Back to Journals » OncoTargets and Therapy » Volume 12
EFEMP2 Inhibits Breast Cancer Invasion And Metastasis In Vitro And In Vivo
Authors Kang N, Zhou J, Xu J, Zhou D, Shi W
Received 28 June 2019
Accepted for publication 4 October 2019
Published 30 October 2019 Volume 2019:12 Pages 8915—8933
DOI https://doi.org/10.2147/OTT.S221219
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Takuya Aoki
Ning Kang,1 Jijun Zhou,2 Jia Xu,1 Dongsheng Zhou,1 Weichen Shi1
1Department of Breast and Thyroid Surgery, Shandong Provincial Qianfoshan Hospital, The First Hospital Affiliated with Shandong First Medical University, Jinan 250013, People’s Republic of China; 2Department of General Surgery, The People’s Hospital of Chengwu, Chengwu 274200, People’s Republic of China
Correspondence: Ning Kang
Department of Breast and Thyroid Surgery, Shandong Provincial Qianfoshan Hospital, The First Hospital Affiliated with Shandong First Medical University, Jinan 250013, People’s Republic of China
Email [email protected]
Background: EGF-containing fibulin-like extracellular matrix protein 2 (EFEMP2) is an extracellular matrix (ECM) glycoprotein, which is regarded as potential prognostic biomarkers in some carcinoma. Little is known about the association of EFEMP2 and breast cancer.
Methods: EFEMP2 expressions in normal breast tissue, benign fibroadenoma, breast cancer, the normal mammary epithelial cell line, and 4 different invasive breast cancer cell lines were evaluated by immunohistochemistry (IHC) or immunocytochemistry (ICC) and real time quantitative reverse transcriptase-polymerase chain reaction (RT-qPCR). Expression and prognostic value of EFEMP2 in breast cancer were verified by the Public databases (Oncomine and Kaplan-Meier plotter database). Lentiviral vector with EFEMP2 cDNA was constructed and used to infect breast cancer cell lines to investigate the effects of EFEMP2 on the biological behavior of breast cancer cells by functional in vitro and in vivo assays.
Results: Down-regulated EFEMP2 expression was found in breast cancer tissues and cells, and low expression of EFEMP2 was associated with poor prognosis in patients with breast cancer. Analysis by the Public database leaded to the same conclusion. Up-regulated EFEMP2 expression significantly hampered the invasion and metastasis abilities of breast cancer cells and the process of epithelial interstitial transformation (EMT) via the Wnt/β-catenin pathway.
Conclusion: EFEMP2 expression was lower in breast cancer and closely related to the prognosis of patients, its anti-oncogenic roles indicated the underlying therapeutic target for the future treatment of breast cancer.
Keywords: EFEMP2, breast cancer, invasion, metastasis, EMT, Wnt/β-catenin signaling pathway
Background
Breast cancer (BC) is the most common malignant tumor and the second most frequent cause of cancer-related mortality in women.1 In 2012, approximately 521,907 breast cancer deaths occurred worldwide, according to the global cancer project (GLOBOCAN, 2012). In the United States, the lifetime incidence of advanced breast cancer (ABC) in women is approximately 12%, and based on this estimate, more than 266,120 new cases of ABC are expected in 2018.2 Currently, breast cancer treatment consists of comprehensive therapies including surgery, radiotherapy, chemotherapy, endocrine therapy, and biological targeting agents. With improvement in diagnostic techniques and treatment therapeutics, breast cancer related mortality is declining with an overall rise in ABC survival. However, advanced breast cancer is currently virtually incurable and the main objective of the treatment is palliative care, focused on controlling the rapid progression of the disease, improving quality of life, and prolonging survival. Using molecular biotechnology techniques, a few genetic markers, such as breast cancer type 1 (BRCA1), breast cancer type 2 (BRCA2), and deleted in breast cancer 1 (DBC-1), have been established,3 but specific prognostic and therapeutic BC markers have not been observed. Multiple new, targeted drugs have been developed to treat patients with ABC; however, optimal therapies are yet to be determined. Thus, it is necessary to study the mechanism of invasion and metastasis of breast cancer and to find new therapeutic targets.
EGF-containing fibulin-like extracellular matrix protein 2 (EFEMP2) is a protein that is encoded by the EFEMP2 gene in humans. EFEMP2 (also known as MBP1 or fibulin-4) is relatively small in size (50–60 kDa), contains five tandem repeats of calcium-binding epidermal growth factor-like (cbEGF) modules, a unique fibulin C-terminal domain, and belongs to a seven-member fibulin family of extracellular matrix (ECM) glycoproteins.4–6 EFEMP2 plays an essential role in elastogenesis, as EFEMP2 knockout mice die perinatally from vascular abnormalities, such as the severe rupture of intact elastic fiber assembly.7–9 In addition to the significance of EFEMP2 in elastic fiber formation, recent work has focused on its relation to cancer. In certain kinds of cancer,10–14 EFEMP2 is a candidate oncogene with high expression, suggesting poorer prognosis. Conversely, in prostate cancer and endometrial carcinoma, EFEMP2 was identified as an anti-oncogene where it played a protective role in suppressing cancer progression.15,16 Research on the association between EFEMP2 and cancer is in its infancy, as little is known about the underlying oncogenic or anti-oncogenic functions of EFEMP2 in human breast cancer. In this study, we aimed to clarify the effects of EFEMP2 on the progression of breast cancer and to determine the roles of EFEMP2 in the proliferation, invasion, and metastasis of breast cancer cells.
Methods
Cell Lines
MDA-MB-468, BT474, BT549, MCF-7 (human breast cancer cell lines), and HBL-100 (normal breast epithelial cell line) were obtained from the cell bank of typical culture preservation committee of the Chinese Academy of Sciences. All cells were cultured in Gibco™ Dulbecco’s Modified Eagle Medium: Nutrient Mixture F-12 (DMEM/F-12) media supplemented with 10% Gibco™ fetal bovine serum (FBS, Australia origin) and 1% Gibco® Antibiotic-Antimycotic (10,000 units/mL of penicillin, 10,000 µg/mL of streptomycin, and 25 µg/mL of Gibco Amphotericin B), and kept under 37 °C and at 5% CO2.
