Back to Journals » Breast Cancer: Targets and Therapy » Volume 14
Economic Evaluation of Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer in the United States
Received 13 September 2022
Accepted for publication 29 November 2022
Published 9 December 2022 Volume 2022:14 Pages 453—463
DOI https://doi.org/10.2147/BCTT.S389696
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Pranela Rameshwar
Yitian Lang,1 Bin Wu,2 Xiaoyan Liu1
1Department of Pharmacy, Huangpu Branch, Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, People’s Republic of China; 2Medical Decision and Economic Group, Department of Pharmacy, Ren Ji Hospital, South Campus, School of Medicine, Shanghai Jiaotong University, Shanghai, People’s Republic of China
Correspondence: Xiaoyan Liu, Department of Pharmacy, Huangpu Branch, Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine, No. 58 Puyu East Roa, Huangpu Districtd, Shanghai, 200011, People’s Republic of China, Email [email protected]
Purpose: Recently, the DESTINY-Breast04 trial revealed that significant benefits in both overall survival (OS) and progression-free survival (PFS) in patients with HER2-low advanced or metastatic breast cancer treated with trastuzumab deruxtecan (T-DXd) compared with chemotherapy. The current study assessed the cost-effectiveness of T-DXd from the perspective of the United States payer.
Methods: We developed a partitioned survival model to project the disease course of breast cancer. The OS and PFS data were derived from the DESTINY-Breast04 trial. We extrapolate the survival data beyond the follow-up time to assess the long-term survival prognosis. Direct medical costs and utility data were collected. The incremental cost-utility ratio (ICUR) was the primary outcome that evaluated the cost-effectiveness of a therapy regimen. One-way sensitivity and probabilistic sensitivity analysis were implemented to explore the uncertainty of outputs.
Results: In the base-case, the ICUR of T-DXd versus chemotherapy is $346,571.8/QALY and $337,789.4/QALY for all patients group and hormone-receptor-positive (HR+) subgroup, respectively. One-way sensitivity analyses revealed that the hazard ratio of OS, the unit cost of T-DXd, and body weight had a relatively large impact on the base-case result. Probabilistic sensitivity analyses showed that the likelihood that T-DXd was cost-effective is 14.5% and 12.6% for all patients group and HR+ subgroup, respectively.
Conclusion: The cost-effectiveness analysis suggested that, at current price, trastuzumab deruxtecan is unlikely to be a preferred option for patients with HER2-low breast cancer at a threshold of $150,000/QALY from a US payer perspective.
Keywords: cost-effectiveness, HER2-low breast cancer, trastuzumab deruxtecan, partitioned survival approach
Introduction
Globally, breast cancer is the most common malignancy among women. Based on the latest GLOBOCAN data, 2.26 million new breast cancer cases were diagnosed worldwide in 2020, which accounts for 11.7% of new diagnosed cancers.1 Nevertheless, treatment decisions are usually based on histopathological factors, and four major breast cancer subtypes are recognized in clinical practice with prognostic and predictive relevance, including luminal A, luminal B, human epidermal growth factor receptor 2(HER2)-positive, and triple-negative breast cancer.2 HER2-positive tumors account for around 10–20% of all breast cancers, and more than 80% are HER2-negative.3 Approximately 60% of HER2-negative breast cancers express low levels of HER2. In the past, patients with HER2-low-expressing tumors did not appear to benefit from HER2-targeted therapies, like trastuzumab adjuvant therapy.4 However, the HER2-targeted antibody-drug conjugates (ADC) seem to break this barrier.5 Recently, the DESTINY-Breast04 trial revealed that significant benefits in both overall survival (OS, 23.4 vs 16.8 months, hazard ratio=0.64, P=0.001) and progression-free survival (PFS, 9.9 vs.5.1 months, hazard ratio=0.5, P<0.001) in patients with HER2-low advanced or metastatic breast cancer treated with trastuzumab deruxtecan (T-DXd) compared with chemotherapy.6 T-DXd structurally composed of a humanized, anti-HER2 IgG1 monoclonal antibody, a cleavable peptide linker, and a topoisomerase I inhibitor payload (DXd, a derivative of the camptothecin analogue exatecan).7,8 T-DXd can effectively target tumor cells that express low levels of HER2 and release its cytotoxic payload to neighboring tumor cells, which can significantly enhance the efficacy of drug. And T-DXd is the first approved targeted therapy drug by the US Food and Drug Administration for HER2-low breast cancer.9
Malignant tumors greatly affect the quality of life of patients. The Global Burden of Disease (GBD) reported that the disability-adjusted life-years (DALYs) of breast cancer ranked first among female malignancies.10 And novel antineoplastic agents led to a high economic burden. It is, therefore, imperative that clinicians and policymakers take cost-effectiveness into account when making medical decisions so that limited resources can be allocated as efficiently as possible. T-DXd showed better therapeutic advance compared with chemotherapy, the cost-effectiveness of the regimen is still not evaluated. The current study investigated the cost-effectiveness of T-DXd for HER2-low advanced or metastatic breast cancer.
