Back to Journals » Nature and Science of Sleep » Volume 14
EEG Activation Does Not Differ in Simple and Complex Episodes of Disorders of Arousal: A Spectral Analysis Study
Authors Mainieri G, Loddo G , Castelnovo A, Balella G, Cilea R, Mondini S , Manconi M , Provini F
Received 27 January 2022
Accepted for publication 24 May 2022
Published 7 June 2022 Volume 2022:14 Pages 1097—1111
DOI https://doi.org/10.2147/NSS.S360120
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ahmed BaHammam
Greta Mainieri,1,* Giuseppe Loddo,2,* Anna Castelnovo,3– 5 Giulia Balella,1 Rosalia Cilea,6 Susanna Mondini,7 Mauro Manconi,3,4,8 Federica Provini1,7
1Department of Biomedical and NeuroMotor Sciences, University of Bologna, Bologna, Italy; 2Department of Primary Care, Azienda AUSL di Bologna, Bologna, Italy; 3Sleep Medicine Unit, Neurocenter of Southern Switzerland, Lugano, Switzerland; 4Faculty of Biomedical Sciences, Università della Svizzera Italiana, Lugano, Switzerland; 5University Hospital of Psychiatry, University of Bern, Bern, Switzerland; 6Neurology Unit, “Morgagni-Pierantoni” Hospital, AUSL Romagna, Forlì, Italy; 7IRCCS Istituto delle Scienze Neurologiche di Bologna, Bologna, Italia; 8Department of Neurology, University Hospital, Inselspital, Bern, Switzerland
*These authors contributed equally to this work
Correspondence: Federica Provini, IRCCS, Institute of Neurological Sciences of Bologna, Bellaria Hospital, Via Altura, 3, Bologna, 40139, Italy, Tel +39 051 4966829, Email [email protected]
Purpose: Disorders of arousal (DoA) are characterized by incomplete awakening from NREM sleep, with the admixture of both deep sleep and wake EEG activity. Previous observations suggested that changes in EEG activity could be detected in the seconds preceding DoA episodes. The aims of this work were to characterize the topography of EEG spectral changes prior to DoA episodes and to investigate whether or not behavioral complexity could be predicted by changes in EEG immediately preceding behavioral onsets.
Patients and Methods: We collected 103 consecutive video-polysomnographic recordings of 53 DoA adult patients and classified all episodes as simple, rising and complex arousal movements. For each episode, a 5-second window preceding its motor onset (“pre-event”) and a 60-second window from 2 to 3 minutes before the episodes (“baseline”) were compared. Subsequently, a between-group comparison was performed for the pre-event of simpler versus the more complex episodes.
Results: Spectral analysis over 325 DoA episodes showed an absolute significant increase prior to DoA episodes in all frequency bands excluding sigma, which displayed the opposite effect. In normalized maps, the increase was relatively higher over the central/anterior areas for both slow and fast frequency bands. No significant differences emerged from the comparison between simpler and more complex episodes.
Conclusion: Taken together, these results show that deep sleep and wake-like EEG rhythms coexist over overlapping areas before DoA episodes, suggesting an alteration of local sleep mechanisms. Episodes of different complexity are preceded by a similar EEG activation, implying that they possibly share a similar pathophysiology.
Keywords: parasomnia, disorders of arousal, neurophysiology, spectral EEG
Introduction
Disorders of arousal (DoA) are currently included in the group of NREM sleep parasomnias as undesired events characterized by incomplete awakening from NREM sleep.1 They are characterized by inappropriate judgement of the environment, absent or inadequate responsiveness, limited or absent mental content and partial amnesia of the event. DoA encompass 3 main clinical entities, namely, confusional arousal, sleep terrors and sleepwalking.1 Only a few studies have systematically assessed DoA semiology,2–6 but a conclusive agreement is still lacking. A recent video-polysomnographic (VPSG) analysis of 184 episodes attempted to classify DoA motor episodes, identifying three specific patterns of increasing complexity.7 These motor patterns went from simple arousal movements (SAMs), involving head with or without limb/trunk elevation, to rising arousal movements (RAMs), in which patients sit up in bed, to complex arousal movements (CAMs), implicating getting out of bed and sleepwalking.7 In comparison to RAMs and CAMs, SAMs are more difficult to differentiate from physiological arousal-related phenomena and represent the real challenge for sleep experts. In a given subject, SAMs are often an initial small fragment of a more complex episode, but appear more frequently as isolated events across the night sleep of the patient. This finding led to the speculation of a possible common neurophysiologic background for the different motor manifestations.7
Since the first electroencephalographic (EEG) description,8 it became clear that most DoA episodes arise from N3 stage of NREM sleep,9–11 the deeper stage of sleep, characterized by a reduced response to external stimuli as well as decreased awareness of the environment.12 Capturing an in vivo episode of sleepwalking, a SPECT study disclosed a “dissociation” between more activated areas, such as posterior cingular cortex and anterior cerebellum, and areas with decreased blood flow, such as fronto-parietal associative cortices, suggesting the selective activation of thalamo-cingulate networks with persistent inhibition of other thalamo-cortical pathways.13 Since then, some pre-surgical stereo-EEG studies in patients with refractory epilepsy recorded by chance DoA episodes.14–17 The picture resulting from these studies indicates the persistence of deep sleep rhythms over anterior, fronto-parietal associative cortices and hippocampi, responsible for the unawareness and frequent amnesia during DoA episodes, together with fast EEG rhythms more typical of wakefulness over the cingulate cortices, motor areas, amygdala, and thalami, which in turn account for the motor and emotional activation during the episodes.11,16 Moreover, both slow and fast EEG activities have been recorded even in the same brain areas, adding further complexity to DoA neurophysiology.17 These local wake/sleep phenomena account for the dissociation of the arousal process, for which NREM parasomnias are currently under the name of DoA.9,10,18 In addition, DoA are also considered to have a dysfunctional N3 stage, characterized by an inability to maintain slow wave sleep (SWS), an increased fragmentation of this stage and an abnormal response to sleep deprivation, which facilitates the occurrence of more complex episodes.6,9 Stereo-EEG studies offer excellent temporal and spatial resolution but, albeit providing impressive results, they are usually performed in other clinical entities and the subset of explored cerebral areas is strictly dependent on the epileptic focus localization. In addition, even if all these studies carefully rejected arousals with a possible seizure activity, the involvement of epilepsy in their observation cannot be completely ruled out.17 Over the last years, some EEG studies specifically targeting subjects with DoA tried to characterize the neurophysiologic activity preceding DoA episodes19–29 by means of visual, spectral power or connectivity analysis. Overall, most studies focused on slow wave activity, analyzed on fronto-central leads. A common finding across the studies is the increase in delta power, especially in frontal areas, in the seconds immediately preceding an episode, with variability in the chosen time window (Table 1).20,22,24–27 The main limitations of these works include different methodological designs, involving nights after a sleep deprivation protocol or not homogeneous criteria for EEG frequency bands.27 In addition, these studies mostly included small samples of patients and number of episodes analyzed. The events taken into account were often the most complex behavioral episodes available in their recordings and a comparison with less elaborate behaviors was not performed.