Tumor Tissues Samples
With the informed consent of the patients, 375 breast tissue specimens were obtained from Qilu Hospital, Shandong Province Hospital and Qianfo Hill Hospital of Shandong Province between 2005 and 2015. These samples consisted of 50 normal breast tissue, 60 breast fibroadenomas, and 265 breast cancer samples. All breast cancer patients had their disease stage diagnosed according to the 8th edition of the American Joint Committee on Cancer (AJCC) tumor-node-metastasis (TNM) staging system, and none of the patients had received preoperative radiation or chemotherapy. This study was approved by the Institutional Medical Ethics Committee of Shandong University. All specimens were reviewed by two pathologists.
Immunohistochemistry And Immunocytochemistry
For immunohistochemistry (IHC) of the samples, formalin-fixed, paraffin-embedded tissue blocks were cut into 5-μm thick sections. After deparaffinization in xylene and rehydration in gradient ethanol, antigen retrieval was performed in antigen repair solution (0.01 M citrate buffer, pH 6.0) at high pressure for 1–2 min. After cooling to room temperature, tissue sections were washed with phosphate buffer saline (PBS) 3 times, and then stained with the selected streptavidin–biotin–peroxidase (SP) staining method. For immunocytochemistry (ICC) of the samples, the cells, when in the logarithmic growth phase, were digested by trypsin, suspended in the complete culture medium, and seeded into cell culture dishes covered with coverslips. After 24 h of adhesion, cells were fixed with 4% paraformaldehyde for 30 min, washed with PBS 3 times, and then stained by the same SP staining method as for the IHC. The procedures of SP staining were as follows: All sections and coverslips were treated with 3% H2O2 to extinguish endogenous peroxidase activity and incubated with normal goat serum to block intrinsic non-specific biotin-binding. Then, rabbit monoclonal antibody against human EFEMP2 (ab125073, Abcam) incubation was performed at 4°C overnight and followed by incubation with anti-mouse secondary antibody for 30 min at room temperature. Color development was performed with 3′,3-diaminobenzidine tetrahydrochloride (DAB reagent kit, ZSGB-BIO, ZLI-9017, China) and hematoxylin counterstaining. The presence of brown granules in the cytoplasm or stroma was identified as positive expression of EFEMP2. For the negative control, the primary antibody was replaced by normal serum.
Evaluation Of The Staining Results Of IHC And ICC
To evaluate the EFEMP2 staining results in IHC and ICC, a semiquantitative grading system based on staining intensity and number of positive cells was used in this study.17 The positive staining intensity was scored from 0 to 3 (no stain scored 0, week stain scored 1, medium stain scored 2 and strong stain scored 3). The percentage of positively stained cells relative to the entire detection area cells was scored from 0 to 4 (0% positive cells scored 0, 1–25% positive cells scored 1, 26–50% positive cells scored 2, 51–75% positive cells scored 3 and 76–100% stained cells scored 4). Combining the positive staining intensity score with the positive staining cell percentage score gave the total semi-quantitative grading system score of 0–7. To facilitate statistical analysis, the total score of slices with low EFEMP2 expression is less than or equal to 3. The total score of slices with high EFEMP2 expression is greater than or equal to 4. All slices were blindly evaluated by two researchers, and if there was a difference in the score, a consensus was reached by discussion or by inviting a third expert to arbitrate.
Oncomine And Kaplan–Meier Plotter Analysis
Oncomine (https://www.oncomine.org) is the world’s largest oncogene chip database and integrated data mining platform, aimed at exploring genetic information about cancer. We used this tool to compare EFEMP2 expression between clinical breast cancer specimens and normal control samples. A Kaplan–Meier plotter (http://kmplot.com/analysis/) was used to assess the relevance of gene expression on clinical outcomes of breast cancer patients. A background database was established using the gene expression data and relapse free and overall survival information downloaded from the Gene Expression Omnibus (GEO) (Affymetrix HGU133A and HGU133+2 microarrays), European Genome-phenome Archive (EGA) and the Cancer Genome Analysis (TCGA).18 This online tool was used to assess the correlation between EFEMP2 expression and breast cancer patient survival.
Total RNA Extraction And Real-Time Quantitative Reverse Transcription Polymerase Chain Reaction (RT-qPCR)
RNAiso Plus (TaKaRa) was used to get total RNA, then reverse transcription was performed to generate complementary DNA (cDNA) using the PrimeScriptTM RT reagent Kit with gDNA Eraser (TaKaRa). Real Time PCR was performed using the TB Green™ Premix Ex Taq™ II kit (TaKaRa) by LightCycler/LightCycler 480 System (Roche Diagnostics). The volume of each PCR reaction system was 20 µl, and it consisted of 10 µl TB Green Premix Ex Taq II (Tli RNaseH Plus), 0.8 µl of each primer (forward and reverse primers, final concentration 0.4 µM), 2 µl cDNA and 6.4 µl sterile water. RT-qPCR was performed according to the manufacturer’s instructions. Takara Biotechnology Co., Ltd designed and synthesized the forward and reverse primers. The primer sequences are described in Table 1. The convenient 2(-Delta C(T)) method was used for the analysis of RT-qPCR data.19
 |
Table 1 The Sequence Of Primer In RT-qPCR |
Western Blotting
When 90% confluency was attained, the cells were collected by trypsin digestion, and lysed by radioimmunoprecipitation assay (RIPA) lysis buffer with phenylmethylsulfonyl fluoride (PMSF) solution (RIPA:PMSF=100:1), in an ice bath for 30 min. While in the ice bath, the cells were shaken every 5 min to ensure complete cell lysis. After centrifugation at 4 °C and 12,000 rpm for 30 min, the supernatant was carefully absorbed into a new EP tube, and then the protein concentration was determined using the BCA Protein Assay Kit (Solarbio). The supernatant volume was recorded as V, and 1/3 V of 4× loading buffer was added into the EP tube. Then, the tube was immersed in a metal bath at 100 °C for 10 min and was stored in a −20 °C refrigerator for short-term use. Protein samples (40 µg/lane) were separated using 10% sodium dodecyl sulfate polyacrylamide gel (SDS-PAGE) electrophoresis. The electrophoretic separated protein was transferred to a polyvinylidene difluoride (PVDF) membrane. After blocking with 5% bovine serum albumin (BSA) for 1 h, the membrane was incubated with primary antibodies (the dilution ratio was 1: 1000) at 4 °C in a shaker overnight. The following day, after washing with tris-buffered saline with Tween 20 (TBST), the membrane was incubated with the corresponding secondary antibodies at room temperature for 1 h. The enhanced chemiluminescence (ECL) method by the Bio-Rad ECL kit (Solarbio) was performed to develop the positive blots of the proteins on the membrane, and the gray-scale value of the images was measured by Image J (National Institutes of Health, USA).
