Back to Journals » Risk Management and Healthcare Policy » Volume 8
Economic burden of Clostridium difficile in five hospitals of the Florence health care system in Italy
Authors Poli A, Di Matteo S, Bruno GM , Fornai E, Valentino MC , Colombo GL
Received 15 June 2015
Accepted for publication 8 September 2015
Published 18 November 2015 Volume 2015:8 Pages 207—213
DOI https://doi.org/10.2147/RMHP.S90513
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Frank Papatheofanis
Anna Poli,1 Sergio Di Matteo,2 Giacomo M Bruno,2 Enrica Fornai,1 Maria Chiara Valentino,2 Giorgio L Colombo2,3
1Vigilanza e Controllo Infezioni Correlate all'Assistenza, Ospedale Piero Palagi, Azienda Sanitaria di Firenze, Firenze, Italy; 2SAVE Studi – Health Economics and Outcomes Research, Milan, Italy; 3Department of Drug Sciences, University of Pavia, Pavia, Italy
Introduction: Despite the awareness about the increasing rates of Clostridium difficile infection (CDI) and the economic burden arising from its management (prolonged hospitalization, laboratory tests, visits, surgical treatment, environmental sanitation), few studies are available in Italy on the economic costs directly attributable to the CDI. The Florence health care system has designed a study with the aim of describing the costs attributable to the CDI and defines the incremental economic burden associated with the management of this complication.
Methods: We conducted a retrospective study in five hospitals of the Florence health care system. The enrolled population included all patients who were hospitalized during the year 2013 with a diagnosis of CDI. Of the 187 total cases reported in 2013, 69 patients were enrolled, for whom the main cause of hospitalization was directly attributable to CDI.
Results: We enrolled 69 patients (19 males and 50 females), with a mean age of 82.16 years (minimum 46 to maximum 98). The total number of hospitalization days observed was 886 (12.8 per patient on average). The data from this study show that the mean total incremental cost for a patient with CDI was €3,270.52 per year. The hospital stay length is the most significant cost parameter, having the largest influence on the overall costs, with an impact of 87% on the total cost. The results confirm the costs for the management of CDI in five hospitals of the Florence health care system are in line with data from the international literature.
Conclusion: The economic impact of CDI is most evident in the extension of the duration of hospitalization and emergency recurrences requiring new therapeutic options with the need to develop and implement new diagnostic and therapeutic algorithms in clinical practice.
Keywords: cost of illness, burden of disease, pharmacoeconomics, cost analysis
Introduction
Clostridium difficile is one of many types of bacteria that are normally found in the colon. A large expansion of its population occurs after the consumption of antibiotics which kill off other indigenous bacterial flora. C. difficile infection (CDI) is one of the major causes of diarrhea in hospitalized patients and its incidence and severity are rising, often leading to death.1–3 Beside the recent increase in the use of antibiotics, other factors that influence the risk for CDI are gastrointestinal surgery, serious underlying illness, a weakened immune system (due, for example, to chemotherapy or HIV/AIDS), and advanced age.4 The prevalence of C. difficile spores in the environment is relatively high in hospitals, where subjects are frequently exposed to antibiotics.3,5,6
In recent years, CDI has become one of the most important public health problems worldwide; in fact, increases not only in the incidence of infections but also their severity, recurrence, and mortality have been observed.7 From a study conducted in 14 European countries in 2005, Barbut et al reported a mean CDI incidence of 2.45 cases per 10,000 patient-days,8 whilst a more recent European survey, conducted in November 2008, collected data from 106 laboratories in 34 European countries and reported a mean CDI incidence of 4.1 per 10,000 patient-days per hospital.9 Both studies reported a high variability in incidence of CDI among hospitals and countries. Epidemiological data from Italy regarding CDI rates derive mainly from the European survey conducted in 2008, with only five Italian hospitals involved, which included 57 CDI cases on 533 tested patients.9 A small study in Bolzano, in the north of Italy, reported 13 cases of nosocomial CDI from 163 tested patients.10