Materials and Methods
The Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement, published in 2013 and updated in 2022, was designed as guidance to help authors report economic evaluation accurately. The current economic evaluation adhered to the CHEERS 2022 checklist (see Supplemental Table 1).11,12
Model Structure
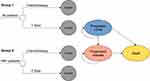
We developed a decision analytic model for T-DXd and chemotherapy to assess their cost-effectiveness from the perspective of the United States payers. A partitioned survival approach (PartSA) was used to simulate disease development in patients with HER2-low advanced breast cancer. The simulated patients with unresectable, locally advanced or metastatic HER2-low breast cancer who had received chemotherapy or at least one line of endocrine therapy, which is consistent with the DESTINY-Breast04 trial.6 And the baseline of patients in the DESTINY-Breast04 trial can see Supplemental Table 2. In this partitioned survival model, the health state was divided into three mutually exclusive stages, including progression free (PF) survival, progressed disease (PD), and death. The PF is assumed as a default state, which could progress into a PD state or death state. The proportion of the cohort at any model time point in each health state can be calculated from the reconstructed PFS and OS curves.13
The analysis included two groups. Group 1 corresponds to the analysis for all patients (all randomized patients including HR-positive and HR-negative) and group 2 is for the patients with HR-positive. In each of the groups, the patients receive one of the following therapy regimens: (1) chemotherapy; (2) T-DXd. The two regimens and the doses strategies are kept with the DESTINY-Breast04 trial. The chemotherapy regimen included eribulin, capecitabine, nab-paclitaxel, gemcitabine, or paclitaxel. Therein, eribulin was administrated at a dose of 1.4 mg/m2 body surface area (BSA) intravenously on days 1 and 8 of each 21-day cycle, capecitabine was administrated at a dose of 1000 mg/m2 orally twice daily on days 1 to 14 of each 21-day cycle, nab-paclitaxel was administrated at a dose of 100 mg body surface area (BSA) per square meter on days 1, 8, and 15 of each 21-day cycle, gemcitabine was used at a dose of 800 to 1200 mg/m2 intravenously on days 1, 8 and 15 of each 28-day cycle, or paclitaxel was administrated at a dose of 175 mg/m2 intravenously on day 1 of every 21-day cycle.14 The T-DXd regimen was administrated at a dose of 5.4 mg/kg intravenously every 21-day cycle.6 In each group, disease progression, unacceptable toxic effects, or death will end the current therapy regimen. The three-week model cycle was adopted to make cost estimates easier, and a 10-year time horizon was determined to ensure that more than 90% of the whole cohort enter the absorbing state, and the long-term outcomes can be assessed. The decision tree and model structure are shown in Figure 1.
