Back to Journals » Clinical Interventions in Aging » Volume 16
Effect of 5 Years of Exercise Intervention at Different Intensities on Brain Structure in Older Adults from the General Population: A Generation 100 Substudy
Authors Pani J, Reitlo LS, Evensmoen HR, Lydersen S, Wisløff U, Stensvold D, Håberg AK
Received 4 May 2021
Accepted for publication 21 July 2021
Published 12 August 2021 Volume 2021:16 Pages 1485—1501
DOI https://doi.org/10.2147/CIA.S318679
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Nandu Goswami
Jasmine Pani,1,2 Line S Reitlo,1,2 Hallvard Røe Evensmoen,2 Stian Lydersen,3 Ulrik Wisløff,4,5 Dorthe Stensvold,4,6 Asta K Håberg1,2
1Department of Neuromedicine and Movement Science, Faculty of Medicine and Health Sciences, NTNU (Norwegian University of Science and Technology), Trondheim, Norway; 2Department of Radiology and Nuclear Medicine, St. Olavs Hospital, Trondheim University Hospital, Trondheim, Norway; 3Department of Mental Health, Faculty of Medicine and Health Sciences, NTNU (Norwegian University of Science and Technology), Trondheim, Norway; 4Department of Circulation and Medical Imaging, Faculty of Medicine and Health Sciences, NTNU (Norwegian University of Science and Technology), Trondheim, Norway; 5School of Human Movement & Nutrition Sciences, University of Queensland, Queensland, Australia; 6Department of Cardiology, St. Olavs Hospital, Trondheim University Hospital, Trondheim, Norway
Correspondence: Asta K Håberg
Department of Neuromedicine and Movement Science, Faculty of Medicine and Health Sciences, NTNU (Norwegian University of Science and Technology), Trondheim, N-7491, Norway
Tel +47 90 25 91 47
Email [email protected]
Purpose: The aim was to examine the effect of a 5-year exercise intervention at different intensities on brain structure in older adults from the general population partaking in the randomized controlled trial Generation 100 Study.
Participants and Methods: Generation 100 Study participants were invited to a longitudinal neuroimaging study before randomization. A total of 105 participants (52 women, 70– 77 years) volunteered. Participants were randomized into supervised exercise twice a week performing high intensity interval training in 4× 4 intervals at ∼ 90% peak heart rate (HIIT, n = 33) or 50 minutes of moderate intensity continuous training at ∼ 70% of peak heart rate (MICT, n = 24). The control group (n = 48) followed the national physical activity guidelines of ≥ 30 min physical activity daily. Brain MRI at 3T, clinical and cardiorespiratory fitness (CRF), measured as peak oxygen uptake, were collected at baseline, and after 1, 3, and 5 years of intervention. Brain volumes and cortical thickness were derived from T1 weighted 3D MRI data using FreeSurfer. The effect of HIIT or MICT on brain volumes over time was investigated with linear mixed models, while linear regressions examined the effect of baseline CRF on brain volumes at later time points.
Results: Adherence in each group was between 79 and 94% after 5 years. CRF increased significantly in all groups during the first year. Compared to controls, the HIIT group had significantly increased hippocampal atrophy located to CA1 and hippocampal body, though within normal range, and the MICT group greater thalamic atrophy. No other effects of intervention group were found. CRF across the intervention was not associated with brain structure, but CRF at baseline was positively associated with cortical volume at all later time points.
Conclusion: Higher baseline CRF reduced 5-year cortical atrophy rate in older adults, while following physical activity guidelines was associated with the lowest hippocampal and thalamic atrophy rates.
Keywords: CNS, aging, limbic, brain reserve, morphometry
Plain Language Summary
This study investigated the effect of 5 years of supervised exercise twice weekly compared to a control group following the national guidelines recommending 30 min of physical activity daily on brain structure across 5 years. Older adults (70–77 years) from the general population were invited. The supervised exercise group was assigned to either high intensity interval training, HIIT, consisting of 10 minutes of warm-up followed by 4×4 minutes intervals at ~90% of peak heart rate or near exhaustion intermingled with 3 minutes of active breaks, or moderate intensity continuous training, MICT, of 50 minutes of continuous activity at ~70% of peak heart rate, or medium exhaustion. At inclusion into the study and after 1, 3 and 5 years of HIIT, MICT or guideline-based recommended physical activity, the participants underwent clinical and physical testing, and brain MRI. Brain volumes were derived from T1 weighted 3D MRI scans acquired on the same scanner with the same protocol across the 5-year period.
Both the effect of group (HIIT, MICT or control) and the effect of cardiorespiratory fitness, measured as VO2-peak, on brain volumes were investigated. We also investigated the HIIT&MICT group combined versus the control group. The link between baseline cardiorespiratory fitness and later brain volume was also assessed.
In all groups, participants adhered well (79–94%) and to a similar extent to their assigned exercise or physical activity regime. Unexpectedly, we found that the HIIT as well as the combined HIIT&MICT group had markedly smaller hippocampal volume at the end of the intervention, while the MICT as well as the combined HIIT&MICT group had markedly smaller thalamic volume. This was not due to accelerated degree of tissue loss in the supervised training groups but due to lower-than-expected tissue loss in the group following the national physical activity guidelines. We found no effect of increasing cardiorespiratory fitness on brain volumes after age 70, but there was a positive effect of having high cardiorespiratory fitness at inclusion into the study for later cortical volume for all groups. In summary, brain structure across 5 years was preserved the best in older adults who followed the national physical activity guidelines and in those with higher VO2 at inclusion.
Introduction
A string of failed Alzheimer’s disease (AD) drug trials1 and the global population aging have led to an increased focus on finding effective measures to avoid onset or delay development of dementia. High cardiorespiratory fitness (CRF) measured as peak oxygen uptake (VO2peak), exercising as well as physical activity in general are among the preventive measures associated with dementia risk reduction.2–4 The positive effect of exercise training on the brain is assumed to be associated with higher CRF.5 Observational studies using brain MRI measures as proxies for brain health and AD risk report positive associations between CRF and brain structures involved in the pathophysiology of AD such as the hippocampus and cortex.6–10 However, there are contradictory findings regarding the presence of such positive effects. Randomized controlled trials (RCT) and other exercise intervention studies in community dwelling and hospital cohorts of younger and older adults report predominantly positive effects of moderate intensity exercise on the brain, with greater hippocampus and cortex volume in the exercise group compared to controls.11–15 However, no effects16–19 and negative effects20,21 have also been described in the same brain structures in similar cohorts. It should be noted that the RCTs and exercise interventions that report positive effects on brain structures followed the participants for maximally one year while those that find no effects were longer, lasting up to 24 months.
Intervention studies including brain MRI in older adults have implemented moderate intensity exercise training despite that high intensity training provides larger cardiovascular health effects at all ages.22,23 It has been suggested that high intensity interval training (HIIT) is superior in preserving brain structure and function compared to moderate training levels, although potential risks of HIIT for the brain of older adults are discussed.24,25 Thus, both duration and intensity of exercise intervention to better preserve or improve brain structure remain to be determined.26
It is therefore timely to assess the effect of long-term exercise training at different exercise intensities on brain structure in older adults.27 The Generation 100 Study is a 5-year RCT investigating the effects of 5 years of HIIT, moderate-intensity continuous training (MICT) or following the national health authorities’ guidelines for physical activity on overall mortality.28 The RCT Generation 100 Study found a trend towards reduced mortality after HIIT compared to the other two groups.29
The aim of this study in the Generation 100 Study was to examine the effect of the exercise intervention on changes in brain structure with 3 Tesla (T) MRI in a group of Generation 100 Study participants from inclusion and after 1, 3 and 5 years of intervention. We expected an effect of group over time on hippocampal and cortical volumes with the HIIT group having the lowest degree of age-related atrophy, followed by the MICT group. Other brain structures included in the analyses were caudate, thalamus and white matter volumes, regions understudied in exercise interventions. We also investigated the effect of CRF, measured objectively as VO2peak, on these brain structures during the intervention, as well as the predictive value of baseline CRF on brain volumes across the intervention.
