Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 15
Economic Burden of Chronic Obstructive Pulmonary Disease (COPD): A Systematic Literature Review
Authors Iheanacho I, Zhang S, King D , Rizzo M , Ismaila AS
Received 17 October 2019
Accepted for publication 24 January 2020
Published 26 February 2020 Volume 2020:15 Pages 439—460
DOI https://doi.org/10.2147/COPD.S234942
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Ike Iheanacho,1 Shiyuan Zhang,2 Denise King,3 Maria Rizzo,1 Afisi S Ismaila2,4
1Meta Research, Evidera, London, UK; 2Value Evidence and Outcomes, GlaxoSmithKline plc., Collegeville, PA, USA; 3Value Evidence and Outcomes, GlaxoSmithKline plc., Brentford, UK; 4Department of Health Research Methods, Evidence and Impact, McMaster University, Hamilton, ON, Canada
Correspondence: Afisi S Ismaila
Value Evidence and Outcomes, GlaxoSmithKline plc., 1250 South Collegeville Road, Collegeville, PA 19426-0989, USA
Tel +1 919 315 8229
Email [email protected]
Background and Objectives: Chronic obstructive pulmonary disease (COPD) affects over 250 million people globally, carrying a notable economic burden. This systematic literature review aimed to highlight the economic burden associated with moderate-to-very severe COPD and to investigate key drivers of healthcare resource utilization (HRU), direct costs and indirect costs for this patient population.
Materials and Methods: Relevant publications published between January 1, 2006 and November 14, 2016 were captured from the Embase, MEDLINE and MEDLINE In-Process databases. Supplemental searches from relevant 2015– 2016 conferences were also performed. Titles and abstracts were reviewed by two independent researchers against pre-defined inclusion and exclusion criteria. Studies were grouped by the type of economic outcome presented (HRU or costs). Where possible, data were also grouped according to COPD severity and/or patient exacerbation history.
Results: In total, 73 primary publications were included in this review: 66 reported HRU, 22 reported direct costs and one reported indirect costs. Most of the studies (94%) reported on data from either Europe or North America. Trends were noted across multiple studies for higher direct costs (including mean costs per patient per year and mean costs per exacerbation) being associated with increasingly severe COPD and/or a history of more frequent or severe exacerbations. Similar trends were noted according to COPD severity and/or exacerbation history for rate of hospitalization and primary care visits. Multivariate analyses were reported by 29 studies and demonstrated the statistical significance of these associations. Several other drivers of increased costs and HRU were highlighted for patients with moderate-to-very severe COPD, including comorbidities, and treatment history.
Conclusion: Moderate-to-very severe COPD represents a considerable economic burden for healthcare providers despite the availability of efficacious treatments and comprehensive guidelines on their use. Further research is warranted to ensure cost-efficient COPD management, to improve treatments and ease budgetary pressures.
Keywords: chronic obstructive pulmonary disease, cost of illness, healthcare utilization, review, systematic literature review, economic burden
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by incurable, progressive airflow restriction and has various clinical forms, including emphysema and chronic bronchitis.1 Periods of time in which patients experience an acute deterioration in their respiratory symptoms, known as exacerbations, are a common manifestation of the disease.1 A debilitating yet preventable multifactorial disease, COPD is estimated to affect over 380 million people worldwide.2
The goals of COPD pharmacologic therapy are to provide symptomatic relief, improve health status and exercise tolerance, and prevent and treat exacerbations.1 While therapeutic regimens are adapted according to the needs of each individual patient, most pharmacologic maintenance treatments can be categorized as either inhaled corticosteroids (ICS), long-acting muscarinic antagonists (LAMA) or long-acting β2-agonists (LABA),1 and are often administered as combinations. As an example, triple therapy with an ICS, LAMA and LABA has been shown to improve lung function and patient-reported outcomes, including exacerbation risk, compared with ICS/LABA or LAMA monotherapy.1 Despite recent advances within the field and the availability of guideline-recommended treatments, current management is inadequate for many patients with COPD; this unmet need, coupled with the high prevalence of the condition, suggests a high clinical and economic burden, and this has been demonstrated in a number of studies.