Recent studies are focused on the comparison between different therapies for the treatment of CD infection.11,12 Despite the awareness about the infection rates from C. difficile and the economic burden derived from the management of CDI (extended hospitalization, rehospitalization, laboratory tests, medications, and surgical treatment), few studies are available about the economic costs of CDI, and a recent review investigating costs associated with CDI reported studies conducted from 1980 to April 15, 2009 in only four countries, not including Italy (USA, Canada, UK, and Ireland).13 In US-based studies, incremental costs were estimated to range between $2,871 and $4,846 per case of primary CDI.13–15 C. difficile places a significant burden on the health care system. However, there are few data defining the cost of CDI for health care systems, and much of the burden needs to be fully quantified. These costs will only continue to increase as the incidence of CDI continues to increase. Fully understanding the burden placed by CDI on health care is important in order to ensure that adequate resources are dedicated to CDI treatment and prevention efforts are cost-effective.16 The surveillance system at the Florence health care system for the management and control of CDI discloses epidemiological data in line with other international and European studies.3,5,6 The latest surveillance data show a worrying spread of multiresistant strains, resulting in increased mortality.17,18
Patients and methods
We conducted a retrospective study in the five hospitals of the Florence health care system in Florence, Italy. The enrolled population included all patients who were hospitalized during the year 2013 (from January to December) with a diagnosis of CDI disease at admission. Sixty-nine patients of the 187 total cases reported in 2013 had a diagnosis of CDI confirmed (detection of toxigenic C. difficile-specific – GDH antigen) and they were included in our analysis. The objective of the analysis was to describe the costs attributable to the CDI and to define the incremental cost associated with managing this important complication in terms of comorbidities and treatment options/adopted procedures. All data were collected from three different sources: from the Argos program (digital medical records), the Epi Info computer database system to collect data on CDI surveillance, and data from the Hospital Discharge Register (HDR). In accordance with Italian privacy law (code concerning the protection of personal data, 30 June 2003, n.196) patients were assigned identification numbers for the study, thus eliminating the patient health service codes and avoiding the risk of identifying patients personally.
Cost analysis
Cost analysis was completed under the hospital point of view. The costs analyzed were hospital stay, additional measures taken (transferred to intensive care unit [ICU] or isolation measures) for controlling the CDI, antibiotic therapy (vancomycin and metronidazole used individually or in combination), and diagnostic tests (X-ray, ultrasound, magnetic resonance imaging, computerized tomography), as well as costs associated with other types of interventions directly attributable to the CDI episode. Surgery performed for the management of CDI (colectomy, ileostomy, colostomy) were also included in the used resources during the design phase of the study, but the analysis of medical records showed that no surgery was performed on enrolled patients. As shown in Figure 1 the main cost was represented by the hospitalization (87,2%).
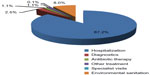  | Figure 1 Costs in Clostridium difficile infection management as percent of total cost. |
Table 1 shows the breakdown of hospital direct costs due to hospitalization divided into inpatient hospital and intensive care department length of stay (LOSs), alongside the corresponding amount in euros. The cost of 1 inpatient day was €198.51; the cost of 1 day in intensive care was €873.95. In addition to the items listed in Table 1, the cost of hospitalization also included the cost of sanitizing all bed accessories, sanitation of dedicated instruments (sphygmomanometer, stethoscope, thermometer, etc), sanitation of all reusable materials used by the company in charge of the environmental hygiene (carts, buckets, etc), and disposal of medical waste. The costs valued in this analysis were derived from the Nomenclatore Tariffario of Tuscany Region from 2013.19
  | Table 1 Cost analysis of hospitalization and other health care costs in Clostridium difficile infection management |
The cost for environmental sanitation for inpatients was divided into the following cost items: patient unit €6.25 and hospital room and bathroom €14.20. ICU cost change if patient recovered in the ICU department rising up to €15.93 per patient. Specialist advice was estimated at €15.00 based on the Tuscany Region Nomenclatore Tariffario from 2013.19 Table 1 also shows costs related to blood transfusions, transfusion of plasma, immunologic examinations for blood components, transfusion procedures, and venipuncture. The therapeutic path “Surveillance, Prevention, Diagnosis, and Treatment of Clostridium difficile in the Hospital” (AS/PR/051) of the Florence health care system follows the instructions of Document Address SIMPIOS, pending the approval of the regional procedure which provides for the introduction of new algorithms diagnostic.20 The chapter on antibiotic treatment provides the following guidelines.21
