSS was not statistically different in our study. In addition, tissue trauma could induce the systemic inflammatory response to surgery,1 which is the main determinant of perioperative patient recovery compared to neuroendocrine changes associated with the injury stress response.41 However, there was no significant difference detected among the three groups for postoperative recovery in our study.
There are some limitations in our study. The kit is composed of four parts, and the matching degree is not adequately high, so the sharpness of the needle tip is not sufficient, and the puncture resistance is relatively large. With the further improvement of the product, the total procedure times in group LAI-II will be further shortened, and the procedure will be more convenient. In our previous application, it was found that the balloon shrank before the tip of the internal needle breaking the peritoneum when a kit was used for geriatric patients with loose subcutaneous tissue and it was also present in the current study. In the future, it is needed to improve performance such as appropriately reducing the pressure of the air bubble of the syringe. On the other hand, VAS scores were lower in the LAI-II group than in the other two groups in the early postoperative period but no difference on statistics. Then, studies with larger sample sizes may be needed to verify the above results. Finally, we did not detect relevant inflammatory factors and merely observed the effect of analgesia methods on recovery among the three groups within 48 h after surgery. Studies with long-term results are needed in the future. Considering the trend of SILC as a day surgery, the matter of which convenient analgesic methods can be used to replace PCIA should be taken into consideration in the future.
Conclusion
Our results suggest that incision infiltration with local anesthetic at the trocar sites is an analgesia method with good control of postoperative pain, quick anesthesia procedure time, and low impact on staff workload in SILC. Ultrasound-guided RSB and conventional local anesthetic infiltration were similar regarding postoperative pain management in SILC. Furthermore, incisional anesthetic infiltration using a local anesthetic injection kit better reduces the rescue analgesic requirement compared with conventional local wound infiltration and RSB for patients undergoing SILC.
Data Sharing Statement
All necessary data supporting our findings have been presented within the manuscript. The datasets used and/or analyzed during the current study are available for anyone who wishes to access them for reasonable request. The data will be accessible from the corresponding author.
Ethics Approval and Informed Consent
The authors declared that all the study patients provided written informed consent and that this study was conducted in accordance with the Declaration of Helsinki. This trial was approved by the ethics committee of the current hospital (No. 2021-007-01).
Consent for Publication
All the authors have read this article and gave agreements to publish it.
Acknowledgments
We acknowledge the assistance of American Journal Experts (AJE) for English language editing.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Applied Medicine Research Project Fund of Hefei Health Committee (grant number 2019-ZC-2) and Postgraduate Innovation Research and Practice Program of Anhui Medical University (grant number YJS20230088). The authors had full access to all data in the original study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Lirici MM, Tierno SM, Ponzano C. Single-incision laparoscopic cholecystectomy: does it work? A systematic review. Surg Endosc. 2016;30(10):4389–4399.
2. Casaccia M, Ponzano M, Testa T, et al. Single-port cholecystectomy for cholecystitis versus non-cholecystitis. Jsls. 2022;26(3):00020.
3. Furukawa K, Asaoka T, Mikamori M, et al. Single-Incision Laparoscopic Cholecystectomy: a Single-Centre Experience of 1469 Cases. J Gastrointest Surg. 2022;26(4):831–836.
4. Haueter R, Schütz T, Raptis DA, et al. Meta-analysis of single-port versus conventional laparoscopic cholecystectomy comparing body image and cosmesis. Br J Surg. 2017;104(9):1141–1159.
5. Ali Alshahri TM, Abounozha S, Ibrahim R. Is single port laparoscopic cholecystectomy superior to standard cholecystectomy in post-operative pain? Ann Med Surg. 2021;63:102123.
6. Lyu Y, Cheng Y, Wang B, et al. Single-incision versus conventional multiport laparoscopic cholecystectomy: a current meta-analysis of randomized controlled trials. Surg Endosc. 2020;34(10):4315–4329.
7. Philipp SR, Miedema BW, Thaler K. Single-incision laparoscopic cholecystectomy using conventional instruments: early experience in comparison with the gold standard. J Am Coll Surg. 2009;209(5):632–637.