Previous publications have indicated that COPD is associated with a substantial economic burden, both in terms of direct costs to healthcare systems and indirect costs to society.1,3–5 In the United States (US), for example, direct costs of COPD were estimated to be $32 billion in 2010, with indirect costs (incurred by lost working days, for instance) accounting for an additional $20.4 billion.3
While evidence on the economic burden of COPD exists, it generally comprises individual studies that are limited by factors such as population characteristics, geographical location and clinical setting. To better understand the current COPD-related burden in settings where patients are likely to be managed as per globally recognised treatment algorithms, such as from the Global Initiative for Chronic Obstructive Lung Disease (GOLD),1 we performed a comprehensive systematic literature review (SLR) of real-world observational studies in patients with moderate-to-very severe disease and/or those with a history of exacerbations, conducted across a number of industrialized countries during the last decade. This evidence was obtained to address two research questions. Firstly, what is the global economic burden associated with moderate-to-very severe COPD? This was examined with a specific focus on regions with high utilization of healthcare resources, such as Australia, Europe, Japan and North America? Secondly, what are the key drivers of economic burden in moderate-to-very severe COPD?
Materials and Methods
Literature Search
This SLR was conducted using processes similar to those outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines6 to identify articles assessing both the economic and/or humanistic burden of COPD. This review summarizes the evidence of COPD’s economic burden only; humanistic outcomes will be reported in another publication. Searches were conducted to capture publications of interest from January 1, 2006 to November 14, 2016 using the electronic literature databases Excerpta Medica Database (Embase), via embase.com, MEDLINE (via embase.com) (Supplementary Table 1) and MEDLINE In-Process (via ncbi.nlm.nih.gov/pubmed) (Supplementary Table 2).
Supplemental grey literature searches of proceedings from the following relevant conferences that took place in 2015 and 2016 were also conducted: European Respiratory Society (ERS), American Academy of Allergy, Asthma, and Immunology (AAAAI), American Thoracic Society (ATS) and International Society for Pharmacoeconomics and Outcomes Research (ISPOR).
Bibliographies of identified SLRs on the economic burden of COPD were examined for any additional relevant publications and to validate the electronic searches.
Study Selection
During the first level of review, titles and abstracts were screened by two investigators independently using pre-defined inclusion and exclusion criteria (Supplementary Table 3). To be included, articles needed to be English-language observational studies published between January 1, 2006 and November 14, 2016 examining adult patients with moderate-to-very severe COPD (defined as post-bronchodilator forced expiratory volume in 1 s/forced vital capacity [FEV1/FVC] of <0.70, plus 50%≤FEV1<80% predicted [moderate], 30%≤FEV1<50% predicted [severe], or FEV1<30% [very severe], or in accordance with GOLD recommendations or other measures employed by investigators), or with a history of frequent exacerbations (≥2 exacerbations in the previous year that required treatment with oral/systemic corticosteroids and/or antibiotics) or exacerbation-related hospitalizations, in the context of healthcare resource utilization (HRU) and costs. Full-text articles for abstracts deemed to be relevant during the first level of review were retrieved and reviewed. Data were restricted to nine countries of interest: Australia, Canada, France, Germany, Italy, Japan, Spain, the United Kingdom (UK), and the United States (US). None of the exclusion criteria and all protocol-specified inclusion criteria needed to be met for inclusion. All rejected records were re-reviewed as a quality-assurance step. At both review stages, any discrepancies were resolved through discussion between the two reviewers and/or by a third, more senior, investigator.
Data Extraction and Synthesis
A standardized data extraction template was used to collect key data elements from the Consolidated Standards of Reporting Trials (CONSORT) checklist (www.consort-statement.org), including study type, study characteristics, population/subpopulations of interest, patient characteristics and categories of outcomes reported. Each entry was validated by a second researcher and a third, more senior, investigator was involved as a quality-assurance step to confirm extractions or resolve disagreements.
Given the nature of the data, it was not appropriate to conduct a quantitative analysis; however, studies were grouped according to the type of economic outcome (direct costs, costs per exacerbation/hospitalization, indirect costs and HRU). Throughout this manuscript, costs are presented as mean (standard deviation [SD]) unless otherwise indicated.
Where possible, data were also grouped according to population characteristics: those with moderate-to-very severe COPD as defined by FEV1% predicted, and/or those with a history of exacerbations and moderate-to-very severe COPD not defined by FEV1% predicted.
Although no formal quality assessment was conducted on these economic studies, aspects related to the quality of the outcomes of these studies were considered in our interpretation of the evidence, including, for example, sample size and study design.