Materials and Methods
The Generation 100 Study
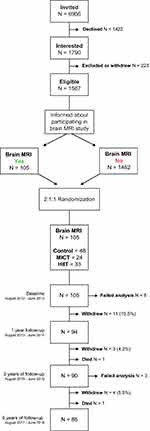
The Generation 100 Study is a registered RCT (NCT01666340, ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT01666340) conducted in a general population of older adults aged 70–77 years (born between 1936 and 1942) and registered in the National Population Registry as citizens of Trondheim municipality. A total of 6966 older adults received a personal invitation letter to participate in the Generation 100 Study and 1790 were interested in participating. Of these, 174 withdrew before or during the initial examination and 49 were excluded. In total, 1567 older adults were eligible for participation. Participants were stratified according to sex and cohabitation status before randomized 2:1:1 into control (N=780), HIIT (N=400) and MICT (N=387) groups. The randomization procedure was performed by the Unit for Applied Clinical Research, NTNU, using a web-based system. The Generation 100 Study was approved by the Regional Committee for Medical Research ethics, Central Norway (2012/381 B) and complies with the Declaration of Helsinki. Participants gave their written informed consent and agreed to receive invitations to other studies. Exclusion criteria were somatic or psychiatric disease (including dementia), any issue precluding exercise intervention, and participation in other exercise training studies. Details on the study design and protocols can be found in Stensvold et al.28 Baseline data collection started in August 2012 and lasted till June 2013. Follow-ups were performed 1, 3 and 5 years after baseline data collection with 5 years data collected between August 2017 and June 2018.
Brain MRI Study
The present study investigates the effect of the Generation 100 exercise intervention on structural brain health. Information was provided to all Generation 100 Study participants (N=1567) before randomization, and 105 MRI compatible participants volunteered to take part in this study on brain structure. Exclusion criteria were standard MRI contraindications, such as medical electrical implants. Of the 105 participants included in this study, 48 were in the control group, 24 in MICT and 33 in the HIIT group, reflecting the distribution between the groups in the Generation 100 Study. The participants were scanned at baseline, 1, 3 and 5 years after inclusion in connection with the clinical examinations in the Generation 100 Study. The brain MRI study was approved by the Regional Committee for Medical Research Ethics, Central Norway (2012/849) and was performed in accordance with the Declaration of Helsinki. All participants gave their written informed consent.
Exercise Intervention and Adherence
The HIIT group was instructed to warm up for 10 minutes followed by 4×4 minutes intervals at 85–95% of peak heart rate or minimum 16 on the Borg 6–20 rating of perceived exertion scale,30 interleaved with 3 minutes of active breaks. The MICT group was prescribed 50 minutes of continuous activity at 70% of peak heart rate or 13 on the Borg 6–20 scale. Participants in the HIIT and MICT groups met twice weekly to their respective supervised exercise classes. The supervised exercise classes were performed indoor or outdoor and included, eg, walking and running in different types of terrains as well as aerobics.28 HIIT and MICT participants could exercise individually if able to follow their assigned exercise regime after receiving instructions. All participants in the HIIT and MICT groups were required to meet for mandatory intensity-specific supervised spinning session every 6th week to ensure compliance with exercise intervention. In the mandatory classes, they exercised with a heart rate monitor to make sure that they exercised at the prescribed exercise intensity. The control group was instructed to follow the Norwegian health authorities’ physical activity recommendations of at least 30 minutes of moderate intensity physical activity every day.28 Adherence to the prescribed exercise intervention or national guidelines was calculated from physical activity questionnaires at 1, 3 and 5 year follow-up. The questionnaire included questions on exercise frequency, intensity and duration. Frequency was assessed on a scale from 0 to 5 times per week based on the question “How often do you exercise?” with reply options “Never” (0 times), “Less than once a week” (0.5 times), “Once a week” (1 time), “2–3 times per week” (2.5 times) and “Almost every day” (5 times). For duration, the question was “For how long do you exercise each time?” with reply options “Less than 15 minutes” (7.5 minutes), “15–29 minutes” (22.5 minutes), “30 minutes to 1 hour” (45 minutes) and “More than 1 hour” (60 minutes), giving a range from 7.5 to 60 minutes per session. Minutes per week used for exercise were calculated multiplying the average frequency and duration.29 Mean intensity of exercise was assessed with the Borg 6–20 RPE scale.30
Adherence to the exercise intervention was defined as fulfillment of at least 50% of the prescribed exercise sessions according to the RCT protocol.29 Thus, adherence to HIIT was defined as exercising at least ≥30 minutes ≥15 on the Borg scale per week. MICT adherence was defined as at least ≥30 minutes at 11–14 on the Borg scale per week. Adherence to control (physical activity recommendations) was defined as at least ≥75 minutes of physical activity per week, intensity was not considered for this group. Percentage adherence to assigned program was calculated for each group as number of participants adhering to the prescribed exercise program divided by total number of participants in the group at that time point multiplied by 100.
Demographic Data and Clinical Measurements
Data on date of birth, sex, level of education (primary school, high school and university), cohabitation status, current smoking (yes/no), sleep, health-related quality of life and psychological health were obtained from questionnaires at baseline, 1, 3 and 5 years. Health-related quality of life was obtained with the Short Form health survey (SF-8) questionnaire and both physical and mental component summary scores are reported.31 Psychological health was assessed with a Norwegian validated version of the Hospital Anxiety and Depression Scale (HADS).32,33 Total HADS score was reported.34,35 The Norwegian validated version of the Montreal Cognitive Assessment (MoCA) was administered at 5-year follow-up to evaluate dementia.36 The cut-offs are derived from a large Swedish population with a score of 21 for primary, 22 for secondary and 24 for higher education.37 Total score was reported.
Clinical measurements encompassed height, body weight, body mass index (BMI), body composition (Inbody 720, BIOSPACE, Body Analysis AS, South-Korea), waist circumference, blood pressure and resting heart rate (RHR). Fasting blood samples were obtained and high sensitivity C-reactive protein (hsCRP), glycated hemoglobin (HbA1c), glucose, high-density lipoprotein cholesterol (HDL), low-density lipoprotein cholesterol (LDL), total cholesterol (TC), and triglycerides (TG) levels were measured. At the same time points CRF was assessed objectively as VO2peak using graded maximal exercise testing on a treadmill (NInclusion=102, N5-year=78) or exercise bike (NInclusion=3, N5-year=7). Since some participants did not meet the criteria for maximal oxygen uptake during the study period, the term VO2peak was used. Grip strength was measured with the JAMAR Hydraulic Hand Dynamometer (Lafayette Instrument Company, Lafayette, IN, USA). For details on CRF and grip strength assessment see Supplemental Materials.
