Back to Journals » Therapeutics and Clinical Risk Management » Volume 19
Economic Evaluation for Palbociclib Plus Fulvestrant vs Ribociclib Plus Fulvestrant and Abemaciclib Plus Fulvestrant in Endocrine-Resistant Advanced or Metastatic Breast Cancer in Italy
Authors Colombo GL , Valentino MC , Fabi A, Dieci MV, Caruggi M, Bruno GM , Lombardi G, Di Matteo S
Received 11 October 2022
Accepted for publication 12 March 2023
Published 28 March 2023 Volume 2023:19 Pages 301—312
DOI https://doi.org/10.2147/TCRM.S391769
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Garry Walsh
Giorgio Lorenzo Colombo,1 Maria Chiara Valentino,2 Alessandra Fabi,3 Maria Vittoria Dieci,4,5 Mauro Caruggi,2 Giacomo Matteo Bruno,2 Gloria Lombardi,6 Sergio Di Matteo2
1Department of Drug Sciences, University of Pavia, Pavia, Italy; 2S.A.V.E. Studi Analisi Valutazioni Economiche S.r.l., Health Economics & Outcomes Research, Milan, Italy; 3Precision Medicine Breast Unit, Scientific Directorate, Department of Women, Children and Public Health Sciences, “A. Gemelli” IRCCS, Roma, Italy; 4Department of Surgery, Oncology and Gastroenterology, University of Padova, Padova, Italy; 5Oncology 2, Veneto Institute of Oncology IOV-IRCCS, Padova, Italy; 6Real World Solutions, IQVIA Solutions Italy S.r.l, Milan, Italy
Correspondence: Giorgio Lorenzo Colombo, Email [email protected]
Background: To date, no study evaluated the cost-effectiveness of palbociclib (PAL) plus fulvestrant (FUL) vs ribociclib (RIB) plus FUL and abemaciclib (ABM) plus FUL in Italy. Cost-effectiveness analysis comparing the three cyclin-dependent 4/6 kinase inhibitors in combination with endocrine therapies for the management of postmenopausal women with HR+, HER2- advanced or metastatic breast cancer in Italy was developed.
Material and Methods: To assess the cost-effectiveness of PAL plus FUL vs RIB plus FUL and ABM plus FUL, a cost-minimization has been carried out with a conservative scenario considering three CDK4/6 inhibitors with equal effectiveness in terms of overall survival (OS) (MAIC, Rugo et al 2021). Adverse events (AEs) associated with all therapies were obtained from clinical trials. Ad-hoc analysis was performed to estimate the cost-effectiveness considering the quality-of-life (QoL) data (Lloyd et al 2006).
Results: Cost-minimization inputs were drugs, visits and exams, AE monitoring and best supportive care (BSC) before the progression state, active and BSC in the progression and terminal phase of the last two weeks of life. Given the comparability of PAL, RIB and ABM in terms of efficacy, this analysis demonstrated slight economic savings over a lifetime for PAL. Results showed saving per patient of € 305 (lifetime) when PAL is compared with RIB; for PAL vs ABM a saving of € 243 (lifetime) in a conservative scenario. Results of a budget impact analysis showed a potential savings of € 319,563 for PAL vs RIB and € 297,544 for PAL vs ABM. When QoL data were considered, results may favor PAL due to the lower impact of AE with savings and improvement in the QoL related to fewer AE.
Conclusion: From the Italian perspective, a cost-saving profile associated with the use of PAL+FUL for the management of advanced/metastatic HR+/HER2- breast cancer compared to RIB+FUL and ABM+FUL emerged.