8. Kamei H, Ishibashi N, Nakayama G, et al. Ultrasound-guided rectus sheath block for single-incision laparoscopic cholecystectomy. Asian J Endosc Surg. 2015;8(2):148–152.
9. Garg P, Thakur JD, Garg M, et al. Single-incision laparoscopic cholecystectomy vs. conventional laparoscopic cholecystectomy: a meta-analysis of randomized controlled trials. J Gastrointest Surg. 2012;16(8):1618–1628.
10. Raff M, Belbachir A, El-Tallawy S, et al. Intravenous oxycodone versus other intravenous strong opioids for acute postoperative pain control: a systematic review of randomized controlled trials. Pain Ther. 2019;8(1):19–39.
11. Barazanchi AWH, MacFater WS, Rahiri JL, et al. Evidence-based management of pain after laparoscopic cholecystectomy: a PROSPECT review update. Br J Anaesth. 2018;121(4):787–803.
12. Molfino S, Botteri E, Baggi P, et al. Pain control in laparoscopic surgery: a case-control study between transversus abdominis plane-block and trocar-site anesthesia. Updates Surg. 2019;71(4):717–722.
13. Wu L, Wu L, Sun H, et al. Effect of ultrasound-guided peripheral nerve blocks of the abdominal wall on pain relief after laparoscopic cholecystectomy. J Pain Res. 2019;12:1433–1439.
14. Dingeman RS, Barus LM, Chung HK, et al. Ultrasonography-guided bilateral rectus sheath block vs local anesthetic infiltration after pediatric umbilical hernia repair: a prospective randomized clinical trial. JAMA Surg. 2013;148(8):707–713.
15. Gurnaney HG, Maxwell LG, Kraemer FW, et al. Prospective randomized observer-blinded study comparing the analgesic efficacy of ultrasound-guided rectus sheath block and local anaesthetic infiltration for umbilical hernia repair. Br J Anaesth. 2011;107(5):790–795.
16. Kitamura N, Iida H, Maehira H, et al. Postoperative analgesic effect of ultrasound-guided rectus sheath block and local anesthetic infiltration after laparoscopic cholecystectomy: results of a prospective randomized controlled trial. Asian J Endosc Surg. 2022;15(1):29–35.
17. Visoiu M, Scholz S, Malek MM, et al. The addition of clonidine to ropivacaine in rectus sheath nerve blocks for pediatric patients undergoing laparoscopic appendectomy: a double blinded randomized prospective study. J Clin Anesth. 2021;71(110254):19.
18. Fu H, Fu Y, Xu X, et al. Ultrasound-Guided Rectus Sheath Block Combined with Butorphanol for Single-Incision Laparoscopic Cholecystectomy: what is the Optimal Dose of Ropivacaine? J Pain Res. 2020;13:2609–2615.
19. Chung W, Yoon Y, Kim JW, et al. Comparing two different techniques of rectus sheath block after single port laparoscopic surgery in benign adnexal mass patients: surgical versus ultrasonography guidance-A randomized, single-blind, case-controlled study. Eur J Obstet Gynecol Reprod Biol. 2017;217:29–33.
20. Yu JM, Sun H, Wu C, et al. The Analgesic Effect of Ropivacaine Combined With Dexmedetomidine for Incision Infiltration After Laparoscopic Cholecystectomy. Surg Laparosc Endosc Percutan Tech. 2016;26(6):449–454.
21. Xue Q, Chu Z, Zhu J, et al. Analgesic Efficacy of Transverse Abdominis Plane Block and Quadratus Lumborum Block in Laparoscopic Sleeve Gastrectomy: a Randomized Double-Blinded Clinical Trial. Pain Ther. 2022;11(2):613–626.
22. Luna RA, Nogueira DB, Varela PS, et al. A prospective, randomized comparison of pain, inflammatory response, and short-term outcomes between single port and laparoscopic cholecystectomy. Surg Endosc. 2013;27(4):1254–1259.
23. Modir H, Yazdi B, Piri M, et al. An investigation of the effects of dexmedetomidine and fentanyl as an adjuvant to ropivacaine on pain scores and hemodynamic changes following laparoscopic cholecystectomy. Med Gas Res. 2021;11(3):88–93.