Brain MRI
At baseline and after 1, 3 and 5 years, participants underwent the same standardized MRI protocol acquired on one 3T Magnetrom Skyra scanner (Siemens AG, Erlangen, Germany) equipped with a 32-channel head coil. The scans used in this study included a high resolution 3D T1-weighted MPRAGE (TR=1900; TE=3.16; FOV=256×256; slice thickness=1mm; gap=0mm) and a 3D T2-weighted (TR=3200; TE=412; FOV=250×250; slice thickness=1mm; gap=0mm) scan.
Image Processing
The T1-weighted scans were analyzed in the Freesurfer suite v. 6.0 (http://surfer.nmr.mgh.harvard.edu/), for details see Fischl.38 The images were processed using the longitudinal stream39 to ensure low inter-subject variability.40 Visual quality control of all Freesurfer outputs was performed, and scans that failed this control were excluded from the analyses. Total cortex volume and cortical thickness were derived using cortical surface-based analysis.41 For the hippocampus, thalamus, caudate and total cerebral white matter volumes, the volumes from right and left hemisphere were combined. Hippocampal subfields were derived with the longitudinal hippocampal subfield algorithm in the developmental version of Freesurfer v. 6.0.40,42 The hippocampal subfield volumes were combined into three regions: CA1, CA3 (CA2 + CA3) and dentate gyrus (GC-DG + CA4)43 to obtain more reliable measures.42,44 Subsequently, the long axis volumes, ie, hippocampal head, body and tail volumes, were obtained. The right and left hippocampal subfields and long-axis volumes were combined.
Intracranial volume (ICV) was estimated in SPM8 (http://www.fil.ion.ucl.ac.uk/spm) with the automatic reverse brain mask method45 using the 3D T1- and T2-weighted images. All brain volumes were corrected for ICV with the residual method.46 The automatic reverse brain mask method improves the accuracy of the ICV measurement45 and the residual method has been shown to be superior to the proportion method in removing the effects of ICV.46 The combined use of the automatic reverse brain mask method and the residual ICV correction method requires smaller sample sizes compared to the FreeSurfer ICV estimation to detect group differences in brain volumes.45
Statistical Analysis
Sample Characteristics
Demographic and clinical characteristics at baseline were compared between the Generation 100 Study participants and those also participating in the brain MRI study using the Pearson chi squared test, t-test, and Mann–Whitney U-test as appropriate. Pearson chi squared, one-way ANOVA and Kruskal–Wallis test were used to compare the demographic and clinical characteristics at baseline between the HIIT, MICT and control group in the brain MRI study. Additionally, demographic and clinical characteristics known to be affected by exercise and to influence the brain (eg, blood glucose regulation, body fat distribution, sleep, depression) are shown at each follow-up time point for the three groups (Supplementary Tables 2–4). Comparisons of adherence to training regime was compared between groups at each follow up time point using Pearson chi squared test. Missing values constituted less than 5% of the data and were likely randomly missing.
Longitudinal Change in Brain Volumes in the Control, MICT and HIIT Groups
The main analysis (Model 1) assessed the effect of the exercise intervention group on brain structures across the 5-year period using linear mixed models. Linear mixed models are optimal for longitudinal data because they account for within- and between-subjects variability and allow missing values in one or more time points without the exclusion of the participant. Model 1 was performed with brain structure (corrected for ICV using the residual method) as the dependent variable, participant as random effect, time and, time*group interaction as dummy variables with baseline and the control group as references, thus adjusting for baseline value of the outcome variable as recommended by Twisk, Bosman, Hoekstra, Rijnhart, Welten, Heymans.47 The analyses were also adjusted for sex, education and age at baseline. A second model (Model 2) was performed adding the measured CRF values at each time point as covariates to Model 1. For hippocampus, both models were repeated with hippocampal subfield and long-axis volumes as dependent variable. As a sensitivity analysis, the linear mixed models were performed without adjusting for baseline value of the outcome variable. We also performed the analysis with the supervised exercise groups (MICT&HIIT) combined into one group.
CRF Associations with Brain Volumes Across Control, MICT and HIIT Groups
To investigate group effects on CRF over time, we used a linear mixed model with CRF as the dependent variable, participant as random effect, time, and time*group interaction as dummy variables with baseline and the control group as references, adjusting for sex, education and age at baseline (ie, similar as Model 1 above). The same analysis was also performed in the combined exercise group (MICT&HIIT).
The role of CRF per se on brain volumes was assessed after collapsing the three groups into one. Linear regressions were run to investigate if CRF at baseline predicted brain volumes at 1, 3 and 5 years with age at baseline, sex and education as covariates. Presence of localized cortical thickness effects of CRF was also investigated. Cortical thickness is associated primarily with clinical and environmental factors, while cortical surface area is mainly under genetic control.48 To investigate if baseline CRF predicted cortical thickness, we performed a general linear model analysis in MATLAB R2018a (https://www.mathworks.com/products/matlab.html) with CRF at baseline as predictor, and age, sex and education as covariates and cortical thickness of each hemisphere at 1, 3 and 5 years as the dependent variable. Localized cortical thickness maps were smoothed with a full-width-half-maximum Gaussian kernel of 30 mm. To correct for multiple comparisons, the p-value maps of the two hemispheres were combined and thresholded to a false discovery rate (FDR) of 5% across the whole brain.
For all statistical analyses in RStudio49 (including the function “lmer” in the “lme4” package50) and SPSS 25,51 a p value of <0.05 was considered statistically significant. Correction for multiple comparisons was not implemented as per the analysis protocol. The p values should be interpreted keeping this in mind.
Results
Demographics and Clinical Measures for Generation 100 Study and Brain MRI Participants
The participants in the brain MRI sample were slightly healthier than those in the Generation 100 Study at baseline with significantly higher CRF and HDL level, combined with lower TG, HbA1c levels, and HADS score. The brain MRI participants were also slightly younger and had higher educational attainment (Supplementary Table 1).
Clinical and Exercise Characteristics of Brain MRI Participants
At inclusion, there were 53 men and 52 women. Their mean age was 72 years and 64.4% had a university/college education. The total dropout rate after 5 years was 19.0%. The largest drop out was during the first year (10.5%) (Figure 1). Those who withdrew had lower education compared to those who remained in the study (Primary school 20% vs 9%, High school 45% vs 27%, University 40% vs 64%, respectively, p=0.012), but did not differ in other clinical characteristics (data not shown). Two participants (one man and one woman) in the HIIT group died of cancer.