 |
Table 1 EEG Quantitative Studies in DoA Analyzing the Pre-Episode Period |
The primary objective of this study was to characterize the EEG activity preceding DoA episodes by means of a whole scalp and broad-band EEG spectral analysis in a large population of DoA patients. The secondary aim was to compare the EEG activity prior to different types of motor episodes, in order to test our hypothesis that minor DoA episodes might be fragments of the major ones.
Materials and Methods
Study Sample
All patients included in the study had been consecutively referred to the Sleep Center of the Department of Biomedical and NeuroMotor Sciences, University of Bologna, and IRCCS Istituto delle Scienze Neurologiche di Bologna, in a time period ranging from 2007 to 2021 and evaluated by a sleep disorder specialist (FP). All patients included had a clinical diagnosis of DoA according to the ICSD – III criteria confirmed by VPSG recording of at least one DoA episode.7 Exclusion criteria were the presence of neurological or psychiatric disorders, the presence of psychotropic therapy and/or the presence of other sleep disorders. Clinical data including age, sex, family history of parasomnia, age of onset and frequency of episodes at the observation, and principal type of nocturnal manifestation, were collected. Patients underwent at least two nocturnal home-VPSGs (XLTEK Trex HD, Natus Medical Incorporated®, video-camera Handycam HDR-CX700, Sony, 12.3 Megapixel resolution). All patients were instructed to keep their habitual sleep routine in the week preceding the exam. None of them underwent a sleep deprivation schedule.
Video-polysomnographic recordings were performed using standard bipolar EEG (according to the International 10–20 system) and included 19 electrodes (Fp1, Fp2, F3, F4, F7, F8, Fz, C3, C4, Cz, T3, T4, T5, T6, P3, P4, Pz, O1, and O2), electrocardiogram, electro-oculogram, chin and both anterior tibialis electromyography, thoracoabdominal plethysmography bands, and synchronized audio-video recording.
Sleep stages were scored in 30s epochs according to the AASM criteria.30 Nocturnal sleep data were collected for all patients.
The study was approved by our local Ethical Committee “Comitato Etico Interaziendale Bologna-Imola, CE-BI”, code 17176/2017. All subjects gave written informed consent to the study protocols, in agreement with the Convention of Helsinki.
Episodes Analysis
All full-night VPSG recordings were independently reviewed by two sleep specialists (GM, GL) who checked for the global quality of the recordings, including artifacts, technical issues and presence of disturbing noises and/or lights. For the aim of the study, only episodes with clear visibility on the video recordings were included. Each DoA episode was subclassified according to Loddo et al motor classification7 as:
- SAMs or pattern I, when movements implied head flexion/extension (IA) alone or in conjunction with limbs flexion/extension (IB) and partial trunk flexion/extension (IC);
- RAMs or pattern II, involving complete trunk flexion/extension and sitting up in bed;
- CAMs or pattern III, characterized by getting up, leaving the bed and walking.
Inter-observer discordances on the classification of episodes were sorted out by a third observer, a sleep specialist with expertise in nocturnal motor episodes semeiology (FP).
The beginning of each episode was time marked with either the first electromyographic activation or the first visible movement (including eye opening) on the video analysis. The end of the episode was set as the last visible movement on the video review. Collected features for each episode included the arising sleep stage, the sleep cycle and the duration.
EEG Analysis
All recordings were conducted on a 32-channel polygraph, using vertex referencing and digitized at a sampling rate of 256 Hz. Recordings were acquired with DC filter set up to filter out drift and slow components.