Lentiviral Transfection For Up-Expression Of EFEMP2
Construction of the lentivirus EFEMP2 cDNA vector was performed by Genechem Co. (Shanghai, China). In order to explain the role of EFEMP2 in breast cancer cell growth and invasion, the expression of EFEMP2 in breast cancer cells MCF-7 and BT-474 were upregulated by lentivirus infection. According to the experimental instructions provided by Genechem Co., before transfection, logarithmic growth cells were conventionally cultured in a 24-well plate for 24 h. Virus quantity was calculated with an MOI (MOI, average virus active units/cell at transfection) value of 100. After 12 h, virus mixed culture medium was replaced with fresh medium with 10% FBS. After 72 h of conventional culture, the infection efficiency was examined under a fluorescence microscope. The changes of EFEMP2 gene expression after transfection were detected by RT-qPCR and Western blotting.
Cell Growth Curve Assay
A cell growth curve is an important index for determining cell viability. Cells that grew to almost complete confluency, were digested with trypsin and suspended in fresh medium. Cells were enumerated and inoculated in a 24-well plate (1× 104/well). After 24 h, the average cell count per day was calculated for 3 wells and recorded for 7 consecutive days. The growth curves were drawn from the results of the cell counts.
Cell Plate Clone Formation Assay
The ability of a cell to form clones reflects two significant characteristics: cell population dependence and proliferation ability. Not every adherent cell can proliferate and clone, but the cloned cell must be adherent and proliferative. Monolayer cells in the logarithmic growth period were sampled and digested with 0.25% trypsin to form a single cell suspension. After multiple dilutions of the cell suspension, 500 cells were inoculated into a 6-well plate and gently rotated to disperse evenly. The plate was placed in a 37 °C, 5% CO2 and saturated humidity cell incubator for 2 weeks. After the 2 weeks, when the clones were visible to the naked eye, clones were fixed for 30 mins using 4% paraformaldehyde. The clones were then stained for 10 min using hematoxylin. After washing the plate under a gentle stream of water and air drying, the plate was inverted and covered with a transparent film with mesh. The clones were directly enumerated by counting with the naked eye.
Boyden Chamber Invasion And Migration Assay
The Boyden chamber consists of two compartments separated by a polycarbonate membrane containing uniformly sized pores of 8.0 µm. The Boyden chamber assay is a widely accepted and useful technology to study chemotaxis of leukocytes or other migratory cells. For the Boyden chamber migration assay, 200 µl serum-free medium containing 2×105 cells was placed in the upper compartment and complete medium with 20% FBS was added to the lower compartment. After conventional incubation for 12 h, the cell source chamber was removed, and the excess cells on the upper side of the filter (those that did not migrate across the filter) were removed by gently wiping. The entire filter was then submerged in a fixation solution (4% paraformaldehyde) for 30 min and stained using a Hematoxylin–Eosin staining kit (Solarbio). Using a microscope set at 200× magnification, the number of stained cells in 5 fields on the lower side of the filter were enumerated, and the average count was calculated. For the Boyden chamber invasion assay, the microporous filter was coated with Matrigel (BD BioCoat) to form a bioactive three-dimensional matrix, and the physical characteristics and function of the cell basement membrane were simulated. The procedure was the same as that of the migration assay, except for the incubation time, which was 24 h.
Xenograft Tumor Model In Vivo
Nude, sexually mature female mice, BALB/C/nu/nu strain, were purchased from the National Rodent Experimental Animal Seed Center. The mice were 5–7 weeks old and weighed 18–22 g. They were randomly divided into an overexpression group and a negative control group with 5 in each group. The animals were raised in a specific pathogen free (SPF) environment. Tumor cells in the logarithmic phase of growth were trypsinized, enumerated using trypan blue, and adjusted to the density of 8 × 108/mL. Further a 0.3 mL tumor cell suspension was inoculated subcutaneously on the back of the nude mice. After inoculation, the nude mice returned to the SPF environment. Tumorigenesis and overall health of the nude mice were regularly evaluated and recorded. After 8 weeks, all nude mice were euthanized and the tumor dissected. The long and short diameters of the tumor were measured using a vernier caliper, and the tumor volume was calculated according to the formula, V = long diameter × short diameter2 × 1/2. The animal experiment protocols were approved by the Animal Protection and Use Committee of Shandong University, and they complied with all regulatory guidelines.
Statistical Analysis
Statistical analysis was performed using the SPSS software package (standard version IBM SPSS Statistics 24.0). Pearson’s chi-square or Fischer’s method were used to compare the proportion of EFEMP2 expression among the groups. Quantitative data were evaluated by ANOVA. P < 0.05 was considered statistically significant.
