Back to Journals » Infection and Drug Resistance » Volume 16
eDOTS: Improving the Treatment of Pulmonary Tuberculosis in Xinjiang, China
Authors Guo G, Zheng Y , Ma X, Sun L, Wushouer Q, Jia B, Yusufu M, Wen S, Abudureyimu T, Peng X, Liu Z, Mamut X, Chen Y, Zhang J, Yang Y, Huangfu L, Li J, Zhang W
Received 6 September 2023
Accepted for publication 1 December 2023
Published 7 December 2023 Volume 2023:16 Pages 7497—7505
DOI https://doi.org/10.2147/IDR.S438962
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Sandip Patil
Gang Guo,1,2,* Yanling Zheng,3,* Xuexian Ma,4 Li Sun,5 Qimanguli Wushouer,1 Bin Jia,1 Muladier Yusufu,5 Shu Wen,6 Tuerhong Abudureyimu,6 Xiaowang Peng,6 Zhenjiang Liu,6 Xirizat Mamut,6 Yanggui Chen,7 Jian Zhang,8 Yuling Yang,9 Liusheng Huangfu,9 Jun Li,1 Wenbao Zhang1
1State Key Laboratory Pathogenesis, Prevention and Treatment of High Incidence Diseases in Central Asian, First Affiliated Hospital of Xinjiang Medical University, Urumqi, People’s Republic of China; 2Suzhou Center for Disease Control and Prevention, Suzhou, People’s Republic of China; 3College of Medical Engineering and Technology, Xinjiang Medical University, Urumqi, People’s Republic of China; 4Shache People’s Hospital, Shache, People’s Republic of China; 5Yingjisha County Center for Disease Control and Prevention, Yingjisha, People’s Republic of China; 6Kashgar Regional Lung Hospital, Kashgar, People’s Republic of China; 7Urumqi Municipal Center for Disease Control and Prevention, Urumqi, People’s Republic of China; 8High-Tech District Center for Disease Control and Prevention of Urumqi, Urumqi, People’s Republic of China; 9Xinhuarui Information Co., LTD, Urumqi, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Wenbao Zhang, Tel +86 15276692787, Email [email protected]
Purpose: To increase pulmonary tuberculosis (PTB) treatment adherence in Xinjiang Region, an electronic DOTS (eDOTS) system developed was applied and evaluated.
Methods: An eDOTS system comprised electronic medicine boxes, mobile phones and a central processing platform. Between April and June 2016, persons with active PTB (PAPTB) were recruited from villages and a city and were prescribed a six-month course of antibiotics using either DOTS or eDOTS. Treatment adherence rate and chest X-ray digital radiography (DR) score were used to evaluate usefulness of eDOTS.
Results: A total 167 PAPTB were recruited with 81 participants from villages and 86 from neighbourhoods. Of the 81 village patients, 43 (53%) used eDOTS and 38 (47%) used DOTS. Among the 86 patients from neighbourhoods, 50 (58%) used eDOTS and 36 (42%) used DOTS. After 6 months of treatment, the average treatment compliance of the village patients who used eDOTS were 47.0%± 20.5% compared to 26.7%± 21.1% who used DOTS (t=− 4.475, p< 0.001). The patients using eDOTS from both the villages and city had significantly lower X-ray DR scores than the patients using DOTS by 1.81 points, 95% CI (0.72– 2.90) and 1.05 points, 95% CI (0.15– 1.95), respectively.
Conclusion: eDOTS is an effective means of managing the treatment of active PTB patients through daily reminding and monitoring of patient compliance. Ease of contact with doctors and special education programs encouraged PAPTB to complete their treatment course as required.