Results
Literature Search
After duplicates were removed, the electronic database searches yielded 2343 unique publications (Figure 1). Of these, 979 passed the initial title/abstract screening step, and 409 passed the second level of title and abstract screening. A total of 100 publications were excluded at this stage for not reporting on moderate-to-very severe COPD. Overall, 139 publications met the eligibility criteria for inclusion. An additional six publications were identified through manual review of bibliographies, giving a total of 145 publications reporting data on the humanistic and/or economic burden associated with moderate-to-very severe COPD. The 73 primary publications that reported on COPD’s economic burden are included in this manuscript (Table 1), while studies reporting on humanistic burden will be summarized in a separate publication.
 |  |  |  |  |  |  |
Table 1 Summary of Included Studies (n=73 Studies) |
 |
Figure 1 Identification of studies for inclusion in the systematic literature review (following systematic processes outlined in PRISMA guidelines). Note: Adapted from Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.6Abbreviation: PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses. |
Study Characteristics
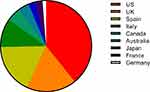
Most of the studies (94%) reported on data from either Europe or North America, with 3% (n=2) reporting on data from Japan and 4% (n=3) from Australia (Figure 2). Of the 73 studies, most reported on HRU (n=66), followed by direct costs (n=22) and indirect costs (n=1) (Figure 3). In total, 29 publications reported multivariate data on drivers/predictors of costs and/or HRU (Figure 3).
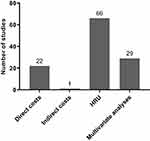 |
Figure 3 Number of studies reporting on each outcome. Abbreviation: HRU, healthcare resource utilization. |
Economic Burden of Moderate-to-Very Severe COPD
HRU
In total, 66 studies reported on HRU, including data regarding hospital admission and length of stay, general practice/primary care visits and intensive care unit (ICU) admission. While more details of HRU in the included studies are included in Supplementary Table 4, in the interests of brevity we have chosen to focus this section on the economic burden of care in the primary, secondary and ICU settings. It must be noted, however, that the studies captured in this review were heterogeneous in nature and are thus challenging to summarize.
In the studies that reported annual hospitalization rates according to COPD severity, COPD-related hospitalizations occurred at a mean annual rate of between 0 and 0.57 in patients with moderate COPD (50%≤FEV1<80% predicted).7–11 Patients with severe COPD (30%≤FEV1<50% predicted) experienced between 0 and 0.44 mean COPD-related hospitalizations per year, while patients with very severe COPD (FEV1<30% predicted) were hospitalized more frequently, from 0 to 0.88 times per year.7–11 In studies where patients’ disease severity was not specified, the highest mean rate of COPD-related annual hospitalizations reported was 1.78 in a population of Spain-based patients who experienced acute exacerbations leading to hospital admission.12 Conversely, the lowest mean rate of COPD-related annual hospitalizations reported was 0.1, in a Spain-based cohort of patients who had experienced <2 exacerbations in the previous year.13
The mean number of annual general practitioner (GP) or primary care visits also varied widely, both within and between COPD severity subgroups. The mean number of visits per patient per year (PPPY) ranged from 2.33 to 12.99 for mild COPD (FEV1≥80% predicted), and from 2.33 to 13.0 for moderate COPD (2.33 visits PPPY was described for patients with “mild-to-moderate COPD” [FEV1≥50% predicted]).8,11,14–17 Patients with severe COPD seemed to visit their GP more frequently, from 2.8 to 15.06 times per year.8,11,14–17 Fewer studies reported the mean number of primary care visits for patients with very severe COPD, but in those that did, the frequency ranged from 3.67 to 12.22 per year.8,11,15 The highest number of GP visits reported was 15.94 per year in a UK-based cohort of patients who experienced ≥2 COPD exacerbations that were moderate-to-severe in severity.18
Only two studies reported length of hospital stay according to COPD severity. One UK-based study reported little variability according to disease severity, with a median stay of 5 days irrespective of whether the patient’s COPD was defined as mild, moderate or severe.11 Length of stay increased to a median of 6 days for patients with very severe disease (FEV1<30% predicted) in this study.11 A study conducted in Spain observed a difference according to COPD severity, with mean length of stay being 8.8 days for patients with mild disease and 16.7 days for patients with severe disease.14 In studies that did not report length of stay according to disease severity, the shortest mean duration reported was 3.24 days (for patients with no diastolic dysfunction) in a US-based study19 and the longest was 13.2 days in a Canada-based study.20 ICU admission rates were not reported according to disease severity, but ranged from 5% of all hospitalized exacerbations in a study conducted in Spain12 to 19% of all hospitalized exacerbations in a US-based study.21
It is also important to note that the review identified several studies showing increased COPD-associated HRU with exacerbation frequency and the severity of COPD symptoms, particularly breathlessness/dyspnea. Exacerbations are a key driver of HRU by patients with COPD and studies captured in this SLR indicated that patients who experienced more exacerbations had more frequent primary care interactions,11,18,22,23 visits to the emergency department (ED),22,23 hospitalizations13,18,22–25 and admissions to the ICU13,22 than patients with fewer exacerbations did. Similarly, a retrospective study conducted in the UK primary care setting noted that the rate of hospitalization for a severe exacerbation within a 24-month observation period was 0.12 for patients with moderate breathlessness, increasing to 0.14 and 0.19 for patients with severe and very severe breathlessness, respectively.10 A similar trend was noted for GP home visits, though rates for GP surgery visits were slightly higher for patients with moderate breathlessness (13.00) than those with very severe breathlessness (12.22).10
Direct Costs
Direct costs were reported by a total of 22 studies and a full summary of these results is presented in Supplementary Table 5. In the interests of brevity, we focus here on mean costs PPPY and mean costs per exacerbation, though differences in currencies and cost years mean that comparisons should be interpreted with caution.