The treatment of the first episode of C. difficile colitis, mild and moderate forms, requires:
- oral metronidazole 0.250 g every 6 hours/0.5 g every 8 hours for 10–14 days; or
- oral vancomycin 0.125 g every 6 hours for 10–14 days; or
- combination therapy: oral vancomycin 0.125 g every 6 hours, oral metronidazole 0.5 g every 8 hours.21
- 125 mg four times a day for 14 days; then
- 125 mg two times a day for 7 days; then
- 125 mg per day for 7 days; then
- 125 mg every 2 days for 8 days (four doses); then
- 125 mg every 3 days for 15 days (five doses).21
The treatment of relapsing forms is as follows. The first relapse is treated like the first episode. From the second relapse, treatment is set as vancomycin “tapered-pulsed dosing”:
Antibiotic therapy (vancomycin €7.69/g, metronidazole €0.20/g) was recorded as the number of grams used in a day multiplied by the duration of administration of the therapy (expressed in total days of hospitalization) (Table 1).21
Statistical analysis
The data for all patients were analyzed through descriptive analysis on the main parameters. Descriptive statistics on study variables used traditional numerical synthesis measurements: mean, standard deviation, median, and maximum and minimum values for the continuous variables, and frequency distributions for the categorical variables. The analysis of variance was used to compare the means of the quantitative variables between patient groups. The main investigated variables were: the patients’ demographic characteristics; stratification by age range; hospitalization; antibiotic therapy; visits; and environmental sanitation. Statistical analysis was conducted using the Statistical Package for the Social Sciences statistical software (SPSS Statistics version 14.0; SPSS Inc., Chicago, IL, USA).
Results
Table 2 shows the main sociodemographic characteristics of the population included in the study. The mean age of the 69 patients examined (19 males [28.0%] and 50 females [72.0%]) was 82.16 years. Sixty-six patients were over 65 years of age, and three patients were under 65 years of age. Regarding the clinical area of hospitalization, 84.0% of the patients were hospitalized in the medical area; 6.0% were admitted to the surgical area; 4.0% to the short observation unit; 3.0% were hospitalized in the ICU; and, finally, 3.0% were hospitalized in the infectious disease area. The total number of hospitalization days observed was 886 (12.8 per patient on average), ranging from a maximum of 35 days to a minimum of 1 day (in this case, the patient died after 1 day of hospitalization).
  | Table 2 Patients’ characteristics |
Table 3 shows that the cost of a single inpatient day was €198.51; multiplied by the total days in hospital excluding ICU stays (855), it makes a total cost of €169,726.05. We added the cost of care per day in high-risk departments (€873.95), with a total ICU cost of €27,092.45 (31 days), and the total cost for the hospital was €196,818.50 (Table 3), with a mean cost per patient of €2,852.45 and a mean cost for LOS of €222.15 (hospitalization) (Table 5). The total cost of different antibiotic therapies was given by the unit cost of each therapy multiplied by the days of hospitalization and was €2,481.80 in total (Table 3), with a mean cost per patient of €35.60. The cost for sanitation was calculated by the number of days of hospitalization in inpatient hospitalization plus those in ICU departments multiplied by the daily cost for environmental sanitation, with a total cost of €17,978.58; the mean cost per patient was €260.56 (Table 5).
The total costs of transfusion therapy amounted to €2,580.00, with an average cost (divided by 48 patients) per patient transfused of €53.75. Table 4 also highlights the costs for diagnostic tests per patient and specialist advice (infectious diseases, surgery, and nephrology).
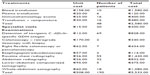  | Table 4 Other health care costs per number of patients in Clostridium difficile infection management |
Finally, Table 5 shows the total costs incurred by the patients participating in the study, divided into hospitalization costs, diagnostic exam costs, antibiotics costs, costs for blood transfusions/plasma infusion of fluids, specialist consultancy costs, and environmental sanitation costs. The analysis of medical records showed that nine patients were treated with vancomycin and 13 patients with metronidazole; 35 patients were put on a combination therapy of the two drugs; and 12 patients did not receive antibiotic therapy or were given different antibiotics other than those for the treatment of C. difficile. As shown in Table 5, the mean cost of CDI per patient was €3,270.52. The most significant cost driver was hospitalization, with a total of €196,818.50, followed by environmental sanitation at €17,978.58, costs of diagnostic procedures at €5,533.00, other treatments at €2,580.00, and antibiotic therapy at €2,481.80.