Keywords: cost effectiveness, cost-minimization, metastatic breast cancer, palbociclib, ribociclib, abemaciclib, second-line endocrine therapy, Italy
Background
Breast cancer is the most common tumour, based on the incidence in female population, and is the main cause of cancer mortality in women worldwide.1 In 2019, in Italy, it affected 53,000 women and about 500 men.2 In Italy, breast cancer accounts for 30% of female cancers, followed by cancers of the colorectal (12%), lung (12%), thyroid (5%) and body of the uterus (5%).2 Despite great advances in detection and management, breast cancer has a serious economical global impact.1
Breast cancer is a complex disease, it has four main distinct molecular subtypes: luminal A and B, human epidermal growth factor receptor 2 (HER2)-enriched, basal-like.3 Luminal represents the most widespread type of breast cancer and is typified by the expression of the estrogen receptor (ER) and/or the progesterone receptor (PR), which can be effectively targeted with endocrine treatment. Sequential hormone therapies are the standard treatment regimens for hormone receptor positive (HR+)/HER2-negative (HER2-) advance and metastatic breast cancer (MBC) patients. However, there are patients who have inherent or gathered resistance to endocrine therapy (ET).4 The last few decades have seen great advances in the development of new effective therapies. Among the available therapies, cyclin-dependent 4/6 kinase inhibitors (CDK4/6) represent a major step forward in the treatment of patients who are affected by advance and HR+/HER2- MBC. The task of CDK4/6 is to coordinate cell cycle advancement through a reversible combination with cyclin D, which leads to Rb phosphorylation and consequently activates pivotal transcription factors that contribute to cell cycle progression.5 CDK4/6 have an essential role in cell cycle regulation to make these kinases effective targets for the development of a therapeutic strategy against malignancy, especially in breast cancer. The progress of CDK 4/6 inhibitors has radically modified the treatment of HR+/HER2- MBC. The CDK4/6 inhibitors palbociclib, ribociclib and abemaciclib, when added to ET, significantly prolong progression-free survival (PFS) in endocrine-sensitive and endocrine-resistant advance and HR+/HER2- MBC patients.5
In the endocrine-resistant setting, these drugs have been approved in combination with fulvestrant (FUL) given the results of randomized Phase III studies6–8 which show a significant advantage in PFS. Every agent is well accepted, and nearly all of the toxicities related to this class of drugs are mainly manageable without effort. Adverse events reported are a non-significant number.9
Since there are no direct evidence from head-to-head clinical trials, in order to determine relative efficacy of treatments, indirect treatment comparison (ITC) methods can be used. Higher overall survival (OS) is shown in separate randomized controlled trials in relation to placebo (PBO) + FUL for PAL+FUL in PALOMA-3 (HR: 0.81; 95% CI: 0.64–1.03), ABM+FUL in MONARCH 2 (HR: 0.76; 95% CI: 0.61–0.95) and RIB+FUL in MONALEESA 3 (HR: 0.72; 95% CI: 0.57–0.92). However, amongst publications that impact the final results, they do not take into consideration the relevant variability in patient populations as important prognostic variables. In the study of Rugo et al, matching-adjusted indirect comparisons (MAICs) were developed to compare PAL + FUL, ABM + FUL and RIB + FUL in the management of HR+/HER2- MBC in terms of overall survival. After comparing populations based on eligibility criteria, and after adjusting for differences between the studies regarding the baseline characteristics, and taking into account the differences in the control arms between the studies, it was possible to observe an HR reduced by 1.05 (95% CI: 0.76–1, 44) in traditional ITC to 0.87 (95% CI: 0.54–1.40) in MAIC with ABM + FUL and from 1.09 (95% CI: 0.78–1.53) in Traditional ITC at 0.89 (95% CI: 0.48–1.63) in MAIC with RIB + FUL. As no head-to-head clinical trials have been published, these results validated that PAL + FUL have OS that can be considered similar to other inhibitors (CDK4/6i) for treating HR+/HER2- MBC.10
To date, the cost-effectiveness of PAL+FUL compared to RIB+FUL, and ABM+FUL in the context of Italian health care system, has not been evaluated.
In the field of health decision-making, economic evaluations are essential to compare costs and benefits of new health technologies and calculating if their application is an efficient use of resources.11
Therefore, a cost-effectiveness analysis was performed to compare the three CDK4/6 inhibitors in association with FUL for the management of postmenopausal women with advanced HR+/HER2- MBC in Italy.
Methods
The purpose of this study was to evaluate the cost-effectiveness of PAL+FUL, compared with RIB+FUL and ABM+FUL, in postmenopausal patients with advanced HR+/HER2- MBC.
The economic model developed with Microsoft Excel® 2013 presented two different scenarios: in the first scenario a parity of survival data (OS) was assumed given that OS, derived from the MAIC,10 showed a similar efficacy for the three CDK4/6i. Therefore, differences in drug safety, monitoring and costs were considered and a cost-minimization analysis was performed.
In addition, in order to assess the cost-effectiveness of the three drugs taking into account the QoL data (utility parameters) from the study by Lloyd et al 2006 relating to adverse events (AEs), an ad hoc analysis was conducted. Lloyd et al (2006)12 was deemed the best available source for utility in MBC, and it was used in our study to calculate the decrement in utility associated with each AE.
Frequencies of AEs associated with all therapies (ie PAL, ABM and RIB) were obtained from PALOMA-3, MONARCH 2, and MONALEESA-3 trials and were used to populate the pharmaco-economic model. The tool has been adapted to the Italian context from the perspective of the third-party payer. The target population for the model is composed of postmenopausal advanced HR+/HER2- MBC patients.
Model Structure
To evaluate the cost-effectiveness of PAL+FUL, a semi-Markov model was carried out. Three states make up the pharmacoeconomic models: Progression-free (second-line treatment); Progression (subsequent lines of treatment, ie, combination of active treatment (AT) and best supportive care (BSC)); Death.
A discrete monthly cycle was defined as a time interval of 28 days. The rationale behind this choice is the posology of PAL, which is administered on a four-week basis (3 weeks on and 1 week off). Each year is therefore composed of 13 cycles.