Keywords: tuberculosis, infection, drug treatment, prevention/control program
Background
Pulmonary tuberculosis (PTB) is a serious public health disease that challenges health systems around the world, especially in China, which accounts for 15% of the global PTB burden.1–4 Xinjiang Uygur Autonomous Region (Xinjiang) is an area of high endemicity of PTB.5–8 In 2010, the prevalence of persons with active PTB (PAPTB) in Xinjiang was 1526 per 100,000 population, and the prevalence of smear-positive PTB was 433 per 100,000 population, which is higher than the national average (459 per 100,000 population for active PTB and 66 per 100,000 for smear-positive PTB).5,9 PTB cases reported in southern Xinjiang, including the Kashgar, Hotan, Aksu, and Kizilsu Kirghiz prefectures, accounted for 70% of the total PTB cases in Xinjiang.5,8 In 2018, the incidence in these four prefectures (292 per 100,000 population) was 4.8 times higher than the national average (61 per 100,000 population) and was the highest incidence rate in China.10 From 2008 to 2018, Yingjisha County in Kashgar Prefecture had an average annual PTB incidence of 720.56 per 100,000 population, which was the highest in Xinjiang.8
The World Health Organization (WHO) recommends the Directly Observed Therapy Short-Course (DOTS) strategy for stopping TB transmission.11 The strategy was introduced to China in the 1990s, and by 2010, it reduced TB prevalence by half;12 However, the strategy did not reduce TB transmission in Xinjiang. A survey conducted in during 2010–11 showed that the prevalence of PAPTB was 1526 per 100,000 population—2.29 times higher than the prevalence in 1990 (666 per 100,000 population).5 A report has indicated that the poor TB treatment outcome in Xinjiang is likely due to low treatment adherence.13
Effective management of PTB treatment adherence remains challenging. Digital adherence technologies (DATs) including 99 DOTS, medication reminders, and digital remote treatment adherence monitoring devices (evriMED pillboxes), and the drone observed therapy system (DrOTS) have been proposed for facilitating and enhancing treatment adherence of persons with PAPTB.14–17 While digital health interventions may be accepted by some PTB patients, evidence about the effect of these approaches on improving PTB care remains limited.18–21
To improve the treatment adherence in PTB patients, we developed an electronic directly observed therapy short-course (eDOTS) system that reminds PAPTB to take their medication daily, monitors their adherence, and provides an educational program. Here, we report findings from a pilot study of the eDOTS system that was used for PAPTB from remote villages and a city in two areas in Xinjiang, China.
Methods
Patients Screening Process
To determine the usefulness and efficacy of the eDOTS system for managing the treatment of PAPTB, 81 PAPTB from 14 remote villages in Yinjisha County of Kashgar Prefecture in southern Xinjiang and 86 PAPTB from two city neighborhoods in Urumqi City area in northern Xinjiang were enrolled in the pilot. Such a choice was made considering that there were differences in economic and education level between northern and southern Xinjiang and between city and village areas, which may affect the treatment adherence of PTB.
Approximately half of the patients were assigned to the DOTS group, and the remaining half of the patients were assigned to the eDOTS group at the time of diagnosis. All of the patients were between 15 and 75 years old, and they were diagnosed through a clinic or health check program. The diagnosis of active PTB was based on positive chest digital radiography (DR) and the typical symptoms of PAPTB according to the published criteria, such as night sweats, chest pain, persistent cough, and fever for more than two weeks.22 PAPTB retreatment, pregnant women and PAPTB with HIV infection or those using hormone therapy were excluded.
Drug Treatment
Standard anti-TB drug combinations containing isoniazid (H), rifampicin (R), pyrazinamide (Z), and ethambutol (E) were prescribed as the first line of treatment for TB in accordance with the WHO recommendation.23 All of the patients were prescribed two treatment courses, which included a two-month intensive phase with a daily dose of fixed-dose combination tablets containing HRZE and a four-month continuation phase of daily HR.
Intervention Strategy
All of the doctors and staff from the villages and city neighborhoods involved in the eDOTS study received a one-day training session to introduce the device and provide instructions for use. Once a PAPTB diagnosis was confirmed, the patient was registered in the study, and data such as name, ID, age, gender, address, diagnosis information (eg, smear results and radiography), and treatment prescription were recorded. The patient was then assigned to either the DOTS or eDOTS group. Both groups were prescribed anti-TB drugs for 25–26 weeks. All of the patients went to see the doctor once a week to obtain their medicine for the following week. For patients in the DOTS group, the previous week’s medication history was recorded when they reported to the doctor to receive the next-week’s medication. For those in the eDOTS group, the medication history data were automatically recorded and uploaded to the server in the CDC daily.