Clinical Data
Our analysis was based on the DESTINY-Breast04 trial, and the trial published the data on PFS, OS, and safety data.6 Nevertheless, the follow-up time of the trial is not sufficient for the analysis in the setting of a 10-year time horizon. It is therefore necessary to extrapolate beyond the follow-up period of the trial. We digitized the PFS and OS Kaplan-Meier curves of the DESTINY-Breast04 trial to gather the time-to-survival point data. And then, an algorithm that Guyot proposed can obtain pseudo individual participant data (IPD) was used to get the time-to-event data.15 We fitted several candidate survival functions to the generated time-to-event data, including Weibull, Log-logistic, Log-normal, exponential, Gompertz and Gamma distribution.16 The hazard ratio value and 95% confidence interval (CI) of the T-DXd arm versus the chemotherapy arm can be gathered from the DESTINY-Breast04 trial. The projected PFS and OS curve in the T-DXd were estimated by applying the hazard ratios to the chemotherapy arm. The best fit was determined based on the Akaike Information Criterion (AIC) and visual inspection. Additionally, we performed internal validation by comparing the projected clinical outcomes with the DESTINY-Breast04 trial data in terms of median PFS and OS. Treatment-related adverse events (AEs) were also included in the analysis. As the grade 1 to 2 AEs are manageable well, the analysis only considered the grade 3 and greater AEs. The clinical survival data and the incidence of AEs can be seen in Table 1.
 |
Table 1 Key Clinical Data |
Costs and Utilities
The United States payer perspective was adopted in this analysis. Therefore, only direct medical expenditures were included in the cost estimates, which covered the therapy drugs, administration for intravenous injection, management of severe AEs, follow-up, and palliative care. The drug costs in the chemotherapy regimen were collected from the Centers for Medicare & Medicaid Services (CMS) and the average sales price (ASP) that the manufacturer reported was adopted.17 The price of T-DXd was estimated based on the price guide (Price Guide, www.drugs.com/price-guide).18 The average weight of 77.5 kg and height of 1.61 m in women, derived from the National Center for Health Statistics (NCHS) data, were used to calculate the average BSA and the dosage of drugs.19 A 1.86 m2 of BSA was applied. The overall drug costs were calculated according to the predetermined dosing strategy. In the chemotherapy regimen, the eribulin, capecitabine, nab-paclitaxel, gemcitabine, and paclitaxel single agent were assigned according to the proportion of 51.1%, 20.1%, 10.3%, 10.3% and 8.2%, respectively.6 The cost of the chemotherapy regimen is estimated based on the proportions. The cost of administration for intravenous injection was taken from the 2021 Physician’s Fee Schedule.20 The cost related to palliative care, follow-up and best supportive care was derived from published studies.21,22 The expenditure of management of severe (grade 3 and more) AEs was gathered from published studies.22–24 All costs for years prior to 2021 have been adjusted to 2021 US dollars (USD) using the Consumer Price Index (CPI).
Each health state was assigned a health utility value that reflects the stage of progression. Due to the fact that the DESTINY-Breast04 trial did not report the health utility data, highly relevant and robust data derived from relevant studies is obtained.22 The quality of life is assumed to be related to the progressive stage of the tumor. Therefore, the utility values of PF and PD state were estimated to be 0.85 and 0.52, respectively.22 All costs and utilities were discounted at an annual rate of 3%. More detailed values of inputs are summarized in Table 2.
 |
Table 2 Model Costs, Utility Estimates and Other Parameters |
Analyses
In the base-case analysis, the incremental cost-utility ratio (ICUR) was used to evaluate the incremental cost per quality-adjusted life-year (QALY). If the ICUR is below the willingness-to-pay (WTP) threshold, the regimen is deemed as “cost-effective”. The threshold of $100,000–150,000/QALY was usually used for economic analysis in the United States.25 In this analysis, we adopted the threshold of $150,000/QALY to assess the cost-effectiveness of regimens.