The pathway of patients is described in Figure 1. All patients start the pharmacoeconomic model in the “Progression-free” state where they will receive treatment. At each monthly time interval, patients can remain stable or respond to the treatment, progress, or die. Patients are not supposed to change medication without disease progression. If the disease progresses, patients stop the current treatment and pass to “Disease progression state”. Patients will receive subsequent lines of active treatment for a certain number of cycles and then BSC. Patients that have progressed will stay in this state until they die. Terminal care (TC) will be considered during the last 2 weeks of life. Patients can die due to the disease or from any other cause at any time and at any state. “Death” is an absorbing state, meaning that there is no transition out of this state.
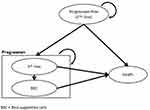 |
Figure 1 Semi-Markov model structure. |
For the purposes of the analysis, it was decided to use a semi-Markov model, in which the area below each survival curve allows to validate patients who remain stable, progress, and die. Taking the palbociclib plus fulvestrant arm as an example, at cycle X, the proportion of patients in the “No disease progression state” will be obtained from the parametric curve for PFS. The number of patients who will be in the “disease progression state” will be the difference between patients who are alive (obtained thanks to the parametric curve for OS at cycle X) and patients who remain stable, while the patients who died at the X cycle will be the inverse of the parametric curve for OS. Moreover, patients in the progressed disease state can be split between two sub-states (ie, active treatment and BSC) by following the flow of patients who progress and by assuming on the duration of the active treatment. Patients progressing receive an active treatment for a maximum of 18.9 cycles before switching to BSC. Overall survival (OS) is estimated by extrapolation of the survival estimates while on second-line treatment. The analysis considered a discount rate of 3%, while in the sensitivity analysis a discount rate of 0.0% and 5.0% is used.13
Comparators
The model allows the comparison between PAL+FUL and RIB+FUL or ABM+FUL.
Efficacy Parameters
The differences in the basic characteristics present in the different studies (PALOMA-3 MONARCH 2 and MONALEESA-3)6–8 were taken into account thanks to the MAIC.10 In order to evaluate and compare the effects of treatment using data from PALOMA-3, MONARCH 2 and MONALEESA-3,6–8 indirect treatment comparison methods (ITC) were used as there are no head-to-head studies in the literature of PAL, ABM and RIB to treat HR +/HER2- MBC. Traditional ITC methods based solely on summary level data can be confusing due to the differences that may exist in patient populations. Through MAICs, it is possible to indirectly compare a treatment effect between studies using individual patient data (IPD) from a study to minimize differences between trials. To achieve this, the IPD needs to be matched and adjusted to the study population summary level data used for comparison. As stated above, in the first scenario, the effectiveness of the three CDK4/6i was considered comparable. Instead, in the second scenario, the efficacy data (OS and PFS) of the MAIC and of Zhang et al study were used.14
Time Horizon
Patients are followed up until death for lifetime horizon (maximum 15 years).
Adverse Events
The AEs included in the model are based on grades 3 and 4, incidence and relevance of management costs. Frequencies of AEs for PAL+FUL and for the comparators are presented in Table 1.
 |
Table 1 Treatment-Related Adverse Event Frequency (Grade 3 and 4)6–8 |
The AEs of the three CDK4/6i were collected from PALOMA-3 for PAL, from MONARCH-2 for ABM and from MONALEESA-3 for RIB.
The cost of AEs in the model is applied in the first cycle of the progression-free state when all patients are alive. We assumed that AEs are mutually independent and have the same incidence rate across the cycles.
To evaluate the management of each AE, the expert opinions of two key opinion leaders were considered to establish how the management of each AE occurs in clinical practice and depending on the type of event, they were valued using the DRG, specialist fees or the simple cost of pharmacological therapy.
Resource Use and Costs
Total costs of patients with advanced or metastatic breast cancer over the model’s time horizon were estimated using the Semi-Markov model structure described above. Costs were assigned to the progression-free and post-progression health states, as well as to the last two weeks of patients’ life (terminal care costs). The year of cost for the analysis is 2022 (For more details, please refer to Supplementary Tables 1 and 2).
Pre-Progression Costs
Drug Acquisition Costs
The cost of the drug in each cycle is based on data from desk research from regional and national databases and public sources.15,16 Costs of the three drugs have been considered with −5–5% Italian mandatory rebate.
For RIB, the dose reduction was considered as indicated in MONALEESA-3 study.7 Specifically, as reported in this study, 62% of patients remained with the full dose (600 mg), 31% experienced a dose reduction and 7% experienced two dose reductions. Not knowing when these reductions occurred, it was assumed that they happened in 85% of the time, while the patient is being treated: initially patients started with 600 mg (full dosage) then 31% had a reduction for 85% of the time of therapy, while for 15% of the time they continued at full dose. As for the 7% of patients who had double reduction, they had it in 75% of the time (for less time as first patient make one reduction, then two). These assumptions were validated by expert opinion.