All data were converted in a European Data Format (EDF) file and subsequently imported in MATLAB (MathWorks Inc., Natick, MA, 2020a). For each episode, 6-minute segments preceding the episode onset were extracted. Each segment was visually inspected with the support of a user graphical interface (https://github.com/CSC-UW/csc-eeg-tools), in order to mark noisy channels and epochs. Additional spectral-based and topographic procedures were used to identify individual channels with distinctly greater power relative to neighboring channels or epochs with clearly deviant spectra or topographies. Each segment was then band-pass filtered (zero-phase digital low-pass and high-pass filter with reflection, 0.5–45 Hz, using the “filtfilt” function of the signal-processing toolbox). Channels marked as bad were interpolated using spherical interpolation, while epochs marked as bad were discarded. The filtered signal was re-referenced to the average of the scalp voltage and divided into consecutive 5-second epochs. Spectral analysis was conducted on clean 5-second epochs (for a total of 360 seconds preceding each episode) using the Fast Fourier Transform (Welch averaged modified periodogram with a Hamming window, 50% overlap). For topographic analysis, spectral density was averaged in 6 frequency ranges (slow wave activity or delta: 0.5–4 Hz; theta: 4–8 Hz; alpha: 8–12 Hz; sigma: 12–16 Hz; beta: 15–25 Hz; and low gamma: 25–45 Hz), in accordance with previous studies.31 Topographic maps of both absolute averages referenced data and subject-normalized data (z-score across channels) were examined.
For the purpose of this study, we compared the time window of 5 seconds before each episode, considered as the “pre-episode” time, with a time frame of 60 seconds from 2 to 3 minutes before the episodes (“baseline” stable N3 sleep without arousal or micro-arousal). The same procedure was applied for episodes arising from N2 sleep. We chose this time window in accordance with other studies, which disclosed an abrupt shift of EEG frequencies in this time frame.15,23
Statistical Analysis
Comparisons of scalp power maps were performed separately for each frequency band. At the scalp level, multiple comparison adjustment was performed using a non-parametric cluster-based permutation test,32 as described in previous works.31,33,34 Specifically, for each performed test, a null distribution was generated by randomly shuffling the group-label of each subject for comparisons. At each iteration of the permutation procedure, the test-statistics was computed for each electrode and the size of the largest significant electrode-cluster (uncorrected p<0.05) was stored in a frequency table. Finally, the 95th percentile (5% significance level) was used as the critical cluster-size distribution threshold. Although this test faithfully addresses the problem of multiple comparisons across an image, it should also be noted that we did not attempt to strictly correct for the issue of multiple testing across different comparisons (for example, across bands and stages) given the exploratory nature of this study.
Results
Sample and Episode Features
A total of 53 patients (25 males, mean age 32.1 ± 15.4 years) were involved in this study. In all, 103 VPSGs were performed: one in 18 patients, two in 26 patients, three in 4 patients, four in 4 patients and five in 1 patient. Clinical features of the sample are summarized in Table 2. Two patients were on levothyroxine and antihypertensive medications, two on levothyroxine alone, one on antihypertensive therapy alone, one was taking a proton pump inhibitor, 2 were on asthma medication spray, and 4 females were on estroprogestinic therapy. Thirty-six percent of patients reported occasional alcohol intake while 9% reported habitual intake (a glass of wine twice per day). Thirty-two percent of patients drank from 1 to 2 coffees per day while 18% drank 3 or more per day. With reference to smoking, 20% were previous smokers, 6% were occasional smokers and 17% were habitual smokers (mean of 8.5 cigarettes per day). Sleep features of the sample are shown in Table 3. The overall number of recorded DoA episodes was 325, 270 SAMs (51 with a pattern IA; 123 with a pattern IB; 96 with a pattern IC), 41 RAMs and 14 CAMs. Episode mean duration, sleep stage and sleep cycle at onset of SAMs, RAMs and CAMs are described in Table 4. A mean of 3.2 episodes per night was recorded. Overall, 88% of episodes arose from N3 stage.
 |
Table 2 Clinical Features of DoA Patients |
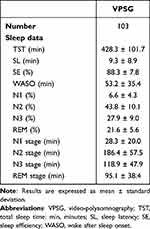 |
Table 3 Sleep Features |
 |
Table 4 Features of SAMs, RAMs and CAMs |
Episodes versus Baseline
In spectral analysis, we observed an absolute significant increase in all frequency bands with the exception of sigma (Figure 1). In normalized maps, a clear-cut dissociation between anterior and posterior areas was observed for theta band, with a relative increase in power over the anterior areas and a relative decrease over the posterior ones. A similar pattern was observed for delta frequency, with the exception of a relative decrease over a small anterior area that included fronto-polar derivations (Fp1 and Fp2) and F8. In addition, also beta band showed a distribution very similar to slow frequencies, prominent over both central and anterior areas. Alpha and gamma bands displayed a similar pattern, being relatively higher over the central areas. Finally, only sigma band showed a significant absolute decrease before the episodes, which showed a distribution typical of sleep spindles in normalized maps, with a relative increase over the vertex and central areas.
These results were not influenced by episodes arising from N2 sleep (Figure S1, Supplemental materials).
Inter-Episode Differences
As illustrated in Figure 2, no differences were detected in the absolute values in any of the EEG frequency bands between SAMs and the major episodes (RAMs and CAMs). In normalized maps, only a significant micro-cluster (Occipital O1, O2 and Pz derivations) was detected over posterior areas in the delta band, decreased in the simpler episodes.
Discussion
Coexistence of Wake/Sleep EEG Rhythms Before DoA Episodes
In the present work we analyzed the EEG spectral activation preceding behavioral episodes in a large sample of DoA patients, collecting over 300 events, further classified and distinguished on the basis of different motor complexity.7 To our knowledge, this is the largest sample of DoA episodes analyzed by means of spectral EEG. In contrast with several previous studies, our analysis was not restricted to some specific EEG leads but involved the whole EEG scalp and the whole EEG band spectrum. Our work demonstrates an absolute increase in power of all frequency bands with the exception of sigma before the motor onset of the DoA episodes.