eDOTS System
The eDOTS system is composed of an electronic medicine box (eBox) for patients, a private mobile phone (management software program I, WeChat App installed, for all of the participants including PAPTB, doctors, nurses, management staff in the local Center for Disease Control and Prevention (CDC), and relatives of the patients), and a central processing platform (management software program II, designed for the CDC), which allows the CDC to manage and analyze the data. Patients are registered in the eDOTS system with the same information required for TB treatment through DOTS, and the system is immediately activated. Every day the eBox sets off an alarm to remind patients to take their medication at a convenient time determined by the patients, normally before breakfast. When the patient opens the eBox, the camera on the eBox takes pictures of the patient’s medication status, which is automatically transferred to the management system. Once the patient has taken the medication, the system records the task as complete. The eBox continues to alarm for 30 minutes if the patient does not open the eBox, after which the system automatically sends a message to the doctor and a relative with a request to remind the patient to take the medication. If the patient has not taken the medication by 5 pm, the system records the patient as “not-taking medicine” (Figures 1 and 2).
 |
Figure 1 eBox structure. Components and function of the eBOX are shown. (A) Open state; (B) Closed state. |
 |
Figure 2 Working flow diagram of eDOTS system. (A) Flow chart using eDOTS, (B) Cartoon using eDOTS. |
Treatment Adherence Measurement
The individual’s weekly treatment adherence rate was calculated as a percentage of the patient’s prescribed number of medications per week. For example, if a PAPTB should take the medication seven times a week but only takes it three times, the PAPTB’s treatment compliance for that week is 3/7 or 42.9%.
PTB DR Score
To evaluate the efficacy of the treatment, we established a PTB DR score system based on the features of chest DR image (Figure S1). Three levels of TB pathology were defined as follows: level 1, showing exudation (0, 1, 2 and 3 scores); level 2, showing infiltration (0, 1, 2, 3 and 4 scores); and level 3, showing density of the PTB lesion (0, 1, 2 and 3 scores), creating a total possible score of 10 (Figure S2). Three experienced radiologists were asked to score each DR image of PAPTB.
Statistical Analysis
Both the adherence rate and PTB DR scores were used to evaluate and compare the efficacy of the interventions used in the villages and city neighborhoods. Normality and homogeneity-of-variance tests of the data for the treatment adherence rates and DR scores were carried out with Kolmogorov–Smirnov test and Levene’s test, respectively. In the case of normal distribution and uniform variance, the difference between DOTS and eDOTS was analyzed by Student’s t-test between the city and village patients. A t-test was applied when PTB DR scores were compared between the DOTS and eDOTS groups. A paired t-test was used to compare the difference in the DR scores within the DOTS or eDOTS groups before and after treatment. DR scores were analyzed and compared among different groups by two-way ANOVA with Tukey’s multiple-comparison test. A chi-square test was used for counting data including gender and ethnic composition ratio. Differences were considered to be significant at p < 0.05.
Results
PAPTB and Interventions
Between April and June 2016, a total 167 PAPTB were randomly selected to participate in the study. Among these patients, 81 were from 14 Kashgar villages. Of those 81, 43 patients were from nine villages and used the eDOTS system. As these 43 patients were identified in the same health check, they were assigned eDOTS at the same time. The remaining 38 patients were from five other villages and were assigned the traditional DOTS system. There was a doctor in each village who took care of the patients there. A total of 86 PAPTB were from Urumqi City neighborhoods. Fifty of the 86 city patients used the eDOTS system and were cared for by one community doctor. The remaining 36 city neighborhood patients used the DOTS system and visited doctors once a week in a hospital in the city (Table 1).