Dose reductions were considered in the calculation of the cost of therapy since the price of RIB is linear with respect to the different packages on the market. With reference to PAL and ABM, the cost of therapy is not impacted by the reduction in doses as the cost of the packages on the market remains unchanged.
Administration
The administration costs are calculated for every cycle and account for outpatient visit, exams and ECG and evaluated, through National tariff, the quantity as per expert opinion suggestion.
Monitoring
The different resources included in the monitoring of patient: CBC, Liver Function Tests, ECG, CT scan, have been quantified and validated by expert opinions. Their costs are taken from the Italian national tariff. Monitoring of patients is assumed to be conducted during the first cycle and at each subsequent cycle thereafter. This is to accommodate the fact that for some drugs monitoring during first cycles may be different.
Adverse Event Costs
AE costs are calculated as a one-off in the first cycle because the frequency of AEs is based on the intention-to-treat (ITT) population. Therefore, the cost of AEs in the model is applied in the first cycle of the pre-progression state when all patients are alive. The unit costs for each AE considered are based on the treatment flowchart given by expert opinion and evaluated through national tariff17 referring to the National Diagnosis-Related Group (DRG) system,18 National Tariffs17 and Farmadati19 with reference to drug prices.
Best Supportive Care Costs
The model also allows for best supportive care (BSC) during pre-progression. This should be the average level of supportive care that should be expected for a patient from the end of the first course of treatment to the onset of progressive disease. The package of care is assumed to be the same for all the treatment strategies. The resources used are composed of community nurse visit and general practice home visit; their costs are valuated through Supplemento ordinario n 38/l Gazzetta Ufficiale Serie Generale n 201 29/8/2016,20 and Italian National Tariff.17
Post-Progression Costs
In the post-progression state, it is assumed that patients stay on alternative active treatment (including everolimus + exemestane, chemotherapy and hormone monotherapy) for a certain number of cycles (eg 12), then move into BSC and then die. Therefore, the post-progression costs include costs while on active treatment, and costs of best supportive care.
The cost of subsequent lines of treatment beyond the second line is estimated as a weighted average of the costs of the regimens most used in the clinical pathway, and the proportion is an assumption based on clinical practice of the opinion leader involved. The costs of the different lines of active treatment and their usage proportion can be found below.
Going into the detail of active treatments, the analysis assessed the use of everolimus + exemestane in 35% of patients with a cost of €1,853.27. Chemotherapy was considered for 60% of patients with a cost of €540.37, and 5% of patients were administered hormone monotherapy with a cost of €25.92.
After progressing, patients stay on active treatment for a specific number of cycles and then they move to BSC before they die, the BSC costs were exposed before, the resources used for the treatment of BSCs according to clinical practice in Italy was valued through expert opinion. Costs related to best supportive care were also provided for the post-progression state, which were calculated in a similar way to the pre-progression, and the only difference is in the number of resources valued.
Terminal Care Costs
They included Hospital (25%), Hospice (15%), and Home (60%). Direct costs for two weeks were respectively €2.839,20 for Hospital17,18 and € 3.983,00 for Hospice.21
Sensitivity Analysis
Two different sensitivity analyses were performed. In the first, an ideal cost-effectiveness analysis was conducted including the efficacy values relating to OS and PFS which were taken, respectively, from MAIC study10 and from Zhang et al14 besides the utility parameters valued in the Lloyd study.12
Moreover, to evaluate the robustness of results, we performed a one-way sensitivity analysis modifying the following parameters by ± 20%: drug acquisition costs, administration costs, monitoring costs, AEs, BSC costs, active treatment costs and terminal care costs.
Results – Cost-Minimization Analysis
The results from the cost-minimization analysis of PAL+FUL versus RIB+FUL and ABM+FUL, over a lifetime horizon, are shown below. The total costs of the two treatment arms are presented by type of cost in Table 2.
 |
Table 2 Results of the Cost Minimization Analysis |
As can be seen from Table 2, in the present cost minimization analysis, it was decided to first compare PAL+FUL vs RIB+FUL, obtaining a saving per patient of €305 considering a lifetime time horizon.
For PAL+FUL versus ABM+FUL, the savings obtainable per patient is €243, again over a lifetime horizon. PAL has less costly monitoring, while the other costs are comparable for the three drugs. The costs related to post progression (active treatment and BSC) and terminal care are considered identical for the three CDK 4/6i.
The driver of this cost minimization analysis is the management of the AEs of PAL+FUL which is less expensive in comparison with RIB+FUL and ABM+FUL. This refers to the improved safety profile of PAL and to the fact that AEs related to PAL are economically less impactful.
PAL is therefore comparable to RIB and ABM in terms of effectiveness and produces slight economic savings over a lifetime.