In order to assess the uncertainty of outputs, we conducted both one-way and probabilistic sensitivity analysis (PSA) for all model inputs. In the projected curve, the 95% CI of the hazard ratio is used for one-way sensitivity analysis. And the range of annual discount rate is from 0 to 8%. Other parameters were assumed to vary by ± 25% of the base-case value. And 1000 iterations of Monte Carlo simulations were used to examine the influence of parameter uncertainty. In each of 1000 simulations, each model input was sampled randomly based on its probability distribution. The Beta distribution is for probabilities of AEs and utility values, the Gamma distribution for the cost inputs, and the log-normal distribution for the hazard ratio of two therapy arms.26 The cost-effectiveness acceptability curves (CEACs) were created to present the likelihood that a therapy regimen was cost-effective at a range of WTP thresholds, and the scatter plots can show the output distribution of each simulation. All models, including Partitioned survival model and the cost-effectiveness model, were programmed through R software (https://www.r-project.org/) with the package of “heemod”.27
Results
Curve Fitting
Survival Curve nodes imply an order of progression from healthier to sicker states, ending in the dead state. The Survival Functions generally should not cross. In this analysis, the distributions that led to crossovers between OS and PFS curves were not included. Combining AIC and visual inspection, the Weibull distribution appears to be the most rational function for extrapolating PFS and OS of chemotherapy regimen in either all patients or the HR-positive group. The reconstructed Kaplan-Meier survival curves and the projected PFS and OS curves of the T-DXd and chemotherapy regimen were generated and shown in Figure 2. The scale and shape parameters of the projected curve of the chemotherapy arm could be seen in Table 1. The projected curves of the T-DXd arm were generated based on the hazard ratio of T-DXd versus chemotherapy. The results for the proportion of patients at each time point can see the supplemental Figure 1. The results of internal validation can see the Supplemental Table 3. Additionally, the fitting curve figures based on other distributions were also generated and can see supplemental Figures 2 and 3.
Base-Case Analysis
The outputs of base-case analysis varied little across all patients group and the HR-positive group. In all patients group, patients who received chemotherapy regimen gained 1.036 QALYs and expended $ 93,554.6. Receiving T-DXd regimen resulted in 1.487 QALYs gained and $ 249,858.5 expended. Compared with the chemotherapy regimen, the T-DXd regimen increased the overall cost by $ 156,304. For effectiveness, the T-DXd regimen showed an increase of 0.451 QALYs compared with the chemotherapy regimen. The ICUR of T-DXd versus chemotherapy is $ 346,571.8 /QALY. In the HR-positive subgroup, patients who received chemotherapy regimen gained 1.111 QALYs and expended $ 96,778. Receiving T-DXd regimen resulted in 1.581 QALYs gained and $ 255,539 expended. Compared with the chemotherapy regimen, the T-DXd regimen increased the overall cost by $ 158,761. For effectiveness, the T-DXd regimen showed an increase of 0.47 QALYs compared with the chemotherapy regimen. The ICUR of T-DXd versus chemotherapy is $ 337,789.4 /QALY. All base-case results are summarized in Table 3.
 |
Table 3 Results of the Base-Case Analysis |
One-Way Sensitivity Analysis
The one-way sensitivity analysis outcomes, represented in the form of tornado diagrams (Figure 3), can illustrate the sensitivity of analysis output to model variables. In all patients group, we can find that, from the diagram of tornado (Figure 3A), the hazard ratio of OS, body weight, and the unit cost of T-DXd were the key driving factors that had a significant impact on ICUR between T-DXd and chemotherapy regimen. The range of the ICUR was from $ 230,767.1 /QALY to $ 582,719.8 /QALY. The unit cost of the T-DXd and the body weight, as key variables affecting the total cost of the T-DXd regimen, can minimize the ICUR. The hazard ratio of OS can affect the effectiveness differences of two regimens by affecting the survival time. The impact of other variables on the ICUR was not prominent. In the HR-positive subgroup, the tornado diagram of T-DXd versus chemotherapy regimen (Figure 3B) showed that the hazard ratio of OS, body weight and the unit cost of T-DXd could yield significant effects on the ICUR. The range of ICUR was from $ 222,317.6 /QALY to $ 613,240.1 /QALY. Other variables had little impact on the reduction of ICUR.
Probabilistic Sensitivity Analysis
Simultaneously sampling all model parameters from probability distributions, the average results of a total of 1000 iterations generally align with the base-case results (see Supplemental Table 4). The CEACs (Figure 4) showed that in all patients, the T-DXd regimen was 14.5% cost-effective at the $150,000/QALY threshold. And the chemotherapy regimen was 85.5% cost-effective at the same threshold. In the HR-positive subgroup, the T-DXd regimen was 12.6% cost-effective at the $150,000/QALY threshold. And the chemotherapy regimen was 87.4% cost-effective at the same threshold.