In total, nine studies presented mean costs PPPY and demonstrated that costs can vary widely across and within countries, and according to patient or disease characteristics.7,15,18,23,24,26–29 The lowest mean costs PPPY were reported for a population of patients with mild COPD in Italy: €1047 PPPY (cost year, 2004).7 The highest costs reported were also found in Italy, not unexpectedly for patients with very severe COPD, whose mean costs PPPY amounted to €38,820 (cost year, not reported [NR]).26
Three studies specifically reported costs according to disease severity, demonstrating clear trends. In two studies based in the UK, costs PPPY increased from £2012–£2087 in patients with moderate disease, to £2092–£2290 in patients with severe disease and to £2258–£2639 in patients with very severe disease (cost year, 2011).10,18 A similar trend was observed in Italy: costs PPPY increased from €2319 in patients with moderate disease to €3752 in patients with severe disease and to €5033 in patients with very severe disease (cost year, 2004).7
Three studies presented mean costs PPPY according to patient or disease characteristics. A study based in Japan observed a large discrepancy in costs according to presence and severity of dyspnea: patients with moderate-to-severe dyspnea (Medical Research Council [MRC] score ≥2) had mean costs PPPY of €6348, compared with only €2797 for patients with mild or no dyspnea (MRC score <2; cost year, 2015).27 A retrospective study based in the UK healthcare setting also noted higher costs PPPY for patients with more severe breathlessness, but the differences according to severity were smaller: £2258 for very severe breathlessness, £2151 for severe breathlessness and £2012 for moderate breathlessness.10 A trend according to increased costs with comorbidities was also noted; as an example, patients with heart disease had higher costs PPPY compared with patients without heart disease in a study conducted in Spain: €2898.66 vs €1672.64, respectively (cost year, NR).28
This SLR also found that annual costs per patient generally increased according to exacerbation frequency. A study conducted in the UK (cost year, 2011), for example, noted that annual per patient costs related to COPD increased from £1523 in patients with no exacerbations, to £2405 in patients with one exacerbation, and to £3396 in patients with ≥2 exacerbations, in the 12 months following study entry.18 The impact of exacerbation history as a driver of direct costs will be explored in greater detail in a later section of this review.
Exacerbations account for a substantial proportion of the direct costs associated with COPD, and this SLR captured seven studies that reported mean costs per exacerbation,20,21,24,29–32 but few studies reported this outcome according to exacerbation severity. Two studies reported mean costs per moderate exacerbation (characterized by the need for management with COPD-specific antibiotics or oral corticosteroids, or a medical diagnosis), which ranged from $269 in the US (US dollars [USD]; cost year, 2011)29 to $641 in Canada (Canadian dollars [CAD]; cost year, 2006).20 Where mean costs were reported for severe exacerbations (defined by the need for hospitalization),20,21,24,29–32 costs were higher than those reported for moderate exacerbations. The lowest reported mean cost for a hospitalized exacerbation was $3164 in Italy (USD; cost year, 2013),30 while the highest was substantially greater: $18,120 in the US (USD; cost year, 2011).29 The wide range of costs across countries is notable, even when being mindful of differences in currencies and cost years.