A Budget Impact Analysis (BIA) was performed to verify the financial impact on the total number of patients who would arrive at the second line of treatment. The BIA and CEA are critical to obtain a comprehensive economic assessment for a health technology or a new drug entering the market.22
The results of a BIA showed how, if usage percentages for PAL were assumed to be 18%,23 ABM at 14%23 and RIB at 12%23 out of a total target population of 8732 patients arriving at the second line,24 total potential savings of € 319.563 for PAL vs RIB would be obtained and €297,544 for PAL vs ABM.
Results – Cost-Effectiveness Analysis
Three measures were estimated in the model to assess the difference in patients’ health incurred by the treatment strategies compared. QALYs measure the effect of both quality and length of life and are considered as the primary health outcome. To estimate the total QALYs, the utility for each state of health is multiplied by the time and number of patients related to that state of health. LYs gained measure the improvement in life expectancy. It is also expressed as additional months of survival. Progression-free life-year (PF-LY) is defined as the length of time during and after treatment in which a patient is living with stable disease.25
A treatment is defined as convenient when the ICER falls below a certain cost per QALY25 threshold. The ICER per accepted earned QALY is different for each country. NICE has set the threshold at around £ 20,000–30,000.26 In Italy, there is no officially established value, and the guidelines of the Italian Association of Health Economics (AIES)27 establish a threshold of € 25,000–40,000. Additional acceptable cost-effectiveness thresholds in Italy are €36,500 and €60,000 calculated in two different studies.28,29
Based on what has been described up to now, the result of the cost-effectiveness analysis showed the dominance of PAL +FUL when compared with ABM+FUL and RIB+FUL in terms of ICER, PF-LYs, and LYs.
This is a conservative scenario that considers the three CDK4/6 inhibitors with equal effectiveness in terms of overall survival (OS) (MAIC, Rugo et al 2021)10 and PFS and considers the quality-of-life data (utility parameters) from the study by Lloyd et al 2006 related to adverse events (AEs).12
Considering the quality-of-life data, a slight dominance of PAL was obtained due to the lower impact of adverse events with consequent savings for NHS and an increase in the quality of life (QoL) per patient.
Sensitivity Analysis
A first sensitivity analysis compared PAL+FUL vs ABM+FUL and PAL+FUL vs RIB+FUL in terms of cost-effectiveness. The three drugs were compared including the analysis of the data relating to OS and PFS10,14 combined with the quality-of-life data according to what emerged from the algorithm used by Lloyd in his study (applicable only 4 of the 13 AEs considered).12 If PAL+FUL vs ABM+FUL were compared, an ICER of €21.912 would be obtained, with an increase in terms of QALY of 0.291. As regards the PF-LY, against an increase of 0.332, a cost per PF-LY equals to €19.214 and a cost for LY equals to €14.851 with an increase in terms of LY equals to 0.429 would be obtained. When compared PAL+FUL and RIB+FUL, an ICER of €24.295 would be obtained, with an increase in terms of QALY of 0.264. As regards the PF-LY, against an increase of 0.350, a cost per PF-LY equals to €18.311 and a cost for LY equals to €17.762 with an increase in terms of LY equals to 0.361 would be obtained. The results obtained are included in the acceptability thresholds and are therefore in favor of Palbociclib.
The uncertainty of the variables and the robustness of the results obtained were verified with deterministic univariate analysis. One-way sensitivity analyses were performed in this second sensitivity analysis to assess the impact of estimated parameters on the costs associated with all treatments (Figures 2 and 3). We tested different scenarios, altering the following inputs one at a time: Discount rate, Drug acquisition costs, Administration costs, Monitoring costs, Adverse events costs, BSC costs, Active treatment costs and Terminal care costs.
 |
Figure 2 Sensitivity analysis palbociclib plus fulvestrant vs ribociclib plus fulvestrant. |
 |
Figure 3 Sensitivity analysis palbociclib plus fulvestrant vs abemaciclib plus fulvestrant. |
Except for the discount rate, for which we used values of 0% and 5%, for the other inputs we considered a 20% increase and a 20% decrease in the sensitivity analysis.
The limited dispersion of the sensitivity analyses demonstrates the strong robustness of the results of this appraisal both when PAL+FUL is compared to RIB+FUL and when compared to ABM+FUL.
Discussion and Conclusion
The purpose of this study was to compare PAL+FUL, ABM+FUL and of PAL+FUL versus RIB+FUL in postmenopausal patients with recurrent HR+/HER2- MBC with a cost minimization and a cost-effectiveness analysis.
The MAIC technique was reported in this study because it considers the data of each patient and allows to adjust the limitations due to indirect comparisons that are based only on aggregated data. MAICs resolve differences in patient baseline characteristics, to smooth out the differences associated with defining clinical responses, to minimize the sensitivity of effect measurement, and to be able to compare clinically relevant dosing schedules.