Results
Expression Of EFEMP2 In Human Breast Tissue Specimens
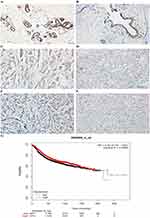
Immunohistochemical results showed that EFEMP2 was mainly located in cell cytoplasm and expressed as brown granules. The expression of EFEMP2 in normal breast (Figure 1A), breast fibroadenoma (Figure 1B), and breast cancer tissues (Figure 1C–F) showed a declining trend with normal breast having the highest expression and breast cancer tissue having the lowest expression. The positive rate of EFEMP2 overexpression in the tissues were 88.0% (44/50), 86.7% (52/60), and 15.8% (42/265), respectively (P < 0.01). High expression of EFEMP2 was negatively correlated with late clinical stage and the histological grade of breast cancer. This means that the expression of EFEMP2 decreased with an increase in clinical stage and histological grade of breast cancer. The expression of EFEMP2 in patients with negative lymph node metastasis was higher than that in patients with positive lymph node metastasis. Factors unrelated to EFEMP2 expression included age, pathological classification, and molecular subtype (Table 2). After analyzing the public Oncomine database, we came to a similar conclusion that compared to normal breast tissue; the mRNA expression of EFEMP2 was significantly reduced in invasive ductal and lobular carcinoma and several invasive special carcinomas, as shown in Table 3. Analysis of the public survival database Kaplan–Meier plotter revealed that the prognosis of breast cancer patients with high EFEMP2 expression was much better than that with low EFEMP2 expression (Figure 1G).
 |
Table 2 The Correlation Between The Expressions Of EFEMP2 And The Clinicopathological Characters Of Breast Cancer |
 |
Table 3 The Differential Analysis Of EFEMP2 mRNA Expression In Normal Breast And Breast Carcinoma Tissue In Oncomine Datasets |
Expression Of EFEMP2 In Human Breast Epithelial Cells And Cancer Cell Lines
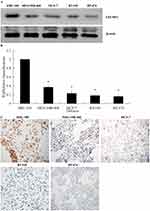
In contrast with breast cancer cell lines (BT-549, BT-474, MDA-MB-468 and MCF-7), the highest expression of EFEMP2 was detected in the normal breast epithelial cell line HBL-100 in mRNA and protein levels, by Western blotting (Figure 2A), real time RT-qPCR (Figure 2B), and ICC (Figure 2C). The MDA-MB-468 and MCF-7 breast adenocarcinoma cell lines and the BT-549 and BT-474 breast ductal cancer cell lines exhibited lower EFEMP2 expression. These results were consistent with the expression of EFEMP2 in breast tissue specimens, which emphasizes that EFEMP2 is highly expressed in normal breast tissues and cells, and that there is a trend of decreasing EFEMP2 expression with an increase in the degree of malignancy in breast cancer cells.
Identification Of EFEMP2 Overexpressed Transfection Efficiencies
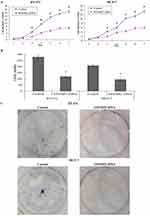
Since EFEMP2 was expressed at lower concentrations in breast cancer cell lines, we planned to increase EFEMP2 expression in the breast cancer cell lines MCF-7 and BT-474 by lentiviral transfection to assess the role of EFEMP2 in the proliferation and invasion of breast cancer cells. After viral transfection, more than 80% of the transfected cells were GFP positive, seen using fluorescence microscopy, which indicates a high transfection efficiency (Figure 3A and B). Real time qPCR and Western blotting confirmed the high EFEMP2 overexpressed transfection efficiency. EFEMP2 cDNA transfected MCF-7 and BT-474 cells had significantly higher EFEMP2 expression compared to the controls, at both the mRNA and protein levels (Figure 3C and D).
Effects Of EFEMP2 On Breast Cancer Cell Migration And Invasive Activities By Boyden Chamber
We performed cell migration and invasion assays using Transwell chambers to detect the effects of EFEMP2 on breast cancer cell invasion and migration capacities. In the Boyden chamber migration assays, fewer cells migrated through the PVPF membrane in the EFEMP2 cDNA transfected groups than in the control group (Figure 4A). In the Boyden chamber invasion assays, the number of cells that invaded through the PVPF membrane and Matrigel in the EFEMP2 cDNA transfected groups were also less than that in the control group (Figure 4B). The average number of EFEMP2 cDNA transfected cells that invaded or migrated were significantly less than that of the control cells (Figure 4C). In conclusion, overexpression of EFEMP2 inhibited the invasion and migration abilities of breast cancer cells.
Effects Of EFEMP2 On Breast Cancer Cell Proliferation And Clonogenic Capacities
Growth curves, an important index to judge cell vitality, and the plate clone formation assay, which reflects cell population dependence and clonogenic potential, were both performed to evaluate the effects of EFEMP2 on breast cancer cell proliferative and clonogenic capacities. Growth curves showed that overexpression of EFEMP2 distinctly inhibited breast cancer cell growth and proliferative capacities (Figure 5A). In the plate clone formation assay, when compared with the control groups, the colony number and size of EFEMP2 cDNA transfected groups were significantly decreased, implying that overexpression of EFEMP2 hampers breast cancer cell clonogenic capacities (Figure 5B and C). There was negative relationship between the expression of EFEMP2 and the proliferative and clonogenic capacities of breast cancer cells, as EFEMP2 overexpression inhibited breast cancer cell proliferation and clonogenicity.