Among participants who remained in the study, there were no differences between the control, MICT and HIIT groups in demographic or clinical characteristics at baseline (Table 1). Similar clinical and demographic characteristics were present in the three groups at 1, 3 and 5 year follow-up (Supplementary Tables 2–4). At 5-year follow up, none of the participants was classified as having dementia (Supplementary Table 4). Adherence across the study period was good for all the three groups, ranging from 71.4 to 94.3%, and there was no significant difference in adherence rate between groups at each time point (Supplementary Table 5).
 |
Table 1 Demographics and Clinical Data for the Control, MICT and HIIT Participants Undergoing Brain MRI at Baseline |
Longitudinal Change in Brain Volumes in the Control, MICT and HIIT Groups
There was limited brain volume loss over time in all groups (Figure 2). Model 1 showed a significant group*time interaction with greater hippocampal atrophy and smaller hippocampal volume in the HIIT compared to the control group at 5 years (Table 2). Over the 5-year intervention period, hippocampal atrophy was −3.9% for control, −5.0% for MICT and −5.5% for HIIT. The estimated average yearly hippocampal atrophy was −0.8% for control, −1.0% for MICT and −1.1% for HIIT.
 |
Table 2 Linear Mixed Model Analyses of Brain Volumes During 5 Years of Intervention (Model 1) in HIIT and MICT Group Compared to Control Group |
 |
Figure 2 Mean and 95% CI for total hippocampal, cortex, thalamus, caudate and white matter volumes (mL) adjusted for ICV at each time point in the control (grey circle), moderate intensity continuous training (MICT) (orange triangle) or high intensity interval training (HIIT) (blue square) groups. See Table 2 and Supplementary Table 7 for results of statistical comparisons. |
The hippocampal subfield analyses likewise revealed a significant group*time interaction with the HIIT group having smaller CA1 volume at 1, 3 and 5 year follow up compared to the control group (Supplementary Table 6). For the hippocampal long axis volumes, a significant group*time interaction was found with HIIT having lower hippocampal body volume at 3-year compared to the control group (Supplementary Table 6). A group*time interaction was present for thalamus with the MICT group having greater atrophy and smaller volume than the control group at 5-year (Figure 2; Table 2). No other group*time effects were present.
Model 2, which included CRF at each time point, showed similar results as Model 1. CRF measurements obtained at each examination were not associated with brain volume at the same time (Supplementary Tables 7 and 8). Similar results were obtained for all the above analyses using linear mixed model without adjusting for baseline values (results not shown).
In the analysis where the MICT&HIIT groups were combined, there was a significant group*time effect with greater hippocampus, thalamus and cortex atrophy in the combined supervised exercise group compared to the control group in both Model 1 and Model 2 (Supplementary Tables 9 and 10). For the hippocampus, greater atrophy was found in CA1 both at 3 and 5 years and in the body at 3 years (Supplementary Tables 11 and 12). No other group*time effects on brain volumes were uncovered (Supplementary Tables 9–12).
CRF Associations with Brain Volumes Across Control, MICT and HIIT Groups
Change in CRF over time in the three groups (Figure 3) showed that CRF was significantly associated with time but not group and there was no group*time interaction (Supplementary Table 13). CRF increased significantly in all groups from baseline to 1 year of intervention. This was followed by a slow decrease across all groups, and at 3 and 5 year follow-up CRF was not significantly different from baseline in any of the groups (Supplementary Table 13). The same result was found when the exercise groups (MICT&HIIT) were combined into one (Supplementary Table 14).
 |
Figure 3 Mean and 95% CI for CRF, objectively measured as VO2peak, at each time point in the control (grey circle), moderate intensity continuous training (MICT) (orange triangle) and high intensity interval training (HIIT) (blue square) groups. See Supplementary Table 13 for results of statistical comparisons. |
Linear regression showed a significant association between CRF at baseline and cortical volume at 1, 3 and 5 years (Table 3). For each VO2peak unit higher at baseline, 1.1 mL of cortex (~0.25% of total cortical volume) was preserved at the end of the intervention (Table 3). However, no localized effects of CRF at baseline on cortical thickness were present at any time point (results not shown). No other associations between CRF and brain volumes were uncovered (Table 3).
 |
Table 3 Linear Regressions Predicting Brain Volumes Across All Participants After 1, 3 and 5 Years of Intervention Based on Baseline CRF |
Discussion
This is the first RCT to investigate the effect of 5 years of exercise intervention at different intensities, on brain volumes in a general population of older adults. Contrary to our hypotheses, 5 years of HIIT or MICT intervention did not lead to larger brain volumes or reduced atrophy at any time point during the study compared to the control group. Rather, we uncovered greater hippocampal atrophy in the HIIT compared to the control group, and thalamic atrophy in the MICT group compared to the control group after 5 years. The greater atrophy rate in the intervention groups became even more notable when analyzing the MICT&HIIT group combined. CRF did not underlay the greater atrophy rate. Indeed, having a higher CRF at baseline was linked to greater cortical volume at all time points across all groups.
This is the first study with an exercise intervention lasting longer than 2 years. The unexpected finding in our study was the negative effect of HIIT and MICT compared to the control group on hippocampal and thalamic volumes, respectively, that emerged during the last part of the 5-year intervention. In the combined MICT&HIIT group, similar effects on both hippocampus and thalamus were observed. Our results are in contrast to previous studies reporting positive or null findings for the effect of exercise on hippocampal and thalamic volumes.12,14,16,18,19,52 However, a decrease in hippocampal volume has been reported in young healthy adults after 6 weeks of HIIT and in patients with schizophrenia compared to controls after 6 months of MICT.20,21 Nevertheless, an overall positive effect in favor of endurance exercise interventions compared to control conditions is reported for the hippocampus in a meta-analysis with adults.27 Including CRF in the statistical model (Model 2) did not change our results. This indicates that HIIT had a negative long-term effect on hippocampal volume irrespective of CRF. Since CRF as well as all other clinical variables previously related to hippocampal volume (eg, anthropomorphic characteristics, blood glucose levels, diabetes, HADS, MoCA, sleep)53 were similar in the three groups at all time points, the most parsimonious interpretation is that HIIT adherence per se caused more notable hippocampal atrophy. The volume loss occurred in the CA1 subfield and the hippocampal body, not in the hippocampal regions previously described as benefitting from exercise intervention in animal models or human studies, such as the dentate gyrus54,55 and the hippocampal head.12 Both CA1 and hippocampal body atrophy are linked to AD.56,57 However, the average hippocampal volume in HIIT at the end of intervention (4.44 ± 0.60% of ICV) was well above the range found in AD (2.88 ± 0.64% of ICV).58 Importantly, the annual hippocampal atrophy rate of 1.1% in the HIIT group is in the range of healthy aging (0.84–1.55% per year).58–61 Indeed, the findings in the HIIT group are not indicative of a pathological process. Rather, the results show that the control group had a very favorable annual hippocampal atrophy rate of 0.8%, which is very low even for healthy aging. Note also that the MoCA scores after 5 years were similar in the control, MICT and HIIT group (Supplementary Table 4), and there was no association between MoCA score and hippocampal volume or interaction between group and MoCA score on hippocampal volume (results not shown). To summarize, the physical activity habits in the control group offered better protection against age-related hippocampal atrophy than HIIT intervention.
There exists evidence for high intensity exercise having a negative effect on the brain, especially in older adults and rodents.24,25 Blood lactate levels are significantly more increased with HIIT than lower training intensities.62 With higher age, the brain’s ability to metabolize lactate in blood which crosses the blood–brain barrier63 is reduced,62 especially under conditions of reduced perfusion.64 Reduced hippocampal perfusion is present in adults over 70 years of age after intense exercise but not in adults under 70 years.13 It is possible that HIIT in the 70+ age group can lead to higher brain lactate levels combined with decreased perfusion of the hippocampus. Lower perfusion leads to lower hippocampal volume over time in humans55 and increased hippocampal lactate levels decrease hippocampal neurogenesis in rodents.65 This could reduce hippocampal volume over time. An alternative explanation is that HIIT is stressful to older adults, as high intensity exercise in animals is found to increase corticosterone levels and reduce hippocampal neurogenesis.66–68 However, since HADS, and the physical and mental component summary score of the quality-of-life questionnaire (SF-8) were similar in all three groups, this seems unlikely.