The cost-minimization analysis refers to a particular type of cost-effectiveness: a method that allows to offset the costs of different interventions (including the costs related to the management of possible consequences due to the intervention), known or which have a medical effect that is equivalent. It is possible to use this type of analysis to establish which therapeutic alternative is the least expensive and which yields a specific health outcome for a certain population.30 Cost minimization is considered as a sub-analysis of the cost-effectiveness ratio. This economic evaluation can be used if, prior to the investigation, there is no reason to expect a therapeutic difference in the results of the procedures under examination.31
Through this study, we wanted to demonstrate that since PAL is comparable to RIB and ABM in terms of efficacy (Rugo et al 2021), it produces slight economic savings over a lifetime horizon. In fact, the results of the analysis showed a saving per patient of €305 (lifetime) when PAL is compared with RIB, while when comparing PAL with ABM, a saving per patient of €243 (lifetime) is generated in a conservative scenario such as the one described above. The results of a BIA showed how, if usage percentages for PAL were assumed to be 18%, ABM at 14% and RIB at 12% out of a total target population of 8732 patients arriving at the second line, total potential savings of €319,563 for PAL vs RIB would be obtained and €297,544 € for PAL vs ABM. Moreover, if the quality-of-life data were also considered, a slight dominance of PAL would be obtained due to the lower impact of AEs with consequent savings for NHS and an increase in the QoL per patient related to fewer adverse events.
As far as we know, this is the first study evaluating the cost-effectiveness (in particular cost-minimization) of PAL and the first study comparing the cost-effectiveness of the three CDK4/6i used in Italy. Wang et al 2132 conducted a study evaluating the cost-effectiveness of ABM + FUL in treating women with advanced HR+/HER2– or MBC with second-line treatment, from the US payer’s perspective. The results of the study were found to be in line with what emerged from the present work, as the comparison between the three CDK4/6i, PAL was found to be the most cost-effective. Furthermore, even in the study conducted by Avxentyev et al,33 the use of PAL versus RIB in the Russian population was found to be the most cost-effective treatment option for postmenopausal HR+/HER2- patients with locally advanced MBC.
HR+/HER2- MBC is not a disease from which the patient can recover, however it can have a long course with multiple lines of sequential effective regimens including, to date, ET alone or associated to CDK4/6i, PI3k inhibitors (e.i. Everolimus) and chemotherapy in further lines. For this reason, when new treatments are introduced more effectively it is very important to demonstrate that QoL is not compromised. Treatment-associated toxicity can adversely affect quality of life. Although the treatment can prolong the time, it takes for the disease to progress thus preventing the effects of the disease.34
This analysis also appears to have some limitations. A noteworthy limitation of the study relates to the use of utilities for QoL estimation that do not derive from an Italian study. Based on the authors’ experience, this is a very common limitation regarding cost-effectiveness analyses in the Italian context, because there is a scarce production of original studies on QoL, in factto date, there is no publication comparing QoL in Italy of the three drugs under analysis. Furthermore, a limitation of the analysis is due to not having been able, due to the lack of information, to consider the utility related to all the adverse events present in the analysis. A further limitation of this analysis is closely related to the use of cost data in some cases, based on DRG rates, rather than real costs.
It would be important to conduct real world studies to confirm the results of this analysis in fact. In the area of reimbursement decisions, health care decision makers are developing policies that integrate data from different sources, valuing the importance of evidence that goes beyond the information gathered in clinical trials, so real-world studies are of great importance.35 Economic evaluation studies that incorporate real-world evidence are critical because they reflect the effectiveness of drugs in real life and show how they translate into the economic value of the drug for the lives of patients.36
Apart from the limitations, however, considering the comparable effectiveness of the three CDK4/6i, the result of the economic analysis showed a cost-saving profile associated with the use of PAL. Moreover, if OS, PFS and quality-of-life data were also considered in the analysis (second scenario), results in favor of PAL could be obtained, because of the lower impact of AEs related to PAL with consequent savings for NHS and an increase in QoL per patient. In conclusion, from the Italian NHS perspective, the result of the economic analysis showed a cost-saving profile associated with the use of PAL+FUL compared to RIB+FUL and ABM+FUL in the treatment of endocrine-resistant HR+/HER2- MBC patients.
Funding
This analysis was sponsored by Pfizer, Italy.
Disclosure
GLC, MCV, MC, GMB, and SDM are employees of S.A.V.E. S.r.l and consultants for different pharmaceutical companies. GLC reports grants from Pfizer. At the time of project initiation and drafting of the paper, GL were employed by IQVIA, which receives professional service fees from a large number of sponsors for a large number of activities in biopharma. MVD reports personal fees for advisory/consultancy role from: Pfizer, Eli Lilly, Novartis, Seagen, Gilead, Exact Sciences, MSD. AF reports personal fees for advisory/consultancy role from: Roche, Novartis, Lilly, Pfizer, MSD, Dompè, Pierre Fabre, Eisai, Sophos, Epionpharma, Gilead, Seagen, Astra Zeneca, Exact Sciences. The authors report no other conflicts of interest in this work.