Effects Of EFEMP2 On The Growth Of Xenotransplantation Tumor In Vivo
The xenograft tumor model was established in nude mice to further investigate whether EFEMP2 affected tumor growth in vivo. EFEMP2 cDNA transfected MCF-7 and BT-474 cells and control cells were each subcutaneously inoculated in 5 nude mice, respectively. EFEMP2 cDNA transfected groups showed a delay in tumor size and growth. Follow up of the growth profile of tumors for 8 weeks is shown in Figure 6A. EFEMP2 overexpression inhibited tumor growth in vivo. The average tumor size in the nude mice subcutaneously inoculated with EFEMP2 cDNA transfected cells was smaller than that of the control group (Figure 6B). By IHC (Figure 6C) and Western blotting (Figure 6D), we identified that the subcutaneous tumors formed by EFEMP2 cDNA-transfected MCF-7 and BT-474 cells had higher EFEMP2 expression than those formed by the control cells. In conclusion, EFEMP2 could impede tumor formation and growth speed in vivo.
Effects Of EFEMP2 On The Key EMT Hallmarks
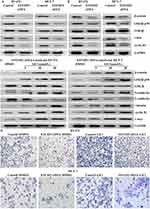
Epithelial–mesenchymal transition (EMT), a cellular program, is crucial for embryogenesis, wound healing, and cancer invasion and metastasis.20 From the results of the Boyden chamber assays, we found that EFEMP2 upregulation inhibited the migration and invasive abilities of breast cancer cells; therefore, we are doubtful as to whether upregulated EFEMP2 will affect the key EMT hallmarks in breast cancer cells. The results of Western blotting (Figure 7A) and real time qPCR (Figure 7B) revealed that EFEMP2 upregulation enhanced the expression of E-cadherin, the epithelial hallmark, which mediates cell–cell interactions. It also repressed the expression of the mesenchymal hallmarks, N-cadherin and Vimentin, and transcription factors, Snail, Slug and Twist, at both the mRNA and protein levels. EFEMP2 overexpression hampers the process of EMT in breast cancer.
Effects Of EFEMP2 On The Wnt/β-Catenin Pathway
A team led by RA Weinberg confirmed that the canonical Wnt signaling pathway was involved in inducing the activation of the cellular EMT process and the maintenance of the final mesenchymal state in an autocrine manner.21 Therefore, we suspected that EFEMP2 upregulation could regulate the process of EMT through the Wnt signaling pathway. After lentiviral transfection, EFEMP2 overexpression decreased the level of GSK3β phosphorylation, and then activated its kinase activity. This promoted the degradation of β-catenin and prevented its accumulation, which decreased the expressions of downstream target oncogenes, such as c-myc and cyclin-D1 (Figure 8A). In the subcutaneous tumors formed by MCF-7 and BT-474 cells transfected with EFEMP2 cDNA as well as the negative control cells, the key hallmarks of the Wnt signaling pathway were detected by Western blotting. The results showed that compared to the control group, the level of GSK3β phosphorylation decreased in the EFEMP2 cDNA transfected groups, and when EFEMP2 was upregulated, β-catenin was degraded, and the expressions of c-myc and cyclin-D1 subsequently decreased (Figure 8B). The EFEMP2 cDNA infected cells were treated with the Wnt signaling activator LiCl (5, 10, and 20 μmol/L) for 48 h. We found that the Wnt signaling activator LiCl could increase the phosphorylation level of GSK3β, and therefore inhibit its kinase activity, prevent β-catenin degradation, promote its accumulation, and increase the expression of c-myc and cyclin-D1. LiCl reactivated the Wnt/β-catenin pathway and promoted the process of EMT in EFEMP2 cDNA-infected cells (Figure 8C), which were hampered by EFEMP2 overexpression. To further verify if EFEMP2 overexpression inhibits the two cell lines through the Wnt/β-catenin signaling, we applied LiCl to the Boyden chamber invasion assay. The results revealed that LiCl could significantly induce cell invasion in comparison to EFEMP2 overexpression, and EFEMP2 overexpression could significantly decrease cell invasive ability induced by LiCl. In the groups where EFEMP2 overexpression was combined with LiCl, a significant inhibition of cell invasion was observed compared to the groups with LiCl treatment alone (Figure 8D). EFEMP2 plays a protective role in the progression of breast cancer, and its upregulation could inactivate the Wnt/β-catenin pathway and inhibit the process of EMT.
Discussion
Our experimental data proved that EFEMP2 expression was downregulated in breast cancer tissue specimens and cell lines, and its low expression was related to poor clinicopathological features and prognosis of breast cancer. Analysis of the public databases (Oncomine and Kaplan–Meier plotter database) verified our conclusions. Further studies revealed that EFEMP2 upregulation inactivated the Wnt/β-catenin pathway and inhibited the process of EMT to prevent breast cancer progression.
In our study, we observed that EFEMP2 expression in breast cancer tissues and cell lines were significantly lower than that in normal tissues and cell lines, and low EFEMP2 expression was related to late clinical stage and high histological grade, positive lymph node metastasis and poor prognosis of breast cancer. The role of EFEMP2 in tumor development remained controversial. Using fluorescence in situ hybridization technique, it was determined that the human EFEMP2 gene was localized on chromosome 11q13, and that gene translocation and rearrangement in this region could lead to multiple human cancers.10 Comparison of human colon tumors and adjacent normal tissue showed that tumors had a 2–7 fold increase at the EFEMP2 mRNA level.10 In cervical and ovarian carcinoma,11,12 EFEMP2 was overexpressed in cancer tissues and significantly related to poor clinicopathological characteristics. EFEMP2 expression was also found to be upregulated in osteosarcoma tissues and highly invasive cell lines, where EFEMP2 acted as a promoter for the development of osteosarcoma.13 EFEMP2 was also significantly upregulated in glioma tissues, and EFEMP2 silencing in glioma cell lines remarkably inhibited cell proliferation, induced cell apoptosis and decreased cell invasive abilities.14 Conversely, in prostate cancer, EFEMP2 was significantly downregulated in the cancer samples, and also was weakly expressed in carcinoma cell lines compared to normal prostate epithelial cells.15 In endometrial carcinoma, EFEMP2 was also decreased in cancer tissues and cell lines, thus suggesting EFEMP2 plays an essential role in suppressing endometrial cancer progression.16 There were conflicting conclusions breast cancer studies. Tao Zuo et al found that EFEMP2 could significantly enhance the invasive ability of breast cancer cells, which depended on the GALNT14 mediating EFEMP2 glycosylation.22 In other studies, FBLN-4 gene expression was downregulated in tumor tissues compared to the adjacent normal tissues and was associated with the histological grade of breast cancer.23 Our study was consistent with the latter studies, and analysis of the Public databases (Oncomine and Kaplan–Meier plotter database) supported our conclusions. EFEMP2 was expressed at lower concentrations in breast cancer tissues and cell lines, and its upregulation inhibited breast cancer invasion and metastasis in vitro and in vivo. The role of the upstream and downstream effectors of EFEMP2 and the proteins that bind to EFEMP2 still remain unclear and need further research is necessary.