Slow frequencies exhibited, in normalized maps, a relative increase over anterior areas (with the exception of an anterior micro-cluster for delta band) and a consequent relative decrease posteriorly. As shown in Table 1, previous spectral EEG studies disclosed an absolute increase of delta power before DoA episodes mainly over the fronto-central derivations, using different time windows and methodology.20,22,24,25,27 Our whole scalp map representation depicts a relatively higher slow wave activity (SWA) over a wide fronto-parietal network, considered to account for the unawareness and the lack of inhibition typical of DoA episodes.9,16 Interestingly, the relative posterior decrease of delta power encountered in our maps reminds of the parieto-occipital “hot zone” considered as a neural signature of dreaming.35 It is well known by now that DoA episodes might be linked to dreamlike mentation, usually more rudimentary, shorter and less elaborate and enriched in details than REM dream content.36–38 From this perspective, we might speculate that the relative posterior decrease of delta band disclosed in our study before the episodes might advocate the presence of dreaming mental content preceding and potentially triggering the episodes of DoA. In our study, along with a significant increase in slow frequencies, we disclosed before DoA episodes the concurrent presence of faster EEG rhythms, such as alpha, beta and gamma, over central and anterior areas (mostly for beta band), in a distribution approximately tracing motor and pre-motor areas. The admixture of sleep and wake activity in DoA, even in the same brain areas in some cases, has been demonstrated in stereo-EEG studies during the motor episode.14–17,39 In particular, the presence of fast EEG rhythms over motor, cingulate and limbic structures has been related to the motor activation and emotional behaviors of DoA.16 Our findings show a pattern characterized by wake-like EEG frequencies within sleep rhythms prior to the onset of the episode, suggesting that the shift toward a partial awakening,24 as well as the preparation for a motor activation,23 precedes the motor event itself. Beta band oscillations have been demonstrated to play a key role in motor control both for pyramidal and extra-pyramidal networks.40 Modifications of beta spectrum might thus suggest the imminent activation of the motor cortex and consequent motor behaviors of DoA. In addition, we found an increase in gamma band power before the episodes, a finding not clearly described in other studies with the only exception of a partial overlap with beta spectrum disclosed in some studies.23,24 Gamma band has been related to active waking processes involving attention41 or oneiric content in REM sleep and has been suggested to underlie dreaming content also in SWS.42 These aspects, however, remain controversial since increases in gamma bands have been disclosed also in states of reduced consciousness such as anesthesia or epileptic seizures.43,44
The only band that showed a significant decrease before the episodes was the sigma one, normally representing the expression of sleep spindles. This finding is shared by physiological arousal in healthy control subjects from N3 sleep, where a blockade of spindles has been demonstrated.45 In addition, sleep spindles have an inverse relationship with slow wave activity and periods with reduced spindle density are correlated with higher arousability.46 Sleep spindles, in fact, are considered to have a protective role for sleep continuity and to be an expression of the thalamic stimuli filtering, disentangling the brain from acoustic and sensory stimulation and ensuring the stability of sleep.46,47 As a consequence, the transitory disappearance of spindles before an arousal has been hypothesized to reflect a time frame in which sensorial transmission improves through the thalamic relay, preparing for a brain state apt to information processing.24,47 As a future direction, to further characterize thalamocortical dysfunction which may contribute to the onset of DoA episodes, the study of K-complexes and sleep spindles may be worth specific attention in these patients.
The Arousal Process in DoA: Where Does It Differ from Physiological Arousal?
The findings from our study delineate a complex interplay between the different EEG bands, confirming their coexistence in different brain areas and the concept that different states of being, exemplified in this case by NREM sleep and wakefulness, may co-occur and become clinically evident in sleep parasomnias.18 The existence of local sleep phenomena, however, has been highlighted also in physiological arousals, with evidence from different stereo-EEG studies of the coexistence of different patterns and EEG rhythms, together with frequent activation of the motor cortex concurrently with an arousal.45,48–51 In addition, a reduction of delta rate during N2 and N3 has been recorded in precentral gyrus in human sleep, independently of arousals,52 suggesting that motor areas are persistently more active during sleep even in normal subjects. The greater activation in motor areas, in fact, is believed to play a key role in providing an adequate response to arousal.48 A spectral analysis focusing on the pre-arousal period in normal subjects, performed on a single channel, revealed an increase and the coexistence of slow (delta, theta) and fast (alpha, beta) EEG frequencies53 in the seconds immediately before the arousal, similar to our results in DoA. Overall, several research studies from the last 30 years on the arousal process recognized the heterogeneity of possible manifestations, going from an autonomic/subcortical level, characterized by heart or respiration changes, to cortical modifications, represented by a “synchronized” or a “desynchronized” response, respectively reflected by slower or faster EEG frequencies.47,53–57 Moreover, recent studies highlighted topographic variability in cortical activation/deactivation patterns during normal arousals,45,58 suggesting that this heterogeneity may depend on several factors comprising the sleep stage, the sleep depth, and internal or external triggers.45,47 In turn, this heterogeneity reflects the double nature of the arousal process, preserving the continuity of sleep, on the one hand, and ensuring a prompt response to a stimulus, on the other.45,47,48,54,58 The clinical result is that one may either keep sleeping or awaken in response to a perturbing stimulus. DoA patients, conversely, appear mentally sleeping but physically awake, a response which embodies at the same time the double nature of the arousal.59 Among studies focused on the pre-arousal activity in DoA (Table 1), only few included controls and showed a higher spectral power in delta band before DoA episodes,19–21 suggesting a greater difficulty in fully awakening in DoA. However, homogeneous studies directly comparing DoA and healthy subjects are lacking and potential differences in the pre-arousal period should be searched in the presence of topographic distribution, different power of EEG bands, along with differences in connectivity pathways. Some studies comparing DoA and healthy subjects discovered slight functional and even structural alterations in DoA during wakefulness or normal sleep, independently of episodes, pointing to increased excitability of motor and cingulate areas,31 impaired inhibitory mechanisms within the motor cortex,60 potentially underpinned by a volumetric reduction of midcingulate cortex.61 These alterations suggest that subthreshold triggers might be sufficient to activate motor responses in these predisposed patients during NREM sleep, leading to increased and dysfunctional arousability. This “dissociated” response, from an evolutionary perspective, has been regarded as a dysfunctional survival reflex, with a prompt motor and emotional activation in response to sudden external threats, together with the persistence of sleep need over other brain areas,16,62 leading to potential traumatic injuries during DoA episodes.