 |
Table 1 Basic Characteristics of Participants |
Adherence in Villages
In the study, a weekly adherence rate was used to evaluate the patient’s adherence. The average treatment adherence rate was 49.8%±26.9% during the first week of using the eDOTS system in the villages and it was 51.1%±28.5% among the village PAPTB using eDOTS during the first six weeks. However, at week 7, due to an internet problem, traditional DOTS had to be used for one week. Once the internet problem was resolved, the eDOTS system showed that the adherence rate dropped to 26.7% ±21.1% during week 8. The low percentage (24.9%±23.9%) of adherence was sustained for five weeks to week 13 when the village doctors were asked to make daily visits to each of these patients. The adherence rate increased to 65.7% ±26.6% at week 20. At week 21, an infectious diseases specialist from a hospital was asked to call all of the patients assigned to the eDOTS system who had not been taking their medication regularly. The adherence rate then increased to 77.8%±17.1% with a peak of 82.0% ±12.3% from week 23 to 26 (Figure 3A). From week 13 to week 26, the average adherence rate was 54.6%±19.1%.
 |
Figure 3 The mean percentage of PAPTB taking anti-TB drug weekly recorded by eDOTS. (A) In village; (B) In city. Dots and error bars denote mean ± SD. |
Adherence in Urumqi City
For the 50 patients from the city neighborhoods who used eDOTS, the adherence rate over the first 12 weeks of treatment was close to 100%. After 12 weeks, the rate dropped slightly, but the overall adherence rate continued to be approximately 90% (Figure 3B).
Over the first six weeks of treatment, the adherence of the village-based patients using eDOTS (51.1% ± 28.5%) was significantly lower than that of the city-based patients using eDOTS (98.9% ± 1.1%; t = −32.781, p < 0.001).
However, both in the Kashgar villages and Urumqi city neighborhoods, the record cards showed that 100% of PAPTB using the traditional DOTS strategy were taking the medicines. We believe that this result lacks objectivity and authenticity.
PTB DR Score
There was no statistically significant difference in the chest DR scores between the PAPTB from the villages and those from the city neighborhoods before treatment. The DR scores of the PAPTB using eDOTS were significantly reduced in both the villages and the city at the end of the intervention period. The comparison of the scores showed that the patients using eDOTS from the villages and the city neighborhoods had significantly lower scores than the patients using DOTS by 1.81 points (95% CI: 0.72–2.90) and 1.05 points (95% CI: 0.15–1.95), respectively, after 25 weeks of treatment. However, regardless of whether DOTS or eDOTS was used, there were significant differences (p < 0.01) in the PAPTB’s PTB scores before and after treatment (Table 2 and Figure 4).
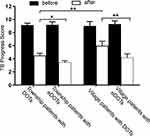 |
Figure 4 Comparison of treatment effect of PAPTB treated with eDOTS or DOTS in village or city. Data are shown as mean ± 95% CI. *p < 0.05, **p < 0.01. |
 |
Table 2 The Results of Comparative Analysis of Digital Radiography Scores Before and After Using DOTS and eDOTS in Different Regions |
The PTB score of the PAPTB from Urumqi City who used eDOTS (3.46 ± 0.57 points) was significantly lower than that of those who used DOTS (4.52 ± 1.45 points) (multivariate ANOVA with Tukey’s multiple-comparison test, p < 0.001). The average PTB score of the PAPTB from the villages who used eDOTS (4.19 ± 1.25 points) was significantly lower than that of those who used DOTS (6.0 ± 1.48) (p < 0.001). At the end of the treatment intervention using eDOTS, there was no difference in the PTB score among the patients from the city neighborhoods and those from the villages (p = 0.51). In contrast, among the patients who used traditional DOTS, the patients from the city neighborhoods had a lower PTB score (4.5 ± 1.6 points) than those from the villages (6.00 ± 1.5 points) (p < 0.001).