We also found increased thalamus atrophy in the MICT group compared to the control group after 5 years in both Model 1 and Model 2. The thalamus is far less studied than the hippocampus in the exercise intervention literature, but one study12 reported a non-significant increase in thalamic volume in both an aerobic moderate intensity exercise and a stretching group. Since the thalamus is a major hub for cortical connections and connects to the hippocampus, the reduction in thalamic volume could reflect changes in connected grey matter regions, as well as changes in white matter microstructure. Again, the association was still present when including CRF (Model 2), suggesting that CRF at time of brain MRI was not associated with thalamus volume.
Since CRF is considered a central mechanism for the benefit of exercise training in the brain,5 factors related to CRF at baseline and increasing CRF could be important determinants of intervention success. It has been shown that sedentary people have larger improvements in CRF as a result of exercise intervention than fit individuals69 and also gain more in terms of health benefits.70,71 Thus, individuals with lower baseline CRF might benefit more from exercise intervention with regard to brain structure. However, studies with similar baseline CRF values as the participants in our study report both positive and no effects of exercise intervention on brain structure in older adults.11–14,16,17 It is therefore unlikely that baseline CRF is a key determinant of intervention success in brain structure. Next, the actual change in CRF due to the exercise intervention could provide a positive effect on brain volumes. But intervention studies with no as well as large increases in CRF report both positive and no association between CRF and brain structure over time.11–13,16,17,72 Thus, it seems unlikely that the relatively high CRF level in our participants at baseline and modest CRF increase compared to some other studies11–14,16 can explain the lack of a positive intervention effect on brain volumes.
We expected and observed an increase in CRF in the exercise groups. However, a similar CRF increase in the control group was unforeseen. The control group had significantly increased CRF after 1 year also in the main RCT study, but the HIIT group still had a higher CRF.29 The participants in the brain MRI study had significantly higher CRF than in the main study at baseline; thus, it is possible that those undergoing brain MRI experienced a similar ceiling effect on their CRF increase irrespective of group. It might also be that people in the control group participating in the brain MRI study trained differently from the controls declining to be part of the MRI study. Since we do not have information on the types of activities performed in the control group, this issue cannot be elucidated further.
The MICT intervention and the control group’s physical activity should in theory have similar intensity, but the amount of time spent to fulfill adherence was different (minimum of 75 min physical activity in control group versus minimum of 30 min MICT in MICT group). The dosage (minutes) of physical activity/training per week may thus be important for brain health. Furthermore, the participants in the control group were in control of their exercise routine and could choose the activity type(s) and intensity (eg, golf, dance, skiing, yoga, strength training), how (eg, alone, with training buddy, a team), where (eg, at home, gym), when and duration (eg, number of days, weekday, time of day). Based on this, we speculate that more time spent being physically active performing an activity chosen by the individual is key to better brain health. Further, our results show that diligently following the physical activity guidelines provides a significant positive CRF effect in healthy older adults. Since only about 32% of Norwegian older adults follow today’s guidelines73 compared to 82–94% in this study, a significant potential exists for increasing brain health and CRF for cardiovascular health in the older population.
The fact that CRF increased and decreased very similarly in the control, MICT and HIIT groups might have precluded uncovering group differences. However, our findings do not support a significant role for CRF increase in brain structure in the 70+ age group (Model 2). Still, a higher CRF at baseline was associated with larger cortical volumes at the 1, 3 and 5 year follow-ups, consistent with results from a 9 year follow-up study in older adults74 and results in older master athletes.75 Taken together, increasing CRF per se did not provide significant positive effects on brain structure in adults over the age of 70. Still, entering old age with a higher CRF appeared beneficial across a 5-year period, but only for cortical volume. Given that lower brain parenchymal atrophy rates are considered advantageous in aging, having a high CRF in the 70+ age group is positive for overall brain structural health in the 70+ age group.
Strengths and Limitations
The strengths of this study include the RCT design, a general population-based sample, allocation of participants to different exercise intensities, participants with similar demographic and clinical health profiles allocated to the three groups, low attrition rate and good adherence to assigned group intervention for the entire 5-year period. The intervention was safe, and none of the participants in the MRI study had adverse events during the 5-year intervention. No participants in the RCT Generation 100 Study had adverse cardiovascular events during the supervised training sessions, but three participants got fractures while training on slippery surfaces.29 Other strengths were the repeated clinical, physiological and brain MRI data collection throughout the study period and MRI scans processed using a highly reliable automated method with longitudinal processing to ensure low inter-subject variability. We adjusted the linear mixed model for baseline values to avoid underestimating or overestimating the effect of the intervention,47 but we also re-ran the models without such adjustment to see if the results differed, which they did not. Limitations include the selection into the study of very healthy older adults with high education and very good physical and psychological health. Even in the main RCT Generation 100 Study those included were more active, healthier and had higher education than the people who decided not to participate in the study.28 The brain MRI sample had even higher education and CRF than those not volunteering for MRI. Thus, our study was in a very selected group of fit older adults. The overall mortality rate, ie, the main RCT outcome, was 4.6% across all groups in the main RCT29 compared to 1.9% in the MRI sample, further underscoring that those participating in the MRI study were the healthiest individuals. The number of subjects agreeing to participate in the brain MRI study was low, but based on our power calculation on yearly average hippocampal atrophy rates we needed at least 26 participants to show that the HIIT group (original hypothesis) had an atrophy rate at the lower end61 and the control group at the higher end60 of that reported in the literature available before study start using 90% power and alpha level of 0.05. Furthermore, the sample sizes in the MICT and HIIT groups were comparable to previous studies and based on group differences reported in those studies, our study should also have been able to uncover an effect of intervention on hippocampal volume.12,14 However, a recent critical systematic review reports no effect or small effects of exercise interventions on brain volumes,76 highlighting the difficulty in estimating the sample size in such studies. Nevertheless, we were able to find a significantly greater hippocampal atrophy rate in the HIIT group, a result contrary to our expectations. The three-group design makes the statistical models more complex and increases variability which might make it more difficult to uncover differences. Still, the combined MICT&HIIT group, had increased, but within normative range, atrophy rate of both the hippocampus and thalamus compared to the control group, which reflects a combination of the results in the two groups separately. Another possible limitation is that we did not correct for multiple comparisons, to avoid type II error as this is the first 5 year exercise intervention study with brain MRI. See Rothman77,78 for a discussion on this controversial topic.