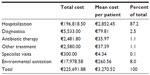  | Table 5 Total costs and mean costs per patient in Clostridium difficile infection management |
Discussion
Over time, cost-of-illness studies have become vitally important to the development of research and policy initiatives aimed at more cost-effectively treating and preventing illness. Despite limited data for Italy, studies to date have indicated that CDI has a significant burden on the health care system. For example, CDI may have resulted in as much as US $4.8 billion excess health care costs in acute-care facilities alone in 2008 in the USA.13,15,16 There are also additional costs for CDI that have yet to be quantified, such as increase in hospitalization rates. Those costs can be avoided if the patient with CDI is isolated in a semiprivate room, this can reduce the transmission of C. difficile and avoid new additional cases of CDI.16 Better understanding of the economic burden of C. difficile can assist various decision makers. Hospital administrators, infection control practitioners, and policy makers could use this information to determine how much to invest in C. difficile prevention and control measures. This information can help policy makers and third-party payers to make insurance coverage and reimbursement decisions. Manufacturers and drug companies can use such information to develop and price C. difficile tests and treatments.22
No significant studies on the economic impact of C. difficile in Italy had been carried out up to now, due to the difficulty in collecting data and developing and promoting studies for identifying the dynamics of the cost-of-illness trends based on the evolution of the epidemiological situation and on incidence and lifetime cost data.15 The results presented in this study demonstrate that the health care unit cost for the management of CDI (€3,270.52 per patient) is similar to those reported by other countries and in other systematic reviews. In particular, in a systematic review of 30 papers from 1980 to 2008, incremental cost estimate results in US-based studies ranged from $2,871 to $4,846 per case for primary CDI and from $13,655 to $18,067 per case for recurrent CDI. Non-US-based studies showed an estimated incremental cost of $5,243 to $8,570 per case for primary CDI and $13,655 per case for recurrent CDI. The economic health care costs of CDI were high for primary and recurrent cases. The high cost associated with CDI justifies the use of additional resources for CDI prevention and control.13,23
There are some limitations to this study, and the results are likely to underestimate total disease costs for various reasons. The first possible limitation concerns the extrapolation of 12-month expenses and the total sample enrolled with respect to other studies. Additional areas of study needed to fully understand the CDI impact on the health care system and society include the costs of recurrent CDI; costs of CDI managed in the outpatient setting; adverse events associated with CDI, particularly in elder individuals; and the impact of CDI on the quality of life. A full appreciation of the burden that CDI has on the health care system is necessary to ensure that adequate resources are allocated to CDI prevention and treatment efforts,16 but, even taking these considerations into account, our results appear to be in line with those of the international cost-of-illness studies performed in other countries. The economic evaluation is centered on the hospitalization and emergency extension in LOS that required new therapeutic options.24,25
The priority problems of current health care systems are: a shortage of available resources to meet general growing health demands; providing comparison tools; developing health care projects and technologies according to criteria of efficacy and convenience; and identifying a scale of priorities to guide the use of public resources. Economic analysis applied to health care activities aims to highlight the most efficient use of the available resources, rather than to reduce expenditure. Economic analysis shows the importance of using suitable tools both for comparing data on the use of medications and health care technologies and for helping doctors and health care professionals to optimize the resources at their disposal.26,27 Adequate analysis of outcomes and costs, as performed for the current treatments for CDI, should be assessed in terms of their incremental cost-effectiveness ratio. Investments must be made into outcomes research to make these tools a constant reference point for decision making by physicians and people in charge of health care policy.