Discussion
Several electronic approaches such as evriMED, MERM, and DrOTS based on DATs have been developed for TB treatment and control. evriMED, a device with medication monitoring and reminder functions, has been used for TB treatment in pilot studies with a small number of patients. However, further evidence on the efficacy of the tool in improving treatment adherence of TB patients needs to be evaluated.24 Medication event and reminder monitor (MERM) is similar to our eDOTS,25 but the impact of MERM on health management outcomes and the cost-effectiveness of this system have not been reported. The DrOTS project uses new drone technology as a drug delivery device to reduce the frequency of patients’ visits to the doctors. However, it is very difficult to ascertain the significance of DrOTS for supporting TB treatment.16 In India, the benefit of the 99DOTS program is encouraging in terms of greater convenience and reduced stigma, but there are obvious challenges in its implementation, including webtool stability, staff training, and better coordination.26,27
Although TB can be cured, the successful treatment depends on the high adherence of PAPTB to anti-TB drugs treatment for at least 6 months.28,29 Failure to meet the adherence levels required for DOTS can prolong the treatment duration and promote drug resistance, relapse, and refractory conditions.30–32 Although DOTS has improved the endemicity rates of TB in China, recent studies have shown that the DOTS strategy has not been effective in increasing treatment adherence in some areas.31–33 Most PAPTB take anti-TB drugs at home with DOTS, and village or city neighborhood doctors distribute medication once a week to these patients. In our study, we observed a drop in treatment adherence to 24.9% amongst the village eDOTS patients after they had to revert to the traditional DOTS system for one week due to the loss of internet service. The lower adherence rate may reflect the true adherence of traditional DOTS in the study areas. This adherence rate is much lower than that in other provinces in China, such as Jiangsu (89%), Anhui (90.5%), and Ningxia (93.3%),33 which may explain why the prevalence of PTB is higher in the study areas in Xinjiang. Therefore, a new approach is urgently needed for DOTS PAPTB treatment in high endemic areas. Our eDOTS system may be a solution as it reminds and monitors PTB patients to take anti-TB drugs every day.
Another challenge of TB treatment and control is the large number of PAPTB cases in the high endemic areas. According to the fifth national TB epidemiological survey in 2010, the prevalence rate of PAPTB among people over 15 years in Kashgar was 2727 per 100,000,2 which means that a large number of doctors are needed to manage the treatment. Taking the example of Yingjisha County, the data of the sixth census conducted in 2010 showed that the total population was 263,616, with a total of 231,336 people living in 122 villages, which equates to an average of 1896 villagers per village. If we used an average prevalence of 2727 per 100,000 residents, there would be an average of 51 PAPTB in each of these villages. Each village typically has one village doctor; thus, it is very difficult for one village doctor to properly care for so many PAPTB using traditional DOTS. We believe eDOTS can help village doctors to manage more PAPTB given that in our trial one doctor was easily able to manage 50 PAPTB using eDOTS in Urumqi City.
In addition, PAPTB need an easy way to contact doctors and social workers for medical and psychological consultation, especially when they have difficulty taking the medication. The eDOTS system offers a tool to link PAPTB with doctors in rural areas. We believe that the ability of doctors to contact PAPTB who want to stop taking their medication is crucial for successful treatment of PAPTB. In our study, through the eDOTS system, the village doctors were able to call the PAPTB who had stopped taking anti-TB drugs at week 13, and in the city, hospital doctors called their patients who wanted to stop their medication at week 21. After these calls, the adherence rate increased to 65.1% and 77.2%, respectively. This indicates that regular communication between doctors and their PAPTB is very important for encouraging patients to take their medication.
It has been shown that education of health workers and patients is important for treatment and control of TB.34,35 Retrospective studies have shown that anti-TB drug–induced liver function damage occurs 4–26 days after administration.36 A study in London showed that 53% of liver damage from anti-TB drugs occurred within two weeks of the start of treatment, and 87.6% occurred within eight weeks.37 Some PAPTB experience side effects such as red urine and tears, facial pigmentation, and abnormal liver function accompanied by nausea, vomiting, and other gastrointestinal symptoms. If the patients do not understand the side effects that arise during the course of treatment, they begin to fear the treatment and stop taking it. The eDOTS system has a voice education program that contains 180 key questions and answers about PTB specifically designed for possible problems that appear while taking anti-TB drugs. We believe the voice education program is suitable for home-based treatment and allows family members, as well as the patient, to be educated so that they can then encourage the PAPTB to finish their treatment course.
Data from the China’s fifth national TB epidemiological sampling survey showed that only 14.4% of active PAPTB were smear-positive.9 In addition, it is well known that after a few weeks of treatment, some cases become smear-negative. Therefore, sputum smears were not used as a parameter for evaluating the treatment efficacy in our study. PTB DR scores were developed and used for identifying the severity of PTB and for indirectly assessing the treatment adherence. Through a comparison of DR scores, we found that the patients using eDOTS both in urban and rural areas showed better therapeutic efficacy than those who used the DOTS system.