To reduce the number of comparisons, we focused on a selection of brain structures regularly reported on as well as understudied in earlier exercise intervention studies. It is possible that there are other brain regions more sensitive to the effects of exercise. The discrepancy between our findings and those reported previously could be due to methodological differences related to participants included (eg, convenience, hospital-based samples), or intervention mode and duration. Shorter interventions report in general positive effects,11–15 while longer interventions report no effects.18,19
Differences related to MRI scanner field strength, scan protocols and analysis can also explain contradictory findings in the literature. For example, positive results on brain structure of exercise intervention are usually reported for MRI scans analyzed with voxel/volume-based morphometry11,12 while no effects with surface-based methods16,18,19 (see also Davatzikos79). Likewise, the statistical models used and covariates included vary greatly between studies. Moreover, control group assignment may have played a role as many previous studies allocate controls to health education, stretching and toning, while the current study had a control group which advised to follow the national physical activity guidelines of at least 30 minutes of physical activity every day. Lastly, increasing CRF and subsequently maintaining CRF during the intervention appeared to be difficult even in fit older adults as here. Indeed, those who volunteered for brain MRI had a high CRF level at inclusion compared to many previous exercise intervention studies.11–14,16,17 Nevertheless, their CRF level was similar to that of Norwegians in their 70s from another general population study,80 indicating that the findings in our study are generalizable to healthy older Norwegian adults who participated in similar studies. Across all groups, CRF only increased during the first year of the intervention and then declined slowly to baseline level at 5 years. This finding might be due to the expected age-dependent decline in CRF as physical activity does not seem to fully counteract the accelerated decline of CRF with increasing age.81
Conclusions
In participants from the Generation 100 Study who volunteered for brain MRI, the individuals in the control group emerged as those with the lowest hippocampal and thalamic atrophy rate, well below that reported in typical aging previously. The greater, but within normal range, hippocampal atrophy in the HIIT and combined MICT&HIIT group is not easily explained but appears to be connected to group assignment. CRF at baseline was associated with greater cortical volume at the end of the intervention, but CRF during the intervention was not linked to brain volumes. Thus, efforts should be directed at increasing CRF before age 70, and then maintaining it through daily physical activity as implemented by the participants in the control group.
Future Directions
In future studies, it would be interesting to investigate the dose–response effect of physical activity and exercising on brain health, and to explore whether there is a ceiling effect of exercise on brain volume. Moreover, the biological mechanisms underpinning the positive and potentially negative effects of exercising need to be determined, cf. the relationship between intensity of exercise, lactate and brain health outcomes. Finally, based on the positive outcome in the control compared to the supervised exercise groups in our study, it is of interest to elucidate the physical activity/exercising habits providing this beneficial effect.
Abbreviations
AD, Alzheimer’s disease; BMI, body mass index; CRF, cardiorespiratory fitness; CRP, C-reactive protein; DBP, diastolic blood pressure; FOV, field of view; HADS, hospital anxiety and depression scale; HbA1c, glycated hemoglobin; HDL, high density lipoprotein cholesterol; HIIT, high intensity interval training; ICV, intracranial volume; LDL, low-density lipoprotein cholesterol; MICT, moderate intensity interval training; MoCA, Montreal Cognitive Assessment; MRI, magnetic resonance imaging; RCT, randomized controlled trial; RHR, resting heart rate; SBP, systolic blood pressure; SF-8, Short Form health survey questionnaire; TC, total cholesterol; TE, echo time; TG, triglycerides; TR, repetition time.
Data Sharing Statement
Because privacy concerns and state regulations, the ethical and governance approvals for this study do not allow the MRI data to be made available in a public repository. Data in this manuscript can be accessed by qualified investigators after ethical and scientific review (to ensure the data is being requested for valid scientific research) and must comply with the European Union General Data Protection Regulations (GDPR), Norwegian laws and regulations, and NTNU regulations. The completion of a material transfer agreement (MTA) signed by an institutional official will be required.
Acknowledgments
The authors thank all the participants for taking part in the study. We thank Torill E Sjøbakk for help with the recruitment, our students Hanne Nikkels and Stine Bjøralt for help with data collection and the radiographers at 3T scanner. Testing of VO2peak was performed at the core facility NeXt Move, Norwegian University of Science and Technology (NTNU). All other clinical measurements were performed at the Clinical Research Facility, St. Olavs Hospital.
Author Contributions
All authors made a significant contribution to the present article, either in one, more than one or all the following areas: conception of the study, acquisition of the data, analysis, interpretation of the results, drafting and critically reviewing the manuscript. All authors revised and critically reviewed the manuscript. There was a joint agreement on which journal the manuscript was submitted to, and all the authors agree to take responsibility and be accountable for the content of this article.
Funding
The Generation 100 Study was supported by the Research Council of Norway, the K.G. Jebsen foundation for medical research, Norway, Norwegian University of Science and Technology (NTNU), Central Norway Regional Health Authority, St. Olavs Hospital, Trondheim, Norway, and the National Association for Public Health, Norway. The brain MR acquisition was supported by Norwegian Advisory Unit for fMRI, Department of Radiology and Nuclear Medicine, St. Olavs Hospital, Trondheim.
Disclosure
The authors have no conflicts of interest.
References
1. Yiannopoulou KG, Anastasiou AI, Zachariou V, Pelidou SH. Reasons for failed trials of disease-modifying treatments for Alzheimer disease and their contribution in recent research. Biomedicines. 2019;7(4):97. doi:10.3390/biomedicines7040097
2. Alty J, Farrow M, Lawler K. Exercise and dementia prevention. Pract Neurol. 2020;20(3):234–240. doi:10.1136/practneurol-2019-002335
3. Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011;10(9):819–828. doi:10.1016/S1474-4422(11)70072-2
4. Tari AR, Nauman J, Zisko N, et al. Temporal changes in cardiorespiratory fitness and risk of dementia incidence and mortality: a population-based prospective cohort study. Lancet Public Health. 2019;4(11):e565–e574. doi:10.1016/S2468-2667(19)30183-5
5. Voss MW. The chronic exercise–cognition interaction: fMRI research. In: McMorris T, editor. Exercise-Cognition Interaction. London, UK: Elsevier Academic Press; 2016:187–209.
6. Gordon BA, Rykhlevskaia EI, Brumback CR, et al. Neuroanatomical correlates of aging, cardiopulmonary fitness level, and education. Psychophysiology. 2008;45(5):825–838.