A Common Mechanism Underlying Episodes of Different Complexity and Its Clinical Inference
Our work demonstrated no significant differences in the spectral EEG preceding simpler and more complex DoA episodes. In a previous paper from our group, SAMs were described as the simpler motor patterns often corresponding to a smaller fragment of the more complex RAMs and CAMs episodes in DoA patients.7 This observation led to the speculation that a similar mechanism could underlie simple and elaborate episodes, and the higher complexity might reflect a greater impairment of the arousal process, responsible for the gating of different motor patterns.7 EEG quantitative studies described in the literature have often focused on more elaborate episodes in smaller samples compared to ours (Table 1). One previous EEG study analyzing the distribution of hypersynchronous delta waves detected by visual EEG inspection did not disclose differences in simple and complex episodes,29 classified according to the different behavioral manifestations.6 Indeed, our results reveal that the overall activation preceding DoA episodes does not differ between simpler and more complex events, suggesting that SAMs share a similar pathophysiological ground with the more elaborate episodes. This finding has relevant clinical implications due to the higher occurrence and probability of recording SAMs during VPSG, hence providing good diagnostic accuracy even in the absence of complex behaviors. Moreover, SAMs could also represent a stable long-life “trait” in DoA subjects, evolving into more complex manifestations when predisposing conditions or particular triggers occur.
In the normalized maps, a small but significant micro-cluster of decreased delta power over a small posterior area was detected in SAMs. Due to the low localizing power of standard EEG montage, this result is difficult to interpret and any consideration would be merely speculative. However, it might be hypothesized that lower posterior slow waves represent higher levels of arousal/activation and therefore a lower possibility of evolving into a longer and more complex episode. In addition, it must be kept in mind that the analysis of EEG spectrum is limited to the pre-episode period, since the episode itself is habitually covered with movement artifacts. Therefore, it is likely that reliable differences between simple and complex episodes have to be searched for in the analysis of the event itself. In this regard, stereo-EEG studies revealed that several brain areas might display both slow and fast EEG rhythms, suggesting that DoA episodes are underpinned by more than an exclusive pattern,17 which might well explain the different complexity and/or the distinctive clinical manifestations. On the other hand, a large study sample is hard to obtain with stereo-EEG studies, hence a systematic analysis focused on the different complexity appears difficult to provide.
In summary, our work provides the identification of a similar pattern of EEG bands distribution in episodes of different complexity, in close proximity to the event, over a large number of DoA episodes. Of note, identifying specific sleep markers before parasomnias might also help differentiate between NREM parasomnias and other motor sleep disorders or sleep-related hypermotor epilepsy (SHE), whose differential diagnosis remains challenging in particular cases or in the presence of only minor events.63 A recent work moved a step in this direction, providing an analysis of the periodic and aperiodic components of EEG power spectrum in DoA and SHE patients, in sleep segments free from episodes.64 However, an analysis of the pre-episode might be helpful to identify distinctive patterns before the motor events, especially when only minor events are recorded, leaving diagnostic uncertainty. In addition, the identification of a specific episode before its occurrence might possibly be helpful, in future research, to develop closed loop techniques65 in order to prevent it.
To date, our sample is the largest dataset of DoA episodes analyzed by means of spectral EEG technique. The study of EEG frequencies, especially SWA, might be influenced by inter-individual differences providing less reliable results, in case of small samples.22 Therefore, collecting over 300 episodes might ensure a more normal distribution and flatten the inter-individual differences in the study of the EEG spectrum. In addition, the whole scalp and broad-band analysis provides some insight into the global dynamic of EEG frequencies before the episodes.
All our patients were recorded following their normal sleep habits in their habitual sleep setting in the absence of any sleep deprivation procedure. If, on the one hand, sleep deprivation elicits more complex episodes, the influence on sleep homeostasis could have altered the EEG spectral analysis, on the other. Moreover, home-based VPSGs minimize the “first night effect”66,67 and provide a “natural” sleep environment possibly allowing for the collection of more events.
Our work has some limitations to disclose. First, the limited number of channels of standard EEG recording limits further and more accurate topographic investigations in our study. In addition, patients in our cohort were not directly questioned at the end of each episode. Therefore, a correlation between clinical parameters (orientation, awareness, recall of dream scenario) with EEG measures was not possible. This kind of approach is highly warranted in the study of NREM parasomnias, since they can be considered an ideal model to address different neurocognitive domains during sleep (memory, attention, oneiric content). Finally, we did not have a control group of healthy sleepers. Extending the analysis to physiological arousals of a control group is essential to deepen and help shed light on current understanding of these fascinating disorders.