EMT can push epithelial cells convert into a series of mesenchymal phenotypes, each showing unique cellular characteristics, including stemness, invasiveness, drug resistance, and the ability to metastasize distant organs, which facilitates tumor metastasis and recurrence.24 Our research revealed that EFEMP2 upregulation in breast cancer cells would suppress the process of EMT, with increased expression of E-cadherin and decreased the expressions of N-cadherin, Vimentin, Snail, Slug, and Twist. To date, there are few studies on the relationship between EFEMP2 and EMT. In human osteosarcoma, EFEMP2 induced EMT to promote cancer invasion and metastasis,13 whereas in endometrial cancer, EFEMP2 inhibited cancer cell invasion and metastasis by blocking the process of EMT.16 EFEMP2 has been postulated to be either a tumor suppressor or promoter depending on the cell type. This dual role is common among other members of the fibulin family, such as fibulin 2 and fibulin 3. Fibulin-2 was a driving factor of lung adenocarcinoma progression and played an important role in the adhesion of tumor cells to collagen.25 However, in nasopharyngeal carcinoma26 and breast cancer,27,28 fibulin-2 had tumor-suppressive effects on cancer cells. Fibulin-3 was highly expressed in ovarian cancer,29 osteosarcoma,30 pancreatic cancer,31 cervical cancer,32 and glioma,33 which promoted cancer cell invasion and metastasis. Conversely, in hepatocellular carcinoma,34 gastric cancer,35 lung cancer,36 endometrial carcinoma,37 breast cancer,38 nasopharyngeal carcinoma,39 and glioblastoma,40 the expression of fibulin-3 was downregulated and inhibited tumor development. One possible reason for the opposing actions is that different tumor microenvironments determine the gene functions.41
Wnt signaling is a highly evolutionarily conserved pathway that was first identified for its role in carcinogenesis, then for its functions in embryonic development and tissue homeostasis.42 The Wnt signaling pathway can induce EMT by inhibiting the phosphorylation of glycogen synthase kinase 3β (GSK3β) and the degradation of β-catenin in the cytoplasm.43,44 The canonical Wnt pathway is involved in the development of breast neoplasms and is accompanied by increased β-catenin expression. For example, in basal-like breast cancer, the repression of Wnt/β-catenin signaling would prevent EMT and inhibit lung metastasis.45,46 In our study, EFEMP2 upregulation depressed the activation of canonical Wnt signaling and impeded EMT progression to inhibit breast cancer cell invasion and metastasis. Similar results have been observed in endometrial carcinoma, where EFEMP2 had the ability to suppress cancer cell proliferation, invasion and metastasis, by inhibiting EMT through the Wnt/β-catenin signaling pathway.16 However, in human osteosarcoma, EFEMP2 played critical oncogene roles in tumor growth by activating Wnt/β-catenin signaling.47 The role of EFEMP2 in tumorigenesis and its possible signaling pathway have not been fully elucidated, so further experimental research is needed.
Conclusion
In our research, low expression of EFEMP2 was closely related to the malignant phenotype and poor prognosis of breast cancer. EFEMP2 would prevent the progression of breast cancer by blocking the Wnt/β-catenin pathway and inhibiting the EMT process. We believe that the study of EFEMP2 could help to inhibit the invasion and metastasis of breast carcinoma more effectively.
Abbreviations
EFEMP2, EGF-containing fibulin-like extracellular matrix protein 2; ECM, extracellular matrix; IHC, immunohistochemistry; ICC, immunocytochemistry; real time RT-qPCR, real time quantitative reverse transcriptase-polymerase chain reaction; EMT, epithelial interstitial transformation; BC, breast cancer; cbEGF, calcium-binding epidermal growth factor-like; DMEM/F-12, Dulbecco’s Modified Eagle Medium: Nutrient Mixture F-12; FBS, fetal bovine serum; AJCC, American Joint Committee on Cancer; TNM, tumor-node-metastasis; PBS, phosphate buffer saline; SP staining, streptavidin-biotin-peroxidase staining; DAB, 3′, 3-diaminobenzidine tetrahydrochloride; RIPA, radioimmunoprecipitation assay; PMSF, phenylmethylsulfonyl fluoride; SDS-PAGE, sodium dodecyl sulfate polyacrylamide gel; PVDF, polyvinylidene difluoride; BSA, bovine serum albumin; TBST, tris-buffered saline with Tween 20; GSK3β, glycogen synthase kinase 3β.
Ethics Approval And Consent To Participate
This study was approved by the Medical Ethics Committee of Human and Animal Institution of Shandong University. All methods were performed in accordance with the relevant guidelines and regulations. We obtained the written informed consents of the patients involved in the study. All mouse experimental procedures were performed in accordance with the Regulations for the Administration of Affairs Concerning Experimental Animals approved by the State Council of People’s Republic of China.
Consent For Publication
We obtained consents for publication from the participants.