7. Erickson KI, Prakash RS, Voss MW, et al. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus. 2009;19(10):1030–1039. doi:10.1002/hipo.20547
8. Verstynen TD, Lynch B, Miller DL, et al. Caudate nucleus volume mediates the link between cardiorespiratory fitness and cognitive flexibility in older adults. J Aging Res. 2012;2012:1–11. doi:10.1155/2012/939285
9. Williams VJ, Hayes JP, Forman DE, et al. Cardiorespiratory fitness is differentially associated with cortical thickness in young and older adults. Neuroimage. 2017;146:1084–1092. doi:10.1016/j.neuroimage.2016.10.033
10. Zotcheva E, Pintzka CWS, Salvesen Ø, Selbaek G, Håberg AK, Ernstsen L. Associations of changes in cardiorespiratory fitness and symptoms of anxiety and depression with brain volumes: the HUNT Study. Front Behav Neurosci. 2019;13:53. doi:10.3389/fnbeh.2019.00053
11. Colcombe SJ, Erickson KI, Scalf PE, et al. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci. 2006;61(11):1166–1170. doi:10.1093/gerona/61.11.1166
12. Erickson KI, Voss MW, Prakash RS, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci USA. 2011;108(7):3017–3022. doi:10.1073/pnas.1015950108
13. Maass A, Duzel S, Goerke M, et al. Vascular hippocampal plasticity after aerobic exercise in older adults. Mol Psychiatry. 2015;20(5):585–593. doi:10.1038/mp.2014.114
14. Niemann C, Godde B, Voelcker-Rehage C. Not only cardiovascular, but also coordinative exercise increases hippocampal volume in older adults. Front Aging Neurosci. 2014;6:170. doi:10.3389/fnagi.2014.00170
15. Tao J, Liu J, Liu W, et al. Tai Chi Chuan and Baduanjin increase grey matter volume in older adults: a brain imaging study. J Alzheimers Dis. 2017;60(2):389–400. doi:10.3233/JAD-170477
16. Jonasson LS, Nyberg L, Kramer AF, Lundquist A, Riklund K, Boraxbekk CJ. Aerobic exercise intervention, cognitive performance, and brain structure: results from the physical influences on brain in aging (PHIBRA) Study. Front Aging Neurosci. 2017;8:336. doi:10.3389/fnagi.2016.00336
17. Matura S, Fleckenstein J, Deichmann R, et al. Effects of aerobic exercise on brain metabolism and grey matter volume in older adults: results of the randomised controlled SMART trial. Transl Psychiatry. 2017;7(7):e1172. doi:10.1038/tp.2017.135
18. Stephen R, Liu Y, Ngandu T, et al. Brain volumes and cortical thickness on MRI in the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER). Alzheimers Res Ther. 2019;11(1):53. doi:10.1186/s13195-019-0506-z
19. Venkatraman VK, Sanderson A, Cox KL, et al. Effect of a 24-month physical activity program on brain changes in older adults at risk of Alzheimer’s disease: the AIBL active trial. Neurobiol Aging. 2020;89:132–141. doi:10.1016/j.neurobiolaging.2019.02.030
20. Scheewe TW, van Haren NE, Sarkisyan G, et al. Exercise therapy, cardiorespiratory fitness and their effect on brain volumes: a randomised controlled trial in patients with schizophrenia and healthy controls. Eur Neuropsychopharmacol. 2013;23(7):675–685. doi:10.1016/j.euroneuro.2012.08.008
21. Wagner G, Herbsleb M, de la Cruz F, et al. Hippocampal structure, metabolism, and inflammatory response after a 6-week intense aerobic exercise in healthy young adults: a controlled trial. J Cereb Blood Flow Metab. 2015;35(10):1570–1578. doi:10.1038/jcbfm.2015.125
22. Milanović Z, Sporiš G, Weston M. Effectiveness of high-intensity interval training (HIT) and continuous endurance training for VO 2max improvements: a systematic review and meta-analysis of controlled trials. Sports Med. 2015;45(10):1469–1481. doi:10.1007/s40279-015-0365-0
23. Swain DP, Franklin BA. Comparison of cardioprotective benefits of vigorous versus moderate intensity aerobic exercise. Am J Cardiol. 2006;97(1):141–147. doi:10.1016/j.amjcard.2005.07.130
24. Calverley TA, Ogoh S, Marley CJ, et al. HIITing the brain with exercise: mechanisms, consequences and practical recommendations. J Physiol. 2020;598(13):2513–2530. doi:10.1113/JP275021
25. Lucas SJ, Cotter JD, Brassard P, Bailey DM. High-intensity interval exercise and cerebrovascular health: curiosity, cause, and consequence. J Cereb Blood Flow Metab. 2015;35(6):902–911. doi:10.1038/jcbfm.2015.49
26. Chen FT, Hopman RJ, Huang CJ, et al. The effect of exercise training on brain structure and function in older adults: a systematic review based on evidence from randomized control trials. J Clin Med. 2020;9(4):914. doi:10.3390/jcm9040914
27. Firth J, Stubbs B, Vancampfort D, et al. Effect of aerobic exercise on hippocampal volume in humans: a systematic review and meta-analysis. Neuroimage. 2018;166:230–238. doi:10.1016/j.neuroimage.2017.11.007
28. Stensvold D, Viken H, Rognmo O, et al. A randomised controlled study of the long-term effects of exercise training on mortality in elderly people: study protocol for the Generation 100 study. BMJ Open. 2015;5(2):e007519. doi:10.1136/bmjopen-2014-007519
29. Stensvold D, Viken H, Steinshamn SL, et al. Effect of exercise training for five years on all cause mortality in older adults—the Generation 100 study: randomised controlled trial. BMJ. 2020;371:m3485. doi:10.1136/bmj.m3485
30. Borg G. Ratings of perceived exertion and heart rates during short-term cycle exercise and their use in a new cycling strength test. Int J Sports Med. 1982;3(3):153–158. doi:10.1055/s-2008-1026080
31. Ware JE, Kosinski M, Dewey JE, Gandek B. How to score and interpret single-item health status measures: a manual for users of the SF-8 health survey. 2001;15(10):5. Lincoln, RI: QualityMetric Incorporated. Available from: https://www.worldcat.org/title/how-to-score-and-interpret-single-item-health-status-measures-a-manual-for-users-of-the-of-the-sf-8-health-survey-with-a-supplement-on-the-sf-6-health-survey/oclc/47005803.
32. Mykletun A, Stordal E, Dahl AA. Hospital Anxiety and Depression (HAD) scale: factor structure, item analyses and internal consistency in a large population. Br J Psychiat. 2001;179:540–544. doi:10.1192/bjp.179.6.540
33. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi:10.1111/j.1600-0447.1983.tb09716.x
34. Bjerkeset O, Mykletun A, Dahl AA, Linaker O. Mortality in relation to self-reported mixed anxiety and depression symptoms–the HUNT study. Nord J Psychiatry. 2007;61(1):6–11. doi:10.1080/08039480601121926
35. Haug TT, Mykletun A, Dahl AA. The association between anxiety, depression, and somatic symptoms in a large population: the HUNT-II study. Psychosom Med. 2004;66(6):845–851. doi:10.1097/01.psy.0000145823.85658.0c
36. Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi:10.1111/j.1532-5415.2005.53221.x
37. Borland E, Nägga K, Nilsson PM, Minthon L, Nilsson ED, Palmqvist S. The Montreal Cognitive Assessment: normative data from a large Swedish population-based cohort. J Alzheimers Dis. 2017;59(3):893–901. doi:10.3233/JAD-170203
38. Fischl B. FreeSurfer. Neuroimage. 2012;62(2):774–781. doi:10.1016/j.neuroimage.2012.01.021
39. Reuter M, Schmansky NJ, Rosas HD, Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage. 2012;61(4):1402–1418. doi:10.1016/j.neuroimage.2012.02.084
40. Iglesias JE, Van Leemput K, Augustinack J, Insausti R, Fischl B, Reuter M. Bayesian longitudinal segmentation of hippocampal substructures in brain MRI using subject-specific atlases. Neuroimage. 2016;141:542–555. doi:10.1016/j.neuroimage.2016.07.020
41. Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–194. doi:10.1006/nimg.1998.0395
42. Iglesias JE, Augustinack JC, Nguyen K, et al. A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: application to adaptive segmentation of in vivo MRI. Neuroimage. 2015;115:117–137. doi:10.1016/j.neuroimage.2015.04.042
43. Mueller SG, Yushkevich PA, Das S, et al. Systematic comparison of different techniques to measure hippocampal subfield volumes in ADNI2. Neuroimage Clin. 2018;17:1006–1018. doi:10.1016/j.nicl.2017.12.036
44. McHugo M, Talati P, Woodward ND, Armstrong K, Blackford JU, Heckers S. Regionally specific volume deficits along the hippocampal long axis in early and chronic psychosis. Neuroimage Clin. 2018;20:1106–1114. doi:10.1016/j.nicl.2018.10.021
45. Hansen TI, Brezova V, Eikenes L, Håberg AK, Vangberg TR. How does the accuracy of intracranial volume measurements affect normalized brain volumes? Sample size estimates based on 966 subjects from the HUNT MRI Cohort. AJNR Am J Neuroradiol. 2015;36(8):1450–1456. doi:10.3174/ajnr.A4299
46. Pintzka CWS, Hansen TI, Evensmoen HR, Håberg AK. Marked effects of intracranial volume correction methods on sex differences in neuroanatomical structures: a HUNT MRI study. Front Neurosci. 2015;9:238. doi:10.3389/fnins.2015.00238
47. Twisk J, Bosman L, Hoekstra T, Rijnhart J, Welten M, Heymans M. Different ways to estimate treatment effects in randomised controlled trials. Contemp Clin Trial Commun. 2018;10:80–85. doi:10.1016/j.conctc.2018.03.008
48. Grasby KL, Jahanshad N, Painter JN, et al. The genetic architecture of the human cerebral cortex. Science. 2020;367(6484). doi:10.1126/science.aay6690
49. R core team (2019). R: A language and environment for statistical computing [computer program]. Vienna, Austria: R Foundation for Statistical Computing; 2019. Available from: http://www.r-project.org/Ref.
50. Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67(1):1–48. doi:10.18637/jss.v067.i01
51. IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp.
52. Rosano C, Guralnik J, Pahor M, et al. Hippocampal response to a 24-month physical activity intervention in sedentary older adults. Am J Geriatr Psychiatry. 2017;25(3):209–217. doi:10.1016/j.jagp.2016.11.007
53. Bartsch T, Wulff P. The hippocampus in aging and disease: from plasticity to vulnerability. Neuroscience. 2015;309:1–16. doi:10.1016/j.neuroscience.2015.07.084
54. Bechara R, Kelly A. Exercise improves object recognition memory and induces BDNF expression and cell proliferation in cognitively enriched rats. Behav Brain Res. 2013;245:96–100. doi:10.1016/j.bbr.2013.02.018
55. Pereira AC, Huddleston DE, Brickman AM, et al. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci USA. 2007;104(13):5638–5643. doi:10.1073/pnas.0611721104
56. Greene SJ, Killiany RJ. Hippocampal subregions are differentially affected in the progression to Alzheimer’s disease. Anat Rec. 2012;295(1):132–140. doi:10.1002/ar.21493
57. Lindberg O, Walterfang M, Looi JC, et al. Hippocampal shape analysis in Alzheimer’s disease and frontotemporal lobar degeneration subtypes. J Alzheimers Dis. 2012;30(2):355–365. doi:10.3233/JAD-2012-112210
58. Jack CR
59. Ardekani BA, Convit A, Bachman AH. Analysis of the MIRIAD data shows sex differences in hippocampal atrophy progression. J Alzheimers Dis. 2016;50(3):847–857. doi:10.3233/JAD-150780
60. Barnes J, Ourselin S, Fox NC. Clinical application of measurement of hippocampal atrophy in degenerative dementias. Hippocampus. 2009;19(6):510–516. doi:10.1002/hipo.20617
61. Fjell AM, Walhovd KB, Fennema-Notestine C, et al. One-year brain atrophy evident in healthy aging. J Neurosci. 2009;29(48):15223–15231. doi:10.1523/JNEUROSCI.3252-09.2009
62. Quistorff B, Secher NH, Van Lieshout JJ. Lactate fuels the human brain during exercise. FASEB J. 2008;22(10):3443–3449. doi:10.1096/fj.08-106104
63. Qu H, Håberg AK, Haraldseth O, Unsgård G, Sonnewald U. 13C MR spectroscopy study of lactate as substrate for rat brain. Dev Neurosci. 2000;22(5–6):429–436. doi:10.1159/000017472
64. Vestergaard MB, Jensen ML, Arngrim N, Lindberg U, Larsson HB. Higher physiological vulnerability to hypoxic exposure with advancing age in the human brain. J Cereb Blood Flow Metab. 2018;40(2):341–353. doi:10.1177/0271678X18818291
65. Kemppainen J, Aalto S, Fujimoto T, et al. High intensity exercise decreases global brain glucose uptake in humans. J Physiol. 2005;568(1):323–332. doi:10.1113/jphysiol.2005.091355
66. Inoue K, Okamoto M, Shibato J, et al. Long-term mild, rather than intense, exercise enhances adult hippocampal neurogenesis and greatly changes the transcriptomic profile of the hippocampus. PLoS One. 2015;10(6):e0128720.
67. Shih PC, Yang YR, Wang RY. Effects of exercise intensity on spatial memory performance and hippocampal synaptic plasticity in transient brain ischemic rats. PLoS One. 2013;8(10):e78163. doi:10.1371/journal.pone.0078163
68. Soya H, Mukai A, Deocaris CC, et al. Threshold-like pattern of neuronal activation in the hypothalamus during treadmill running: establishment of a minimum running stress (MRS) rat model. Neurosci Res. 2007;58(4):341–348. doi:10.1016/j.neures.2007.04.004
69. Storen O, Helgerud J, Saebo M, et al. The effect of age on the v o2max response to high-intensity interval training. Med Sci Sports Exerc. 2017;49(1):78–85. doi:10.1249/MSS.0000000000001070
70. Füzéki E, Banzer W. Physical activity recommendations for health and beyond in currently inactive populations. Int J Environ Res Public Health. 2018;15(5):1042. doi:10.3390/ijerph15051042
71. Kodama S, Saito K, Tanaka S, et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA. 2009;301(19):2024–2035. doi:10.1001/jama.2009.681
72. Niemann C, Godde B, Staudinger UM, Voelcker-Rehage C. Exercise-induced changes in basal ganglia volume and cognition in older adults. Neuroscience. 2014;281:147–163. doi:10.1016/j.neuroscience.2014.09.033
73. Hansen BH, Anderssen SA, Steene-Johannessen J, et al. Fysisk Aktivitet Og Sedat Tid Blant Voksne Og Eldre I Norge - Nasjonal Kartlegging 2014–2015 [Physical activity and sedentary time among adults and older adults in Norway - A national survey 2014-2015]; 2015:IS–2367.
74. Erickson KI, Raji CA, Lopez OL, et al. Physical activity predicts gray matter volume in late adulthood: the Cardiovascular Health Study. Neurology. 2010;75(16):1415–1422. doi:10.1212/WNL.0b013e3181f88359
75. Wood KN, Nikolov R, Shoemaker JK. Impact of long-term endurance training vs. guideline-based physical activity on brain structure in healthy aging. Front Aging Neurosci. 2016;8:155. doi:10.3389/fnagi.2016.00155
76. Hvid LG, Harwood DL, Eskildsen SF, Dalgas U. A critical systematic review of current evidence on the effects of physical exercise on whole/regional grey matter brain volume in populations at risk of neurodegeneration. Sports Med. 2021:1–21. doi:10.1007/s40279-021-01453-6
77. Rothman KJ. Six persistent research misconceptions. J Gen Intern Med. 2014;29(7):1060–1064. doi:10.1007/s11606-013-2755-z
78. Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1(1):43–46. doi:10.1097/00001648-199001000-00010
79. Davatzikos C. Why voxel-based morphometric analysis should be used with great caution when characterizing group differences. Neuroimage. 2004;23(1):17–20. doi:10.1016/j.neuroimage.2004.05.010
80. Loe H, Rognmo Ø, Saltin B, Wisløff U. Aerobic capacity reference data in 3816 healthy men and women 20–90 years. PLoS One. 2013;8(5):e64319. doi:10.1371/journal.pone.0064319
81. Fleg JL, Morrell CH, Bos AG, et al. Accelerated longitudinal decline of aerobic capacity in healthy older adults. Circulation. 2005;112(5):674–682. doi:10.1161/CIRCULATIONAHA.105.545459
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.