References
1. Ferlay J, Colombet M, Soerjomataram I, et al. Cancer incidence and mortality pattern in Europe: estimates for 40 countries and 25 major cancer in 2018. Eur J of Cancer. 2018;103:356–387. doi:10.1016/j.ejca.2018.07.005
2. AIOM-AIRTUM. I numeri del cancro in Italia. Disponibile all’indirizzo; 2019. Available from: https://www.aiom.it/wp-content/uploads/2019/09/2019_Numeri_Cancro-operatori-web.pdf.
3. Aljohar BA, Kilani MA. Breast cancer in Europe: epidemiology, risk factors, policies and strategies. a literature review. Glob J Health Sci. 2018;10:1. doi:10.1038/35021093
4. Tamura K. Differences of cyclin-dependent kinase 4/6 inhibitor, palbociclib and abemaciclib, in breast cancer. Jpn J Clin Oncol. 2019;49:993–998. doi:10.1093/jjco/hyz151
5. Finn RS, Aleshin A, Slamon DJ. Targeting the cyclin-dependent kinases (CDK) 4/6 in estrogen receptor-positive breast cancers. Breast Cancer Res. 2016;18:17. doi:10.1186/s13058-015-0661-5
6. Turner NC, Huang Bartlett C, Cristofanilli M. Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med. 2015;373(17):1672–1673. doi:10.1056/NEJMoa1505270
7. Slamon DJ, Neven P, Chia S, et al. Phase III randomized study of ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: MONALEESA-3. J Clin Oncol. 2018;36(24):2465–2472. doi:10.1200/JCO.2018.78.9909
8. Sledge GW, Toi M, Neven P, et al. The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor-positive, ERBB2-negative breast cancer that progressed on endocrine therapy-MONARCH 2: a randomized clinical trial. JAMA Oncol. 2019;6(1):116–124.
9. Iorfida M, Mazza M, Munzone E. Fulvestrant in Combination with CDK4/6 Inhibitors for HER2- metastatic breast cancers: current perspectives. Breast Cancer. 2020;12:45–56. doi:10.2147/BCTT.S196240
10. Rugo HS, Haltner A, Zhan L, et al. Matching-adjusted indirect comparison of palbociclib versus ribociclib and abemaciclib in hormone receptor-positive/HER2-negative advanced breast cancer. J Comp Eff Res. 2021;10(6):457–467. PMID: 33626934. doi:10.2217/cer-2020-0272
11. Husereau D, Drummond M, Petrou S, et al. Consolidated health economic evaluation reporting standards (CHEERS)—explanation and elaboration: a report of the ISPOR health economic evaluation publication guidelines good reporting practices task force. Value Health. 2013;16(2):231–250. doi:10.1016/j.jval.2013.02.002
12. Lloyd A, Nafees B, Narewska J, Dewilde S, Watkins J. Health state utilities for metastatic breast cancer. BrJCancer. 2006;95(6):683–690.
13. Fiorentino F, Urbinati D. First Italian guidelines for the economic evaluation of health technologies: how do they compare to NICE standards?. GIHTAD. 2021; 14:5. Available from: https://springerhealthcare.it/GIHTAD/wp-content/uploads/2021/07/GIHTAD_Fiorentino_14_5.pdf. Accessed March 15, 2023.
14. Zhang T, Feng F, Zhao W, et al. Comparative efficacy of different targeted therapies plus fulvestrant for advanced breast cancer following progression on prior endocrine therapy: a network meta-analysis. Cancer Manag Res. 2018;10:5869–5880. PMID: 30510455. doi:10.2147/CMAR.S176172
15. Eli Lilly Italia S.P.A. SISTEMA DINAMICO DI ACQUISIZIONE FORNITURA DI FARMACI ED EMODERIVATI XXII APPALTO SPECIFICO. Rep.140/20. Available from: https://www.soresa.it/societatrasparente/Bandi%20di%20gara%20e%20contratti/Contratti/Farmaci%20ed%20Emoderivati/FARMACI/2020/XXII%20APPALTO%20SPECIFICO/rep.%20140_20%20-%20ELI%20LILLY%20ITALIA%20Spa.pdf.
16. Soresa. Available from: https://www.soresa.it/pa/Pagine/Anagrafe/Farmaci-Emoderivati.aspx?Folder=Anagrafiche%20e%20Schede%20Prodotti/Farmaci%20ed%20Emoderivati/2021.
17. Ministero della Salute. (Italian Ministry of Health). Tariffe delle prestazioni di assistenza specialistica ambulatoriale. [inpatient tariffs]. Gazzetta Ufficiale della Repubblica Italiana. Serie N.23; Supplemento N.8 del 28 gennaio; 2013.