In this study, there is a limitation. The purpose of this study is to initially understand the adherence of patients on initial treatment for tuberculosis. Due to the limited number of electronic pill boxes and other factors, the included sample size was not large enough, still, the randomness and representativeness of the sample grouping have been achieved as far as possible, so our research results are of reference value.
Conclusions
The application of eDOTS in remote villages and city neighborhoods showed that the system was effective for improving TB treatment through daily automated reminders and monitoring of PTB anti-TB drugs treatment, and it was especially effective in remote and low-income areas. The system can also provide health education programs for encouraging PAPTB to finish their treatment course. Most importantly, PAPTB can contact doctors and relatives any time through the system for help and advice. eDOTS is an effective tool for improving the prevention and management of TB by increasing PAPTB’s treatment adherence.
Ethical Statement and Informed Consent
Our study is in accordance with the ethical principles that have their origin in the Declaration of Helsinki. The study was reviewed and approved by the Ethical Committee of the First Affiliated Hospital of Xinjiang Medical University in adherence with medical ethics (Ethical approval No. 20150824-01). Informed consent was obtained from participants or guardians of participants under 16 years of age and all personal data were kept confidential. At the same time, we confirmed that all methods were performed in accordance with the relevant guidelines and regulations in China.
Acknowledgments
Thanks to these doctors for their expertise in establishing of PTB X-ray DR score criteria. Bin Jia, Qimanguli Wushouer, Wenya Liu, Jin Wang, Muhebaiti Mehesuti and Astar Wushour for establishing the classification of PTB chest X-ray image and scored the PTB lesion progress. We thank Dr Melissa L Burke (Australian BioCommons), Dr Julie Balon and Ms JO-Ann Bateman (The University of Sheffield) for reading and commenting the article.
Author Contributions
All authors made a significant contribution to the work reported, including the conception, study design, execution, acquisition of data, analysis and interpretation. All authors took part in drafting, revising and critically reviewing the article, and approved the final manuscript. In addition, the authors have agreed on the journal to which this manuscript has been submitted and agree to be accountable for all aspects of the work.
Funding
This study was financially supported by the Project of WHO-TDR small grant (201344525), State Key Laboratory of Pathogenesis, Prevention, Treatment of Central Asian High Incidence Diseases Fund (SKL-HIDCA-2021-JH5, SKL-HIDCA- 2021-JH1), Research Program of First Affiliated Hospital of Xinjiang Medical University (SB-2015-02) and the National Natural Science Foundation Project of China (72174175). The grantors were not involved in the study design, analysis or interpretation, or decision to submit this manuscript.
Disclosure
Gang Guo and Yanling Zheng are co-first authors for this study. All authors declared no conflicts of interest in this work.
References
1. Harding E. WHO global progress report on tuberculosis elimination. Lancet Respir Med. 2020;8(1):19. doi:10.1016/S2213-2600(19)30418-7
2. National Technical Steering Group of the Epidemiological Sampling Survey for TB. Implementing office of the epidemiological sampling survey for TB. The prevalence of pulmonary tuberculosis in a national survey across China in 2010. Chin J Antituberculosis. 2012;35(9):665–668.
3. Onozaki I, Law I, Sismanidis C, et al. National tuberculosis prevalence surveys in Asia, 1990–2012: an overview of results and lessons learned. Trop Med Int Health. 2015;20(9):1128–1145. doi:10.1111/tmi.12534
4. Zumla A, George A, Sharma V, et al. The WHO 2014 global tuberculosis report--further to go. Lancet Glob Health. 2015;3(1):e10–2. doi:10.1016/S2214-109X(14)70361-4
5. Yang JM, Jieensi S, Tai XR, et al. Analysis of tuberculosis epidemiological survey conducted in 2010-2011 in Xinjiang Uygur Autonomous Region. Chin J Antituberculosis. 2013;35(12):960–964.