With the increasing popularity of burden-of-disease studies, the standardization of methods becomes more critical to permit policy makers and the general public to better understand our investment in health care and to drive decisions about future insurance benefits, efforts in curbing and controlling disease and injury, and development of programs to improve the health of the population.28
Despite advances in the diagnosis and treatment of CDI and prevention efforts to reduce the spread of C. difficile, CDI remains a significant challenge to health care systems worldwide. Further advances in prevention of CDI may need to focus on those who continue to be exposed to the organism and who are susceptible. Interventions directed toward this susceptible population, particularly hospitalized patients who receive antibiotics, may be effective. There is moderate evidence on the effectiveness of probiotics to prevent primary CDI, but there are few data to support use in secondary prevention of recurrent CDI.29 Multifaceted national prevention efforts in the UK, including antimicrobial stewardship, patient isolation, hand hygiene, environmental cleaning and disinfection, and audit, resulted in a 59% reduction in CDI cases reported from 2008 to 2012.29 Studies have shown that unrecognized cases of CDI are admitted to health care facilities or transferred from one facility to another and may spread it within a facility via health care workers’ hands.30,31 However, CDI can be prevented. Environmental contamination stems from the fecal–oral transfer of C. difficile spores from the patient to the health care workers’ hands and medical equipment leading to the ingestion of spores by other patients.30,31 Since CDI has been the cause of many large outbreaks in hospital settings, frequent hand washing by health care personnel and frequent cleaning and disinfecting of the patient’s environment are of utmost importance in preventing transmission.
Disclosure
AP has received research and educational grants from Astellas. GLC has served as a consultant on advisory boards for Astellas, LEO Pharma, Sanofi, Merck Sharp and Dohme, DOC Generici, Takeda, and Merck Serono Spa, and has received research and educational grants from Takeda, Gilead Sciences, Merck Sharp and Dohme, and LEO Pharma. The authors report no other conflicts of interest in this work.
References
Gerding DN, Johnson S, Peterson LR, Mulligan ME, Silva J Jr. Clostridium difficile-associated diarrhea and colitis. Infect Control Hosp Epidemiol. 1995;16:459–477. | |
Redelings MD, Sorvillo F, Mascola L. Increase in Clostridium difficile-related mortality rates, United States, 1999–2004. Emerg Infect Dis. 2007;13:1417–1419. | |
Armbruster S, Goldkind L. A 5-year retrospective review of experience with Clostridium difficile-associated diarrhea. Mil Med. 2012;177:456–459. | |
Kinsman G. ‘Responsibility’ as a strategy of governance: regulating people living with AIDS and lesbians and gay men in Ontario. Econ Soc. 1996;25(3):393–409. | |
Zilberberg MD, Shorr AF, Kollef MH. Increase in Clostridium difficile-related hospitalizations among infants in the United States, 2000–2005. Pediatr Infect Dis J. 2008;27:1111–1113. | |
Gouliouris T, Brown NM, Aliyu SH. Prevention and treatment of Clostridium difficile infection. Clin Med. 2011;11:75–79. | |
Cartman ST, Heap JT, Kuehne SA, Cockayne A, Minton NP. The emergence of ‘hypervirulence’ in Clostridium difficile. Int J Med Microbiol. 2010;300(6):387–395. | |
Barbut F, Mastrantonio P, Delmée M, Brazier J, Kuijper E, Poxton I; European Study Group on Clostridium difficile (ESGCD). Prospective study of Clostridium difficile infections in Europe with phenotypic and genotypic characterisation of the isolates. Clin Microbiol Infect. 2007;13:1048–1057. | |
Bauer MP, Notermans DW, van Benthem BH, et al; ECDIS Study Group. Clostridium difficile infection in Europe: a hospital-based survey. Lancet. 2011;377:63–73. | |
Sansone S, Aschbacher R, Staffler M, et al. Nosocomial diarrhoea in adult medical patients: the role of Clostridium difficile in a North Italian acute care teaching hospital. J Prev Med Hyg. 2009;50:117–120. | |