Although cross-trial comparisons are challenging due to differences in currencies and cost years, this SLR indicates that, consistent with the higher rates of HRU noted with increasingly severe disease and COPD symptoms, and more frequent exacerbations, direct costs generally increase alongside COPD and symptom severity, and exacerbation frequency. Specific independent drivers of direct costs will be explored by examining multivariate analyses in a later section of this review.
Indirect Costs
Only one study on indirect costs, conducted in Spain, was identified.28 This study reported on outpatients with moderate-to-very severe COPD (FEV1 <80% predicted) and found a statistically significant difference in the cost of sick leave days PPPY in those without heart disease (€76.61) compared with those with heart disease (€38.33 [cost year, NR]; p<0.05). This SLR did not identify any studies that explored potential predictors of indirect costs in patients with moderate-to-very severe COPD.
Key Drivers of Economic Burden
This SLR identified 29 studies in which multivariate analyses were performed to explore potential drivers of economic burden in patients with moderate-to-very severe COPD. Of these studies, 24 reported multivariate analyses of HRU outcomes (Table 2; Supplementary Table 6) and seven reported direct costs (Table 3; Supplementary Table 7).
 |
Table 2 Multivariate Analyses of HRU (n=24 Studies) |
 |
Table 3 Multivariate Analyses of Direct Costs (n=7 Studies) |
HRU
Independent drivers of more frequent hospitalization for a COPD- or respiratory-related cause included increasing disease severity,33 restricted lung function33 and higher baseline C-reactive protein concentrations.34 Increasingly severe disease was also an independent driver of more frequent non-COPD-related hospitalizations,35 as were the number of previous exacerbations, use of short-acting β2-agonists (SABA) and use of long-term oxygen therapy (LTOT) among other variables.25
Factors independently associated with longer hospital stays for a COPD exacerbation were numerous and were reported in six studies.31,32,36–39 Drivers included the presence of comorbidities such as stroke31 and some cardiac abnormalities,31,32 advancing age and being female.36 Furthermore, specific care-related factors such as a hospital’s size, location or type were also found to be independent drivers of length of stay. Increased hospital size, for example, was associated with longer stays but admission to a teaching hospital was associated with shorter stays compared with a non-teaching hospital.36
Some cardiac abnormalities, such as congestive heart failure and dysrhythmia,32 alongside pneumonia, anemia and anxiety or depression among other comorbidities, were identified as independent drivers of ICU admission.32 Severe COPD (indicated by the use of home oxygen, oral/intravenous steroids, vasopressors or ventilation) and higher B-type natriuretic peptide levels were both found to be drivers of longer ICU stays.38
Eight studies analyzed potential drivers of COPD-related readmission to hospital for patients admitted with an exacerbation of COPD.39–46 Among those that explored readmission for another exacerbation in the shorter term (within 30 days or within 2 months), history of coronary heart disease,46 hospitalizations in the previous year44 and baseline FEV1<30% predicted (vs ≥50% predicted)43 were identified as independent drivers of increased readmission risk, but this is not an exhaustive summary. Interestingly, though perhaps not surprisingly, taking 150 mins of moderate-to-strenuous exercise each week was independently associated with a lower risk of readmission for an exacerbation within 30 days.44 Regular physical activity was also independently associated with lower readmission risk in another study.39 Studies that assessed risks of readmission for another exacerbation in the longer term (with follow-ups ranging from 1 to 6 years) identified congestive heart disease and pulmonary heart disease as independent drivers of increased risk.41 Notably, ischemic heart disease and cardiac arrhythmia were also identified as drivers of increased readmission risk, but only in female patients.41 Being male40,42 and having a history of previous hospital stays40 were similarly associated with an increased risk of readmission. Factors identified as drivers of lower readmission risk for a COPD-related cause in the long term included Hispanic ethnicity and diabetes.40
The majority of factors found to be independently associated with an increased risk of readmission for any cause were comorbidities, ranging from liver disease, paraplegia and substance abuse,47 to neurologic conditions and heart disease.31 Having an increased number of comorbid conditions was also associated with an increased risk of readmission.31
For drivers of primary care utilization, associations between more frequent GP visits and being female, suffering with obesity, depression, anxiety or other comorbidities were noted in one retrospective cohort study.10 Emergency department visits of any cause were found to be independently associated with COPD severity,35 age, comorbidity, exacerbation frequency10 and emotional intelligence,48 among other factors. One study reported on potential drivers of LTOT and found that female gender, white race (vs black or other races), low socioeconomic status and a higher Elixhauser Comorbidity score (among other variables) were independently associated with an increased likelihood of receiving such therapy.49