6. Huang R, Li XY, Xiao H, et al. Application of spatial statistical analysis in tuberculosis study. Chin J Antituberculosis. 2016;38(6):432–435. doi:10.3969/j.issn.1000-6621.2016.06.003
7. Li YH, Wu G, Qi L. Analysis of monthly report of tuberculosis control in Xinjiang in 2005. Endemic Dis Bull. 2006;21(6):29–32. doi:10.13215/j.cnki.jbyfkztb.2006.06.015
8. Zhao Z, Liu NQ, Aihaiti Y, et al. Epidemiological and spatial distribution characteristics of tuberculosis in Xinjiang Uygur Autonomous Region from 2008 to 2018. Chin J Antituberculosis. 2019;41(8):893–899. doi:10.26914/c.cnkihy.2019.061369
9. Technical Guidance Group of the Fifth National TB Epidemiological Survey. The office of the fifth national TB epidemiological survey. The fifth national tuberculosis epidemiological survey in 2010. Chin J Antituberculosis. 2012;34(8):485–508.
10. World Health Organization. Country profiles for 30 high TB burden countries. Global tuberculosis report; 2019. Available from: https://www.who.int/tb/publications/global_report/tb19_Report_country_profiles_15October2019.pdf?ua=1.
11. Frieden TR, Munsiff SS. The DOTS strategy for controlling the global tuberculosis epidemic. Clin Chest Med. 2005;26(2):197–205. doi:10.1016/j.ccm.2005.02.001
12. Wang LX, Zhang H, Ruan YZ, et al. Tuberculosis prevalence in China, 1990-2010; a longitudinal analysis of national survey data. Lancet. 2014;383(9934):2057–2064. doi:10.1016/S0140-6736(13)62639-2
13. Gu XM, Wu WD. Effective evaluation of national tuberculosis control programme in Xinjiang. Bull Dis Control. 2005;20(4):53–54,59.
14. Angella M, Wilson T, Aaron TM, et al. Digital monitoring technologies could enhance tuberculosis medication adherence in Uganda: mixed methods study. J Clin Tuberc Other Mycobact Dis. 2019;17:100119. doi:10.1016/j.jctube.2019.100119
15. Subbaraman R, Mondesert LD, Musiimenta A, et al. Digital adherence technologies for the management of tuberculosis therapy: mapping the landscape and research priorities. BMJ Glob Health. 2018;3:e00101. doi:10.1136/bmjgh-2018-001018
16. Nouvet E, Knoblauch AM, Passe I, et al. Perceptions of drones, digital adherence monitoring technologies and educational videos for tuberculosis control in remote Madagascar: a mixed-method study protocol. BMJ Open. 2019;9:e028073. doi:10.1136/bmjopen-2018-028073
17. Ngwatu BK, Nsengiyumva NP, Oxlade O, et al. Collaborative group on the impact of digital technologies on TB. The impact of digital health technologies on tuberculosis treatment: a systematic review. Eur Respir J. 2018;51(1):1701596. doi:10.1183/13993003.01596-2017
18. Maraba N, Orrell C, Chetty-Makkan CM, et al. Evaluation of adherence monitoring system using evriMED with a differentiated response compared to standard of care among drug-sensitive TB patients in three provinces in South Africa: a protocol for a cluster randomised control trial. Trials. 2021;22(1):389. doi:10.1186/s13063-021-05337-y
19. Leddy A, Ggita J, Berger CA, et al. Barriers and facilitators to implementing a digital adherence technology for tuberculosis treatment supervision in Uganda: qualitative study. J Med Internet Res. 2023;25(25):e38828. doi:10.2196/38828
20. Manyazewal T, Woldeamanuel Y, Holland DP, et al. Electronic pillbox-enabled self-administered therapy versus standard directly observed therapy for tuberculosis medication adherence and treatment outcomes in Ethiopia (SELFTB): protocol for a multicenter randomized controlled trial. Trials. 2020;21(1):383. doi:10.1186/s13063-020-04324-z
21. Thomas BE, Kumar JV, Onongaya C, et al. Explaining differences in the acceptability of 99DOTS, a cell phone-based strategy for monitoring adherence to tuberculosis medications: qualitative study of patients and health care providers. JMIR Mhealth Uhealth. 2020;8(7):e16634. doi:10.2196/16634
22. National Health and Family Planning Commission of the People’s Republic of China. Diagnosis for Pulmonary Tuberculosis (WS288-2017). Beijing: China Standard Press; 2018:1–24.