As the price of T-DXd is a potential variable that can reduce the ICUR, additional probabilistic sensitivity analyses were performed, with prices set at 75% and 50% of the base-value. The additional PSA showed that in all patients, the likelihood of the T-DXd regimen in the 25% and 50% price reduction setting was 30.5% and 68.5% of being cost-effective, respectively. And in the HR-positive subgroup, the likelihood of T-DXd was 33.1% and 71.5%, respectively. More outputs are summarized in Supplemental Table 4 and 5.
Discussion
In women, breast cancer is the most common malignancy and accounts for the majority of cancer deaths. This imposes a significant burden not only on the patients but also on the health-care sectors. The constant emergence of novel therapeutic drugs provides patients with more treatment options and has gradually formed a new treatment pattern. The selection and decision-making of therapeutic drugs are affected by cost-effectiveness, while new antineoplastic drugs are often associated with high price tags. As a new ADC drug, the approval of T-DXd aroused widespread concern, which led us to increasingly recognize its efficacy and addressed a critical unmet treatment need for patients with HER2-low breast cancer. And the new HER2 subtype is understood further by researchers and oncologists. However, the cost-effectiveness of T-DXd in the treatment of HER2-low breast cancer has not been evaluated. As far as we know, this study is the first economic evaluation of T-DXd in advanced or metastatic HER2-low breast cancer from the United States payer perspective.
In this study, we analyzed the cost-effectiveness of different groups in the DESTINY-Breast04 trial. Due to the small sample size of the HR-negative subgroup, the results were not included in this study. Our analysis of all patients group and HR+ subgroup revealed that compared with the chemotherapy regimen, T-DXd was not cost-effective at a threshold of $150,000/QALY. The sensitivity analysis showed that the key variables that driving the results included the hazard ratio value of OS, body weight, and unit price of T-DXd. Among them, weight and unit price of T-DXd will affect the total cost of the T-DXd group. If the values of the two parameters decrease, the total cost of the T-DXd group will be significantly reduced, and then the ICUR across two regimens will be reduced. The hazard ratio value of OS affected the total survival time, and the decrease of the hazard ratio value will increase the total QALYs of the T-DXd regimen. The PSA analysis shows that the results are robust and reliable. In the aforementioned sensitivity variables, the hazard ratio of OS is a non-intervenable variable, while the unit price of T-DXd are intervenable variables, so we also conducted additional scenario analyses on the price strategies. Additional analysis shows that when simulating a 50% price reduction scenario, the ICUR between the two regimens in all patients group and HR+ subgroup is reduced to $113,034.4/QALY and $104,017.1/QALY. The ICUR values of two groups are less than the pre-specified threshold of $150,000/QALY, so the price reduction strategy is an effective way to improve cost-effectiveness. Although the analysis output based on the current price of T-DXd is higher than the WTP threshold in the United States, it is a landmark ADC drug for HER2-low breast cancer patients and its value should not be ignored. In recent years, many pharmacoeconomic evaluations have revealed that the ICURs of many novel antineoplastic drugs have exceeded the willingness to pay threshold compared with chemotherapy regimens. As long as the price of the drug matches its clinical value, it appears to be rational that the ICUR value is slightly higher than the WTP. Also, the economic evaluation can effectively check out the antineoplastic drugs that are priced above the value-based cut point. This is why many countries are advocating a value-based pricing system globally.28 In this pattern, pharmaceutical manufacturers may be encouraged to develop drugs with more meaningful clinical benefits.29
There are several potential weaknesses in this study as well. Due to the fact that patient survival data was taken from the DESTINY-Breast04 trial, only the analysis based on the utility derived from DESTINY-Breast04 was the most accurate. The results of the DESTINY-Breast04 trial on health-related utility values of disease states were not reported. As a result, we had to rely on published literature to gather relatively reliable utility data. Fortunately, utility values do not significantly contribute to the ICUR reduction according to the tornado diagram. A second issue was the uncertainty that was introduced during the digitizing process of the PFS and OS curves. Furthermore, rather than using real data from individual patients, the model’s individual patient data was generated using an algorithm proposed by Guyot.15 Due to the lack of access to a patient-level time-to-event dataset in clinical trials, this method allowed survival analysis based on pseudo-patient-level data. Guyot’s algorithm is frequently used in economic evaluation and has been shown to outperform other methods in terms of performance.30 Thirdly, we tried to fit each arm based on the survival function that has the lowest AIC value. The analysis cannot continue due to the occurrence of crossover between PFS and OS. Therefore, the mode of hazard ratio was adopted. The Gompertz distribution led crossover between OS and PFS curves and not taken into consideration. The Weibull distribution appears to be the most rational function for extrapolating PFS and OS when Akaike information criteria (AIC) and visual inspection are combined.