Conclusion
In conclusion, the activity preceding simpler and more complex episodes is not significantly different, suggesting a common neurophysiologic mechanism that leads to their occurrence, with possible implications for the management of these disorders and their natural history. The results of our work confirm the view of NREM parasomnias as a complex state, with intermingled features of both wakefulness and SWS. Sleep as a local phenomenon has been demonstrated also in normal subjects, reinforcing the consideration that DoA patients embody the ultimate deregulation of a dysfunctional arousal process, where the tip of the iceberg is the clinical motor episode,59 with evidence of the coexistence of wake-like and sleep-like activity even in the same areas.17 The presence of broadband alterations with a specific local topographic distribution in the pre-episode disclosed in our study might provide diagnostic utility as well as a deeper understanding of DoA networks and pathophysiology.
Larger samples including a control group, homogeneous study designs and additional functional analysis techniques are needed to further elucidate DoA pathophysiology.
Acknowledgments
The authors wish to thank Cecilia Baroncini for English editing.
Disclosure
The authors report no conflicts of interest in this work.
References
1. American Academy of Sleep Medicine. International Classification of Sleep Disorders.
2. Blatt I, Peled R, Gadoth N, Lavie P. The value of sleep recording in evaluating somnambulism in young adults. Electroencephalogr Clin Neurophysiol. 1991;78(6):407–412. doi:10.1016/0013-4694(91)90058-c
3. Zucconi M, Oldani A, Ferini-Strambi L, Smirne S. Arousal fluctuations in non-rapid eye movement parasomnias: the role of cyclic alternating pattern as a measure of sleep instability. J Clin Neurophysiol off Publ Am Electroencephalogr Soc. 1995;12(2):147–154.
4. Kavey NB, Whyte J, Resor SR, Gidro-Frank S. Somnambulism in adults. Neurology. 1990;40(5):749–752. doi:10.1212/wnl.40.5.749
5. Derry CP, Harvey AS, Walker MC, Duncan JS, Berkovic SF. NREM arousal parasomnias and their distinction from nocturnal frontal lobe epilepsy: a video EEG analysis. Sleep. 2009;32(12):1637–1644. doi:10.1093/sleep/32.12.1637
6. Joncas S, Zadra A, Paquet J, Montplaisir J. The value of sleep deprivation as a diagnostic tool in adult sleepwalkers. Neurology. 2002;58(6):936–940. doi:10.1212/wnl.58.6.936
7. Loddo G, Sessagesimi E, Mignani F, et al. Specific motor patterns of arousal disorders in adults: a video-polysomnographic analysis of 184 episodes. Sleep Med. 2018;41:102–109. doi:10.1016/j.sleep.2017.08.019
8. Jacobson A, Kales A, Lehmann D, Zweizig JR. Somnambulism: all-night electroencephalographic studies. Science. 1965;148(3672):975–977. doi:10.1126/science.148.3672.975
9. Zadra A, Desautels A, Petit D, Montplaisir J. Somnambulism: clinical aspects and pathophysiological hypotheses. Lancet Neurol. 2013;12(3):285–294. doi:10.1016/S1474-4422(12)70322-8
10. Castelnovo A, Lopez R, Proserpio P, Nobili L, Dauvilliers Y. NREM sleep parasomnias as disorders of sleep-state dissociation. Nat Rev Neurol. 2018;14(8):470–481. doi:10.1038/s41582-018-0030-y
11. Baldini T, Loddo G, Sessagesimi E, et al. Clinical features and pathophysiology of disorders of arousal in adults: a window into the sleeping brain. Front Neurol. 2019;10:526. doi:10.3389/fneur.2019.00526
12. Adamantidis AR, Gutierrez Herrera C, Gent TC. Oscillating circuitries in the sleeping brain. Nat Rev Neurosci. 2019;20(12):746–762. doi:10.1038/s41583-019-0223-4
13. Bassetti C, Vella S, Donati F, Wielepp P, Weder B. SPECT during sleepwalking. Lancet Lond Engl. 2000;356(9228):484–485. doi:10.1016/S0140-6736(00)02561-7
14. Terzaghi M, Sartori I, Tassi L, et al. Evidence of dissociated arousal states during NREM parasomnia from an intracerebral neurophysiological study. Sleep. 2009;32(3):409–412. doi:10.1093/sleep/32.3.409
15. Terzaghi M, Sartori I, Tassi L, et al. Dissociated local arousal states underlying essential clinical features of non-rapid eye movement arousal parasomnia: an intracerebral stereo-electroencephalographic study. J Sleep Res. 2012;21(5):502–506. doi:10.1111/j.1365-2869.2012.01003.x
16. Gibbs SA, Proserpio P, Terzaghi M, et al. Sleep-related epileptic behaviors and non-REM-related parasomnias: insights from stereo-EEG. Sleep Med Rev. 2016;25:4–20. doi:10.1016/j.smrv.2015.05.002
17. Flamand M, Boudet S, Lopes R, et al. Confusional arousals during non-rapid eye movement sleep: evidence from intracerebral recordings. Sleep. 2018;41(10):10. doi:10.1093/sleep/zsy139
18. Mahowald MW, Schenck CH. Insights from studying human sleep disorders. Nature. 2005;437(7063):1279–1285. doi:10.1038/nature04287