Availability Of Data And Material
All data generated or analysed during this study are included in this published article.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work. Conceived and designed the experiments: NK WS. Performed the experiments: NK JZ DZ. Analyzed the data: JX WS. Contributed reagents/materials/analysis tools: JZ JX DZ. Wrote the paper: NK JZ.
Disclosure
The authors declare they have no conflict of interest in this work.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. doi:10.3322/caac.21332
2. McShane TM, Wolfe TA, Ryan JC. Updates on managing advanced breast cancer with palbociclib combination therapy. Ther Adv Med Oncol. 2018;10:1758835918793849. doi:10.1177/1758835918793849
3. Velloso FJ, Bianco AF, Farias JO, et al. The crossroads of breast cancer progression: insights into the modulation of major signaling pathways. Onco Targets Ther. 2017;10:5491–5524. doi:10.2147/OTT.S142154
4. De Vega S, Iwamoto T, Yamada Y. Fibulins: multiple roles in matrix structures and tissue functions. Cell Mol Life Sci. 2009;66:1890–1902. doi:10.1007/s00018-009-8632-6
5. Argraves WS, Greene LM, Cooley MA, Gallagher WM. Fibulins: physiological and disease perspectives. EMBO Rep. 2003;4(12):1127–1131. doi:10.1038/sj.embor.7400033
6. Gallagher WM, Argentini M, Sierra V, Bracco L, Debussche L, Conseiller E. MBP1: a novel mutant p53-specific protein partner with oncogenic properties. Oncogene. 1999;18(24):3608–3616. doi:10.1038/sj.onc.1202937
7. Giltay R, Timpl R, Kostka G. Sequence, recombinant expression and tissue localization of two novel extracellular matrix proteins, fibulin-3 and fibulin-4. Matrix Biol. 1999;18(5):469–480.
8. Choudhury R, McGovern A, Ridley C, et al. Differential regulation of elastic fiber formation by fibulin-4 and −5. J Biol Chem. 2009;284(36):24553–24567. doi:10.1074/jbc.M109.019364
9. Papke CL, Tsunezumi J, Ringuette LJ, et al. Loss of fibulin-4 disrupts collagen synthesis and maturation: implications for pathology resulting from EFEMP2 mutations. Hum Mol Genet. 2015;24(20):5867–5879. doi:10.1093/hmg/ddv308
10. Gallagher WM, Greene LM, Ryan MP, et al. Human fibulin-4: analysis of its biosynthetic processing and mRNA expression in normal and tumour tissues. FEBS Lett. 2001;489(1):59–66. doi:10.1016/s0014-5793(00)02389-9
11. Chen J, Zhang J, Liu X, Fang R, Zhao Y, Ma D. Overexpression of fibulin-4 is associated with tumor progression and poor prognosis in patients with cervical carcinoma. Oncol Rep. 2014;31(6):2601–2610. doi:10.3892/or.2014.3139
12. Chen J, Liu Z, Fang S, et al. Fibulin-4 is associated with tumor progression and a poor prognosis in ovarian carcinomas. BMC Cancer. 2015;15:91.
13. Zhang D, Wang S, Chen J, et al. Fibulin-4 promotes osteosarcoma invasion and metastasis by inducing epithelial to mesenchymal transition via the PI3K/Akt/mTOR pathway. Int J Oncol. 2017;50(5):1513–1530. doi:10.3892/ijo.2017.3921
14. Wang L, Chen Q, Chen Z, et al. EFEMP2 is upregulated in gliomas and promotes glioma cell proliferation and invasion. Int J Clin Exp Pathol. 2015;8(9):10385–10393.
15. Wlazlinski A, Engers R, Hoffmann MJ, et al. Downregulation of several fibulin genes in prostate cancer. Prostate. 2007;67(16):1770–1780. doi:10.1002/pros.20667
16. Wang T, Wang M, Fang S, Wang Q, Fang R, Chen J. Fibulin-4 is associated with prognosis of endometrial cancer patients and inhibits cancer cell invasion and metastasis via Wnt/β-catenin signaling pathway. Oncotarget. 2017;8(12):18991–19012. doi:10.18632/oncotarget.15086
17. Soumaoro LT, Uetake H, Higuchi T, Takagi Y, Enomoto M, Sugihara K. Cyclooxygenase-2 expression: a significant prognostic indicator for patients with colorectal cancer. Clin Cancer Res. 2004;10(24):8465–8471. doi:10.1158/1078-0432.CCR-04-0653
18. Györffy B, Lanczky A, Eklund AC, et al. An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1,809 patients. Breast Cancer Res Treat. 2010;123(3):725–731. doi:10.1007/s10549-009-0674-9
19. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi:10.1006/meth.2001.1262
20. Dongre A, Weinberg RA. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol. 2018;20(2):69–84.
21. Scheel C, Eaton EN, Li SH, et al. Paracrine and autocrine signals induce and maintain mesenchymal and stem cell states in the breast. Cell. 2011;145(6):926–940. doi:10.1016/j.cell.2011.04.029
22. Zuo T, Shan J, Liu Y, Xie R, Yu X, Wu C. EFEMP2 mediates GALNT14-dependent breast cancer cell invasion. Transl Oncol. 2018;11(2):346–352. doi:10.1016/j.tranon.2018.01.021
23. Motalebzadeh J, Mahjoubi F, Nafissi N, Hashemian M, Taheri M, Hosseinpour Y. FBLN-4 and BCRP genes as two prognostic markers are downregulated in breast cancer tissue. Cancer Biomark. 2017;19(1):51–55. doi:10.3233/CBM-160335
24. Zhang Y, Weinberg RA. Epithelial-to-mesenchymal transition in cancer: complexity and opportunities. Front Med. 2018;12(4):361–373. doi:10.1007/s11684-018-0656-6
25. Baird BN, Schliekelman MJ, Ahn YH, et al. Fibulin-2 is a driver of malignant progression in lung adenocarcinoma. PLoS One. 2013;8(6):e67054. doi:10.1371/journal.pone.0067054
26. Law EW, Cheung AK, Kashuba VI, et al. Anti-angiogenic and tumor-suppressive roles of candidate tumor-suppressor gene, Fibulin-2, in nasopharyngeal carcinoma. Oncogene. 2012;31(6):728–738. doi:10.1038/onc.2011.272
27. Tan H, Zhang J, Fu D, Zhu Y. Loss of fibulin-2 expression is involved in the inhibition of breast cancer invasion and forms a new barrier in addition to the basement membrane. Oncol Lett. 2017;14(3):2663–2668. doi:10.3892/ol.2017.6539
28. Fontanil T, Rúa S, Llamazares M, et al. Interaction between the ADAMTS-12 metalloprotease and fibulin-2 induces tumor-suppressive effects in breast cancer cells. Oncotarget. 2014;5(5):1253–1264. doi:10.18632/oncotarget.1690
29. Yin X, Fang S, Wang M, Wang Q, Fang R, Chen J. EFEMP1 promotes ovarian cancer cell growth, invasion and metastasis via activated the AKT pathway. Oncotarget. 2016;7(30):47938–47953. doi:10.18632/oncotarget.10296
30. Wang Z, Cao CJ, Huang LL, et al. EFEMP1 promotes the migration and invasion of osteosarcoma via MMP-2 with induction by AEG-1 via NF-κB signaling pathway. Oncotarget. 2015;6(16):14191–14208. doi:10.18632/oncotarget.3691
31. Seeliger H, Camaj P, Ischenko I, et al. EFEMP1 expression promotes in vivo tumor growth in human pancreatic adenocarcinoma. Mol Cancer Res. 2009;7(2):189–198. doi:10.1158/1541-7786.MCR-08-0132
32. En-Lin S, Sheng-Guo C, Hua-Qiao W. The expression of EFEMP1 in cervical carcinoma and its relationship with prognosis. Gynecol Oncol. 2010;117(3):417–422. doi:10.1016/j.ygyno.2009.12.016
33. Hu B, Thirtamara-Rajamani KK, Sim H, Viapiano MS. Fibulin-3 is uniquely upregulated in malignant gliomas and promotes tumor cell motility and invasion. Mol Cancer Res. 2009;7(11):1756–1770. doi:10.1158/1541-7786.MCR-09-0207
34. Dou CY, Cao CJ, Wang Z, et al. EFEMP1 inhibits migration of hepatocellular carcinoma by regulating MMP2 and MMP9 via ERK1/2 activity. Oncol Rep. 2016;35(6):3489–3495. doi:10.3892/or.2016.4733
35. Zhu XJ, Liu J, Xu XY, Zhang CD, Dai DQ. Novel tumor-suppressor gene epidermal growth factor-containing fibulin-like extracellular matrix protein 1 is epigenetically silenced and associated with invasion and metastasis in human gastric cancer. Mol Med Rep. 2014;9(6):2283–2292. doi:10.3892/mmr.2014.2135
36. Chen X, Meng J, Yue W, et al. Fibulin-3 suppresses Wnt/β-catenin signaling and lung cancer. Carcinogenesis. 2014;35(8):1707–1716. doi:10.1093/carcin/bgu023
37. Yang T, Qiu H, Bao W, et al. Epigenetic inactivation of EFEMP1 is associated with tumor suppressive function in endometrial carcinoma. PLoS One. 2013;8(6):e67458. doi:10.1371/journal.pone.0067458
38. Tian H, Liu J, Chen J, Gatza ML, Blobe GC. Fibulin-3 is a novel TGF-β pathway inhibitor in the breast cancer. Oncogene. 2015;34(45):5635–5647. doi:10.1038/onc.2015.13
39. Hwang CF, Chien CY, Huang SC, et al. Fibulin-3 is associated with tumour progression and a poor prognosis in nasopharyngeal carcinomas and inhibits cell migration and invasion via suppressed AKT activity. J Pathol. 2010;222(4):367–379. doi:10.1002/path.2776
40. Hu Y, Pioli PD, Siegel E, et al. EFEMP1 suppresses malignant glioma growth and exerts its action within the tumor extracellular compartment. Mol Cancer. 2011;10:123. doi:10.1186/1476-4598-10-93
41. Chen L, Sun B, Zhang S, et al. Influence of microenvironments on microcirculation patterns and tumor invasion-related protein expression in melanoma. Oncol Rep. 2009;21(4):917–923. doi:10.3892/or_00000304
42. Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149(6):1192–1205. doi:10.1016/j.cell.2012.05.012
43. Basu S, Cheriyamundath S, Ben-Ze’ev A. Cell-cell adhesion: linking Wnt/β-catenin signaling with partial EMT and stemness traits in tumorigenesis. F1000Res. 2018;7. doi:10.12688/f1000research.15782.1
44. Cai Z, Cao Y, Luo Y, Hu H, Ling H. Signalling mechanism(s) of epithelial-mesenchymal transition and cancer stem cells in tumour therapeutic resistance. Clin Chim Acta. 2018;483:156–163. doi:10.1016/j.cca.2018.04.033
45. Braune EB, Seshire A, Lendahl U. Notch and Wnt dysregulation and its relevance for breast cancer and tumor initiation. Biomedicines. 2018;6(4):E101. doi:10.3390/biomedicines6040101
46. DiMeo TA, Anderson K, Phadke P, et al. A novel lung metastasis signature links Wnt signaling with cancer cell self-renewal and epithelial-mesenchymal transition in basal-like breast cancer. Cancer Res. 2009;69(13):5364–5373. doi:10.1158/0008-5472.CAN-08-4135
47. Li R, Wang L. Fibulin-4 is a novel Wnt/β-Catenin pathway activator in human osteosarcoma. Biochem Biophys Res Commun. 2016;474(4):730–735. doi:10.1016/j.bbrc.2016.05.018
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.