Conclusion
The cost-effectiveness analysis suggested that, at the current price, trastuzumab deruxtecan is unlikely to be a preferred option for patients with HER2-low advanced or metastatic breast cancer at a WTP threshold of $ 150,000/QALY in the United States. Some measures need to be followed to make it a cost-effective choice for the treatment of HER2-low breast cancer, including price strategy, payment strategy, etc.
Data Sharing Statement
All data generated or analyzed during this study are included in this article/Supplementary Material.
Ethics Approval and Informed Consent
This study is based on a literature review and modeling techniques, so it doesn’t require the approval by an institutional research ethics board.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; All authors took part in drafting the article or revising it critically for important intellectual content and agreed to submit to the current journal; All authors gave final approval of the version to be published and agree to be accountable for all aspects of the work.
Funding
This work was supported by authors’ organization and the Wu Jieping Medical Foundation (grant number 320.6750.2021-10-28).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA a Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Tarantino P, Hamilton E, Tolaney SM, et al. HER2-low breast cancer: pathological and clinical landscape. JCO. 2020;38(17):1951–1962. doi:10.1200/JCO.19.02488
3. Schettini F, Chic N, Brasó-Maristany F, et al. Clinical, pathological, and PAM50 gene expression features of HER2-low breast cancer. Npj Breast Cancer. 2021;7(1):1. doi:10.1038/s41523-020-00208-2
4. Fehrenbacher L, Cecchini RS, Geyer Jr JCE, et al. NSABP B-47/NRG oncology phase iii randomized trial comparing adjuvant chemotherapy with or without trastuzumab in high-risk invasive breast cancer Negative for HER2 by FISH and with IHC 1+ or 2+. JCO. 2020;38(5):444–453. doi:10.1200/JCO.19.01455
5. Indini A, Rijavec E, Grossi F. Trastuzumab deruxtecan: changing the destiny of HER2 expressing solid tumors. IJMS. 2021;22(9):4774. doi:10.3390/ijms22094774
6. Modi S, Jacot W, Yamashita T, et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med. 2022;387(1):9–20. doi:10.1056/NEJMoa2203690
7. Keam SJ. Trastuzumab deruxtecan: first approval. Drugs. 2020;80(5):501–508. doi:10.1007/s40265-020-01281-4
8. Andrikopoulou A, Zografos E, Liontos M, Koutsoukos K, Dimopoulos MA, Zagouri F. Trastuzumab deruxtecan (DS-8201a): the latest research and advances in breast cancer. Clin Breast Cancer. 2021;21(3):e212–e219. doi:10.1016/j.clbc.2020.08.006
9. FDA. FDA approves first targeted therapy for HER2-Low breast cancer. FDA; 2022. Available from: https://www.fda.gov/news-events/press-announcements/fda-approves-first-targeted-therapy-her2-low-breast-cancer.