18. Ministero della Salute. (Italian Ministry of Health). Tariffe delle prestazioni di assistenza ospedaliera per acuti [DRG tariffs]. Gazzetta Ufficiale della Repubblica Italiana; Serie N.23; Supplemento N.8 del 28 gennaio; 2013.
19. FARMADATI Italia software Tunnel®. Available from: https://www.farmadati.it/.
20. Supplemento ordinario n 38/l alla Gazzetta Ufficiale Serie Generale n 201 del 29/8/2016. Available from: https://www.gazzettaufficiale.it/do/atto/serie_generale/caricaPdf?cdimg=16G0017700100010110001&dgu=2016-08-29&art.dataPubblicazioneGazzetta=2016-08-29&art.codiceRedazionale=16G00177&art.num=1&art.tiposerie=SG. Accessed March 15, 2023.
21. Tariffe Lazio SAN_DCA_U00411_13_09_2017. Available from: https://www.ausl.latina.it/attachments/article/249/SAN_DCA_U00411_13_09_2017.pdf. Accessed March 15, 2023.
22. Mauskopf JA, Sullivan SD, Annemans L, et al. Principles of good practice for budget impact analysis: report of the ISPOR task force on good research practices— budget impact analysis. Value Health. 2007;10(5):336–347. doi:10.1111/j.1524-4733.2007.00187.x
23. IQVIA. Il mercato Breast HER2- HR+, Studio multiclient, ottobre; 2021.
24. Nuzzolese I, Montemurro F. Attrition in metastatic breast cancer: a metric to be reported in randomised clinical trials? Lancet Oncol. 2020;21(1):21–24. PMID: 31908294. doi:10.1016/S1470-2045(19)30792-2
25. Drummond MF, Sculpher MJ, Claxton K, et al. Methods for the Economic Evaluation of Health Care Programmes.
26. Cleemput I, Neyt M, Thiry N, De Laet C, Leys M. Using threshold values for cost per quality-adjusted life-year gained in healthcare decisions. Int J Technol Assess Health Care. 2011;27(1):71–76. doi:10.1017/S0266462310001194
27. Fattore G; Italian Health Economics Association Associazione Italiana di Economia Sanitaria – AIES. Proposta di linee guida per la valutazione economica degli interventi sanitari in Italia. [Italian guidelines proposal on how to conduct economic evaluation studies of health programs]. Pharmacoecon Ital Res Articles. 2009;11:83–93. Italian. doi:10.1007/BF03320660
28. Lucioni C, Ravasio R. Come valutare i risultati di uno studio farmacoeconomico? PharmacoEcon Ital Res Articles. 2004;3:121–130. doi:10.1007/BF03320630;
29. Messori A, Santarlasci B, Trippoli S, Vaiani M. Controvalore economico del farmaco e beneficio clinico: statodell’arte della metodologia e applicazione di un algoritmo farmacoeconomico. Pharmacoecon Ital Res Articles. 2003;5:53–67. doi:10.1007/BF03320605
30. Cost Minimisation Analysis. York; York health economics consortium; 2016. Available from: https://yhec.co.uk/glossary/cost-minimisation-analysis/.
31. Paravastu SC, Michaels JA. Michaels, in Core Topics in General and Emergency Surgery. Elsevier; 2014.
32. Wang Y, Rui M, Guan X, Cao Y, Chen P. Cost-effectiveness analysis of abemaciclib plus fulvestrant in the second-line treatment of women with HR+/HER2- Advanced or metastatic breast cancer: a US payer perspective. Front Med. 2021;8:658747. PMID: 34150798. doi:10.3389/fmed.2021.658747
33. Avxentyev NA, Lubennikova EV, Frolov M. Pharmacoeconomic analysis of using cyclin-dependent kinase 4 and 6 inhibitors in the first line treatment of HR-positive HER2-negative advanced breast cancer. FARMAKOEKONOMIKA Mod Pharmacoeconomics Pharmacoepidemiol. 2019;12(4):279–290. doi:10.17749/2070-4909.2019.12.4.279-290)
34. Rugo HS, Diéras V, Gelmon KA, et al. Impact of palbociclib plus letrozole on patient-reported health-related QoL: results from the PALOMA-2 trial. Ann Oncol. 2018;29(4):888–894. PMID: 29360932. doi:10.1093/annonc/mdy012
35. Garrison LP, Neumann PJ, Erickson P, et al. Using real-world data for coverage and payment decisions: the ISPOR real-world data task force report. Value Health. 2007;10:326–335. doi:10.1111/j.1524-4733.2007.00186
36. Ruggeri I, Bragato D, Colombo GL, Valla E, Di Matteo S. Cost and appropriateness of treating asthma with fixed-combination drugs in local health care units in Italy. Clinicoecon Outcomes Res. 2012;4:375–382. doi:10.2147/CEOR.S36499
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