24. Hu X, Li J, Zhou R, et al. Dexmedetomidine Added to Local Anesthetic Mixture of Lidocaine and Ropivacaine Enhances Onset and Prolongs Duration of a Popliteal Approach to Sciatic Nerve Blockade. Clin Ther. 2017;39(1):89–97.
25. Andersen JH, Grevstad U, Siegel H, et al. Does Dexmedetomidine Have a Perineural Mechanism of Action When Used as an Adjuvant to Ropivacaine?: a Paired, Blinded, Randomized Trial in Healthy Volunteers. Anesthesiology. 2017;126(1):66–73.
26. Sukegawa S, Higuchi H, Inoue M, et al. Locally injected dexmedetomidine inhibits carrageenin-induced inflammatory responses in the injected region. Anesth Analg. 2014;118(2):473–480.
27. Alexander DJ, Ngoi SS, Lee L, et al. Randomized trial of periportal peritoneal bupivacaine for pain relief after laparoscopic cholecystectomy. Br J Surg. 1996;83(9):1223–1225.
28. Suragul W, Tantawanit A, Rungsakulkij N, et al. Effect of local anaesthetic infiltration on postoperative pain after laparoscopic cholecystectomy: randomized clinical trial. BJS Open. 2022;6(3):876.
29. Kissin I. Preemptive analgesia. Anesthesiology. 2000;93(4):1138–1143.
30. Xuan C, Yan W, Wang D, et al. Efficacy of preemptive analgesia treatments for the management of postoperative pain: a network meta-analysis. Br J Anaesth. 2022;129(6):946–958.
31. Cantore F, Boni L, Di Giuseppe M, et al. Pre-incision local infiltration with levobupivacaine reduces pain and analgesic consumption after laparoscopic cholecystectomy: a new device for day-case procedure. Int J Surg. 2008;6(1):24.
32. Liang M, Chen Y, Zhu W, et al. Efficacy and safety of different doses of ropivacaine for laparoscopy-assisted infiltration analgesia in patients undergoing laparoscopic cholecystectomy: a prospective randomized control trial. Medicine. 2020;99(46):0000000000022540.
33. Bisgaard T. Analgesic treatment after laparoscopic cholecystectomy: a critical assessment of the evidence. Anesthesiology. 2006;104:835–846.
34. Cassuto J, Sinclair R, Bonderovic M. Anti-inflammatory properties of local anesthetics and their present and potential clinical implications. Acta Anaesthesiol Scand. 2006;50(3):265–282.
35. Eriksson AS, Sinclair R, Cassuto J, et al. Influence of lidocaine on leukocyte function in the surgical wound. Anesthesiology. 1992;77(1):74–78.
36. Uchinami Y, Sakuraya F, Tanaka N, et al. Comparison of the analgesic efficacy of ultrasound-guided rectus sheath block and local anesthetic infiltration for laparoscopic percutaneous extraperitoneal closure in children. Paediatr Anaesth. 2017;27(5):516–523.
37. Kauffman JD, Nguyen ATH, Litz CN, et al. Laparoscopic-guided versus transincisional rectus sheath block for pediatric single-incision laparoscopic cholecystectomy: a randomized controlled trial. J Pediatr Surg. 2020;55(8):1436–1443.
38. Beaussier M, El’Ayoubi H, Schiffer E, et al. Continuous preperitoneal infusion of ropivacaine provides effective analgesia and accelerates recovery after colorectal surgery: a randomized, double-blind, placebo-controlled study. Anesthesiology. 2007;107(3):461–468.
39. Lavand’homme PM, Roelants F, Waterloos H, et al. Postoperative analgesic effects of continuous wound infiltration with diclofenac after elective cesarean delivery. Anesthesiology. 2007;106(6):1220–1225.
40. Beloeil H, Mazoit JX. Effect of local anesthetics on the postoperative inflammatory response. Ann Fr Anesth Reanim. 2009;28(3):231–237.
41. Grosu I, P L. Continuous regional anesthesia and inflammation: a new target. Minerva Anestesiol. 2015;81(9):1001–1009.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