19. Espa F, Ondze B, Deglise P, Billiard M, Besset A. Sleep architecture, slow wave activity, and sleep spindles in adult patients with sleepwalking and sleep terrors. Clin Neurophysiol off J Int Fed Clin Neurophysiol. 2000;111(5):929–939. doi:10.1016/s1388-2457(00)00249-2
20. Guilleminault C, Poyares D, Aftab FA, Palombini L, Abat F. Sleep and wakefulness in somnambulism: a spectral analysis study. J Psychosom Res. 2001;51(2):411–416. doi:10.1016/s0022-3999(01)00187-8
21. Zadra AL, Nielsen TA. Topographical EEG mapping in a case of recurrent sleep terrors. Dreaming. 1998;8(2):67–74. doi:10.1023/B:DREM.0000005897.62698.1b
22. Jaar O, Pilon M, Carrier J, Montplaisir J, Zadra A. Analysis of slow-wave activity and slow-wave oscillations prior to somnambulism. Sleep. 2010;33(11):1511–1516. doi:10.1093/sleep/33.11.1511
23. Januszko P, Niemcewicz S, Gajda T, et al. Sleepwalking episodes are preceded by arousal-related activation in the cingulate motor area: EEG current density imaging. Clin Neurophysiol off J Int Fed Clin Neurophysiol. 2016;127(1):530–536. doi:10.1016/j.clinph.2015.01.014
24. Desjardins MÈ, Carrier J, Lina JM, et al. EEG functional connectivity prior to sleepwalking: evidence of interplay between sleep and wakefulness. Sleep. 2017;40:4. doi:10.1093/sleep/zsx024
25. Perrault R, Carrier J, Desautels A, Montplaisir J, Zadra A. Electroencephalographic slow waves prior to sleepwalking episodes. Sleep Med. 2014;15(12):1468–1472. doi:10.1016/j.sleep.2014.07.020
26. Ratti PL, Amato N, David O, Manconi M. A high-density polysomnographic picture of disorders of arousal. Sleep. 2018;41:11. doi:10.1093/sleep/zsy162
27. Camaioni M, Scarpelli S, Gorgoni M, Alfonsi V, De Gennaro L. EEG patterns prior to motor activations of parasomnias: a systematic review. Nat Sci Sleep. 2021;13:713–728. doi:10.2147/NSS.S306614
28. Schenck CH, Pareja JA, Patterson AL, Mahowald MW. Analysis of polysomnographic events surrounding 252 slow-wave sleep arousals in thirty-eight adults with injurious sleepwalking and sleep terrors. J Clin Neurophysiol off Publ Am Electroencephalogr Soc. 1998;15(2):159–166. doi:10.1097/00004691-199803000-00010
29. Pilon M, Zadra A, Joncas S, Montplaisir J. Hypersynchronous delta waves and somnambulism: brain topography and effect of sleep deprivation. Sleep. 2006;29(1):77–84. doi:10.1093/sleep/29.1.77
30. Berry RB, Quan SF, Abreu AR, et al. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. Version 2.6. Darien, IL: American Academy of Sleep Medicine; 2020.
31. Castelnovo A, Riedner BA, Smith RF, Tononi G, Boly M, Benca RM. Scalp and source power topography in sleepwalking and sleep terrors: a high-density EEG study. Sleep. 2016;39(10):1815–1825. doi:10.5665/sleep.6162
32. Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002;15(1):1–25. doi:10.1002/hbm.1058
33. Castelnovo A, Zago M, Casetta C, et al. Slow wave oscillations in schizophrenia first-degree relatives: a confirmatory analysis and feasibility study on slow wave traveling. Schizophr Res. 2020;221:37–43. doi:10.1016/j.schres.2020.03.025
34. Spiess M, Bernardi G, Kurth S, et al. How do children fall asleep? A high-density EEG study of slow waves in the transition from wake to sleep. NeuroImage. 2018;178:23–35. doi:10.1016/j.neuroimage.2018.05.024
35. Siclari F, Baird B, Perogamvros L, et al. The neural correlates of dreaming. Nat Neurosci. 2017;20(6):872–878. doi:10.1038/nn.4545
36. Siclari F, Valli K, Arnulf I. Dreams and nightmares in healthy adults and in patients with sleep and neurological disorders. Lancet Neurol. 2020;19(10):849–859. doi:10.1016/S1474-4422(20)30275-1
37. Oudiette D, Leu S, Pottier M, Buzare MA, Brion A, Arnulf I. Dreamlike mentations during sleepwalking and sleep terrors in adults. Sleep. 2009;32(12):1621–1627. doi:10.1093/sleep/32.12.1621
38. Castelnovo A, Loddo G, Provini F, Miano S, Manconi M. Mental activity during episodes of sleepwalking, night terrors or confusional arousals: differences between children and adults. Nat Sci Sleep. 2021;13:829–840. doi:10.2147/NSS.S309868
39. Sarasso S, Pigorini A, Proserpio P, Gibbs SA, Massimini M, Nobili L. Fluid boundaries between wake and sleep: experimental evidence from Stereo-EEG recordings. Arch Ital Biol. 2014;152(2–3):169–177. doi:10.12871/0002982920142311
40. Assenza G, Capone F, Di Biase L, et al. Oscillatory activities in neurological disorders of elderly: biomarkers to target for neuromodulation. Front Aging Neurosci. 2017;9:189. doi:10.3389/fnagi.2017.00189
41. Martin RA, Cukiert A, Blumenfeld H. Short-term changes in cortical physiological arousal measured by electroencephalography during thalamic centromedian deep brain stimulation. Epilepsia. 2021;62(11):2604–2614. doi:10.1111/epi.17042
42. Steriade M. Grouping of brain rhythms in corticothalamic systems. Neuroscience. 2006;137(4):1087–1106. doi:10.1016/j.neuroscience.2005.10.029