23. World Health Organization. Treatment of tuberculosis: guidelines. WHO guidelines approved by the guidelines review committee. Geneva; 2010. Available from: https://www.ncbi.nlm.nih.gov/books/NBK138748/pdf/Bookshelf_NBK138748.pdf.
24. Marion SB, Francis MP, Kennedy MN, et al. Implementation and effectiveness of evriMED with short messages service (SMS) reminders and tailored feedback compared to standard care on adherence to treatment among tuberculosis patients in Kilimanjaro, Tanzania: proposal for a cluster randomized controlled trial. Trials. 2019;20(1):426. doi:10.1186/s13063-019-3483-3484
25. Liu X, Terrence B, Bruce T, et al. Usability of a medication event reminder monitor system (merm) by providers and patients to improve adherence in the management of tuberculosis. Int J Env Res Pub He. 2017;14(10):1115. doi:10.3390/ijerph14101115
26. Ananya P, Upasna A, Jaya PT, et al. ”99dots” techno-supervision for tuberculosis treatment – a boon or a bane? Exploring challenges in its implementation at a tertiary centre in Delhi, India. Indian J Tuberc. 2020;67(1):46–53. doi:10.1016/j.ijtb.2019.08.010
27. Thakkar D, Piparva KG, Lakkad SG. A pilot project: 99dots information communication technology-based approach for tuberculosis treatment in Rajkot district. Lung India. 2019;36(2):108–111. doi:10.4103/lungindia.lungindia_86_18
28. Nezenega ZS, Perimal-Lewis L, Maeder AJ. Factors influencing patient adherence to tuberculosis treatment in Ethiopia: a literature review. Int J Environ Res Public Health. 2020;17(15):5626. doi:10.3390/ijerph17155626
29. Xu WG, Lu W, Zhou Y, et al. Adherence to anti-tuberculosis treatment among pulmonary tuberculosis patients: a qualitative and quantitative study. BMC Health Serv Res. 2009;9(1). doi:10.1186/1472-6963-9-169
30. Rocha M, Pereira S, Ferreira L, et al. The role of adherence in tuberculosis HIV-positive patients treated in ambulatory regimen. Eur Respir J. 2003;21(5):785–788. doi:10.1183/09031936.03.00077302
31. Tang Y, Zhao MG, Wang YX, et al. Non-adherence to anti-tuberculosis treatment among internal migrants with pulmonary tuberculosis in Shenzhen, China: a cross-sectional study. BMC Public Health. 2015;15(1):474. doi:10.1186/s12889-015-1789-z
32. Yao S, Huang W, Susan VDH, et al. Treatment adherence among sputum smear-positive pulmonary tuberculosis patients in mountainous areas in China. BMC Health Serv Res. 2011;11:11. doi:10.1186/1472-6963-11-341
33. Gong XJ, Li YH, Wang J, et al. Treatment adherence among sputum smear-positive pulmonary tuberculosis patients in Xinjiang, China: a prospective study. RSC Adv. 2018;8(16):8983–8989. doi:10.1039/C7RA11820A
34. Chen W, Li Y, Yang H, et al. Is tuberculosis health education reaching the public in China? A cross-sectional survey in Guizhou Province. BMJ Open. 2016;6(9):e013534. doi:10.1136/bmjopen-2016-013534
35. Wang ZY, Zhang LJ, Liu YH, et al. The effectiveness of E-learning in continuing medical education for tuberculosis health workers: a quasi-experiment from China. Infect Dis Poverty. 2021;10(1):72. doi:10.1186/s40249-021-00855-y
36. Bermingham WH, Bhogal R, Arudi Nagarajan S, et al. Practical management of suspected hypersensitivity reactions to anti-tuberculosis drugs. Clin Exp Allergy. 2022;52(3):375–386. doi:10.1111/cea.14084
37. Prasad R, Singh A, Gupta N. Adverse drug reactions in tuberculosis and management. Indian J Tuberc. 2019;66(4):520–532. doi:10.1016/j.ijtb.2019.11.005
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