10. Kyu HH, Abate D, Abate KH, et al. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2018;392(10159):1859–1922. doi:10.1016/S0140-6736(18)32335-3
11. Husereau D, Drummond M, Petrou S, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS)--explanation and elaboration: a report of the ISPOR health economic evaluation publication guidelines good reporting practices task force. Value Health. 2013;16(2):231–250. doi:10.1016/j.jval.2013.02.002
12. Husereau D, Drummond M, Augustovski F, et al. Consolidated health economic evaluation reporting standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. Value Health. 2022;25(1):3–9. doi:10.1016/j.jval.2021.11.1351
13. Phua LC, Lee SC, Ng K, Abdul Aziz MI. Cost-effectiveness analysis of atezolizumab in advanced triple-negative breast cancer. BMC Health Serv Res. 2020;20(1):581. doi:10.1186/s12913-020-05445-6
14. National Comprehensive Cancer Network®. NCCN clinical practice guidelines in oncology (NCCN Guidelines®) breast cancer version 4; 2022. Avialable from: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.
15. Guyot P, Ades A, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2012;12(1):9.
16. Jackson C. flexsurv: a platform for parametric survival modeling in R. J Stat Softw. 2016;70:1–33. doi:10.18637/jss.v070.i08
17. Centers for Medicare&Medicaid Services. ASP drug pricing files; 2022. Available from: https://www.cms.gov/medicare/medicare-part-b-drug-average-sales-price/2022-asp-drug-pricing-files.
18. Drugs.com. Prices, coupons and patient assistance programs. Available from: https://www.drugs.com/price-guide/.
19. Centers for Disease control and Prevention. Measured average height, weight, and waist circumference for adults aged 20 and over. National Center for Health Statistics: Body Measurements. Available from: https://www.cdc.gov/nchs/fastats/body-measurements.htm.
20. Centers for Medicare and Medicaid Services. Search the Physician Fee Schedule. Available from: https://www.cms.gov/medicare/physician-fee-schedule/search.
21. Wu B, Ma F. Cost-effectiveness of adding atezolizumab to first-line chemotherapy in patients with advanced triple-negative breast cancer. Ther Adv Med Oncol. 2020;12:175883592091600. doi:10.1177/1758835920916000
22. Zhang B, Long EF. Cost-effectiveness analysis of palbociclib or ribociclib in the treatment of advanced hormone receptor-positive, HER2-negative breast cancer. Breast Cancer Res Treat. 2019;175(3):775–779. doi:10.1007/s10549-019-05190-3
23. Agency for Healthcare Research and Quality. HCUPnet data tools. Healthcare Cost and Utilization Project (HCUPnet). Available from: https://datatools.ahrq.gov/hcupnet.
24. Rashid N, Koh H, Baca H, Lin K, Malecha S, Masaquel A. Economic burden related to chemotherapy-related adverse events in patients with metastatic breast cancer in an integrated health care system. BCTT. 2016;8:173–181. doi:10.2147/BCTT.S105618
25. Verma V, Sprave T, Haque W, et al. A systematic review of the cost and cost-effectiveness studies of immune checkpoint inhibitors. j Immunotherapy Cancer. 2018;6(1):128. doi:10.1186/s40425-018-0442-7
26. Briggs AH, Weinstein MC, Fenwick EAL, et al. Model parameter estimation and uncertainty analysis: a report of the ISPOR-SMDM modeling good research practices task force working group-6. Med Decis Making. 2012;32(5):722–732.
27. Filipović-Pierucci A, Zarca K, Durand-Zaleski I. Markov models for health economic evaluation: the R package heemod. ArXiv e-prints. 2017;19(7):A369.
28. Gandjour A. Convergence of decision rules for value-based pricing of new innovative drugs. Expert Rev Pharmacoecon Outcomes Res. 2015;15(2):209–213. doi:10.1586/14737167.2015.972374
29. Goldstein DA, Chen Q, Ayer T, et al. Necitumumab in metastatic squamous cell lung cancer: establishing a value-based cost. JAMA Oncol. 2015;1(9):1293. doi:10.1001/jamaoncol.2015.3316
30. Saluja R, Cheng S, Santos KA, Chan KKW. Estimating hazard ratios from published Kaplan‐Meier survival curves: a methods validation study. Res Syn Meth. 2019;10(3):465–475. doi:10.1002/jrsm.1362
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.