43. Koch C, Massimini M, Boly M, Tononi G. Neural correlates of consciousness: progress and problems. Nat Rev Neurosci. 2016;17(5):307–321. doi:10.1038/nrn.2016.22
44. Murphy M, Bruno MA, Riedner BA, et al. Propofol anesthesia and sleep: a high-density EEG study. Sleep. 2011;34(3):283A–291A. doi:10.1093/sleep/34.3.283
45. Peter-Derex L, Magnin M, Bastuji H. Heterogeneity of arousals in human sleep: a stereo-electroencephalographic study. NeuroImage. 2015;123:229–244. doi:10.1016/j.neuroimage.2015.07.057
46. Fernandez LMJ, Lüthi A. Sleep spindles: mechanisms and functions. Physiol Rev. 2020;100(2):805–868. doi:10.1152/physrev.00042.2018
47. Halász P, Terzano M, Parrino L, Bódizs R. The nature of arousal in sleep. J Sleep Res. 2004;13(1):1–23. doi:10.1111/j.1365-2869.2004.00388.x
48. Latreille V, von Ellenrieder N, Peter-Derex L, Dubeau F, Gotman J, Frauscher B. The human K-complex: insights from combined scalp-intracranial EEG recordings. NeuroImage. 2020;213:116748. doi:10.1016/j.neuroimage.2020.116748
49. Nobili L, Ferrara M, Moroni F, et al. Dissociated wake-like and sleep-like electro-cortical activity during sleep. NeuroImage. 2011;58(2):612–619. doi:10.1016/j.neuroimage.2011.06.032
50. Nobili L, De Gennaro L, Proserpio P, et al. Local aspects of sleep: observations from intracerebral recordings in humans. Prog Brain Res. 2012;199:219–232. doi:10.1016/B978-0-444-59427-3.00013-7
51. Magnin M, Rey M, Bastuji H, Guillemant P, Mauguière F, Garcia-Larrea L. Thalamic deactivation at sleep onset precedes that of the cerebral cortex in humans. Proc Natl Acad Sci U S A. 2010;107(8):3829–3833. doi:10.1073/pnas.0909710107
52. von Ellenrieder N, Gotman J, Zelmann R, et al. How the human brain sleeps: direct cortical recordings of normal brain activity. Ann Neurol. 2020;87(2):289–301. doi:10.1002/ana.25651
53. Sforza E, Jouny C, Ibanez V. Cardiac activation during arousal in humans: further evidence for hierarchy in the arousal response. Clin Neurophysiol off J Int Fed Clin Neurophysiol. 2000;111(9):1611–1619. doi:10.1016/s1388-2457(00)00363-1
54. Parrino L, Halasz P, Tassinari CA, Terzano MG. CAP, epilepsy and motor events during sleep: the unifying role of arousal. Sleep Med Rev. 2006;10(4):267–285. doi:10.1016/j.smrv.2005.12.004
55. Janackova S, Sforza E. Neurobiology of sleep fragmentation: cortical and autonomic markers of sleep disorders. Curr Pharm Des. 2008;14(32):3474–3480. doi:10.2174/138161208786549335
56. Sforza E, Juony C, Ibanez V. Time-dependent variation in cerebral and autonomic activity during periodic leg movements in sleep: implications for arousal mechanisms. Clin Neurophysiol off J Int Fed Clin Neurophysiol. 2002;113(6):883–891. doi:10.1016/s1388-2457(02)00066-4
57. Kuo TBJ, Chen CY, Hsu YC, Yang CCH. EEG beta power and heart rate variability describe the association between cortical and autonomic arousals across sleep. Auton Neurosci Basic Clin. 2016;194:32–37. doi:10.1016/j.autneu.2015.12.001
58. Zou G, Xu J, Zhou S, et al. Functional MRI of arousals in non-rapid eye movement sleep. Sleep. 2019. doi:10.1093/sleep/zsz218
59. Mainieri G, Loddo G, Provini F. Disorders of arousal: a chronobiological perspective. Clocks Sleep. 2021;3(1):53–65. doi:10.3390/clockssleep3010004
60. Oliviero A, Della marca G, Tonali PA, et al. Functional involvement of cerebral cortex in adult sleepwalking. J Neurol. 2007;254(8):1066–1072. doi:10.1007/s00415-006-0489-0
61. Heidbreder A, Stefani A, Brandauer E, et al. Gray matter abnormalities of the dorsal posterior cingulate in sleep walking. Sleep Med. 2017;36:152–155. doi:10.1016/j.sleep.2017.05.007
62. Perogamvros L. An Evolutionary-Emotional Perspective of Insomnia and Parasomnias; 2018.
63. Loddo G, Baldassarri L, Zenesini C, et al. Seizures with paroxysmal arousals in sleep-related hypermotor epilepsy (SHE): dissecting epilepsy from NREM parasomnias. Epilepsia. 2020;61(10):2194–2202. doi:10.1111/epi.16659
64. Pani SM, Fraschini M, Figorilli M, Tamburrino L, Ferri R, Puligheddu M. Sleep-related hypermotor epilepsy and non-rapid eye movement parasomnias: differences in the periodic and aperiodic component of the electroencephalographic power spectra. J Sleep Res. 2021;30(5):e13339. doi:10.1111/jsr.13339
65. Zelmann R, Paulk AC, Basu I, et al. CLoSES: a platform for closed-loop intracranial stimulation in humans. NeuroImage. 2020;223:117314. doi:10.1016/j.neuroimage.2020.117314
66. Edinger JD, Fins AI, Sullivan RJ, et al. Sleep in the laboratory and sleep at home: comparisons of older insomniacs and normal sleepers. Sleep. 1997;20(12):1119–1126. doi:10.1093/sleep/20.12.1119
67. Bruyneel M, Sanida C, Art G, et al. Sleep efficiency during sleep studies: results of a prospective study comparing home-based and in-hospital polysomnography. J Sleep Res. 2011;20(1 Pt 2):201–206. doi:10.1111/j.1365-2869.2010.00859.x
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