Louie TJ, Miller MA, Mullane KM, et al; OPT-80-003 Clinical Study Group. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011;364:422–431. | |
Cornely OA. Current and emerging management options for Clostridium difficile infection: what is the role of fidaxomicin? Clin Microbiol Infect. 2012;18 Suppl 6:28–35. | |
Ghantoji SS, Sail K, Lairson DR, Dupont HL, Garey KW. Economic healthcare costs of Clostridium difficile infection: a systematic review. J Hosp Infect. 2010;74:309–318. | |
Sclar DA, Robison LM, Oganov AM, Schmidt JM, Bowen KA, Castillo LV. Fidaxomicin for Clostridium difficile-associated diarrhoea: epidemiological method for estimation of warranted price. Clin Drug Investig 2012;32:e17–e24. | |
Magalini S, Pepe G, Panunzi S, Spada PL, De Gaetano A, Gui D. An economic evaluation of Clostridium difficile infection management in an Italian hospital environment. Eur Rev Med Pharmacol Sci. 2012;16:2136–2141. | |
Dubberke ER, Olsen MA. Burden of Clostridium difficile on the healthcare system. Clin Infect Dis. 2012;55 Suppl 2:S88–S92. | |
Usiglio D, Marrapodi AM, Lanata M, et al. Incidence and surveillance of infections from Clostridium difficile: the experience at the Galliera of Genoa in the three-year period 2004–2006. Microbiologia Medica. 2009;24(1):25–30. | |
McDonald LC, Owings M, Jernigan DB. Clostridium difficile infection in patients discharged from US short-stay hospitals, 1996–2003. Emerg Infect Dis. 2006;12:409–415. | |
Nomenclatore Tariffario Regionale Delle Prestazioni Specialistiche Ambulatoriali. [Outpatient Formulary List of Medical Acts and Procedures]. Available from: http://www.regione.toscana.it/documents/10180/23313/Nomenclatore+tariffario+regionale/39bc54d6-6ee5-431e-98fe-1a60f2579721?version=1.0. Accessed May 28, 2015. Italian. | |
Sorveglianza (Prevenzione, Diagnosi E Trattamento) Del Clostridium Difficile In Ambiente Ospedaliero. [Surveillance (Prevention, Diagnosis and Treatment) Of Clostridium Difficile In Hospital Environment]. Available from http://www.usl11.toscana.it/dati/all20121121_pohhh011sorveglianzaclostridiuminosprev1.pdf. Accessed October 1, 2015. Italian. | |
Negoziazione e rimborsabilità [Negotiation and reimbursement]. [webpage on the Internet]. Rome: Agenzia Italiana del Farmaco (AIFA); [updated September 11, 2015]. Available from: http://www.agenziafarmaco.gov.it/it/content/negoziazione-e-rimborsabilit%25C3%25A0. Accessed January 23, 2015. Italian. | |
McGlone SM, Bailey RR, Zimmer SM, et al. The economic burden of Clostridium difficile. Clin Microbiol Infect. 2012;18(3):282–289. | |
Wiegand PN, Nathwani D, Wilcox MH, Stephens J, Shelbaya A, Haider S. Clinical and economic burden of Clostridium difficile infection in Europe: a systematic review of healthcare-facility-acquired infection. J Hosp Infect. 2012;81:1–14. | |
Venugopal AA, Johnson S. Fidaxomicin: a novel macrocyclic antibiotic approved for treatment of Clostridium difficile infection. Clin Infect Dis. 2012;54(4):568–574. | |
Eyre DW, Walker AS, Wyllie D, et al; Infections in Oxfordshire Research Database. Predictors of first recurrence of Clostridium difficile infection: implications for initial management. Clin Infect Dis. 2012;55 Suppl 2:S77–S87. | |
Ament A, Evers S. Costs of illness studies in health care: a comparison of two cases. Health Policy. 1993;26:29–42. | |
Drummond M. Cost-of-illness studies: a major headache? Pharmacoeconomics. 1992;2(1):1–4. | |
Clabaugh G, Ward MM. Cost-of-illness studies in the United States: a systematic review of methodologies used for direct cost. Value Health. 2008;11(1):13–21. | |
Evans CT, Johnson S. Prevention of Clostridium difficile infection with probiotics. Clin Infect Dis. 2015 ;60 Suppl 2:S122–S128. | |
Slayton RB, Scott RD, Baggs J, Lessa FC, McDonald LC, Jernigan JA. The cost-benefit of federal investment in preventing Clostridium difficile infections through the use of a multifaceted infection control and antimicrobial stewardship program. Infect Control Hosp Epidemiol. 2015;36(6):681–687. | |
Vassallo A, Tran MC, Goldstein EJ. Clostridium difficile: improving the prevention paradigm in healthcare settings. Expert Rev Anti Infect Ther. 2014;12(9):1087–1102. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