A cross-sectional study among patients with COPD in Spain identified drivers of high HRU, defined as having had one hospital admission for a COPD exacerbation, ≥2 ED visits for COPD exacerbations or ≥2 unscheduled outpatient visits related to COPD in the previous year. Independent drivers included London Chest Activity of Daily Living (LCADL) scale score, leukocyte count and fibrinogen level.50
Direct Costs
Increasingly severe dyspnea was identified as an independent driver of higher annual COPD management costs per patient,27 while older age, receipt of appropriate bronchodilator therapy and higher pre-index COPD-related costs51 were found to be independent predictors of higher COPD-related costs per patient at 6 months after discharge. In two retrospective cohort studies that assessed potential drivers of annual exacerbation costs for patients with chronic bronchitis, numerous independent drivers were identified. Regarding increased annual exacerbation costs per patient, these included being of black race, high income, comorbid cardiovascular disease and comorbid diabetes, among other factors.23 Independent drivers of higher costs per individual exacerbation (of any severity) in patients with chronic bronchitis included Deyo-Charlson’s Comorbidity Index score, the number of exacerbations during the previous year and the number of prescription fills for short-acting muscarinic antagonist (SAMA) therapy, among other factors.29
Potential drivers of costs per hospitalized exacerbation were assessed in three studies conducted in the US.31,32,36 Independent predictors of higher costs included cardiac comorbidities, such as congestive heart failure and ischemic heart disease,32 the nature of the patient’s COPD (emphysema vs chronic bronchitis), as well as advancing age,36 Hispanic race (vs white race), and ICU treatment,31,36 among other factors. Severity score was also described as a predictor of costs, but the nature of this association was unclear in the source publication.31
Discussion
It is important to understand the economic burden associated with moderate-to-very severe COPD and its treatment, particularly given that COPD presents major challenges for patients, healthcare providers and society. Optimal management of patients, their symptoms and exacerbations is one such challenge. This SLR investigated the current impact of COPD on HRU needs and both direct and indirect costs, based on a broad definition of patients whose COPD severity was typically confirmed by FEV1% predicted or assumed (from exacerbation history) to be at least moderate. The analysis was intentionally limited to nine industrialized countries of interest (and to a 10-year time frame) to ensure inclusion of evidence from settings where patients were likely to be receiving guideline-recommended therapeutic management; the US was the most well-represented country.
Fewer studies reported costs compared with those that provided HRU evidence and, considering the inherent variation in geographical location, timing and methodology between the available studies, cross-study comparisons should be interpreted with caution. Overall, however, direct costs were found to generally increase with COPD severity, exacerbation frequency and severity of COPD symptoms. The SLR also found higher rates of HRU, in terms of hospitalization, length of hospital stay and primary care interactions, for patients with more severe COPD, more frequent COPD exacerbations and more severe COPD symptoms.
As shown by the numerous studies that performed multivariate analyses, independent drivers of HRU and costs are diverse. Factors related to disease severity, the frequency and severity of COPD exacerbations, comorbidities and treatment history (among others) were significantly associated with elements of HRU, ranging from hospitalization to LTOT. More severe COPD and a history of frequent exacerbations, for example, were each positively associated with an increased likelihood of hospitalization.25,33,35 Disease severity was also identified as a driver of ED visits,35 and longer hospital or ICU stays.38 These multivariate data support trends reported in other studies, captured both in this SLR and others in the broader literature. As an example, a cross-sectional survey conducted across France, Germany, Italy, Spain and the UK found that moderate-to-severe dyspnea was associated with a significant disease burden in patients with COPD, and was associated with significantly more frequent hospitalizations, specialist and physician consultations, LTOT use and pulmonary rehabilitation (all p<0.0001).52
Examining multivariate analyses of direct costs per patient for COPD also identified dyspnea severity,27 and exacerbation frequency29 and severity23 as significant drivers. This was perhaps to be expected: a more intensive treatment regimen may be required to manage symptoms and/or exacerbations and thus is likely to carry a burden of increased costs. These results are in keeping with the SLR of economic burden in COPD by Srivastava et al (2015), which found that direct and indirect costs per patient increased with symptoms, dyspnea severity and disease duration.53 The data are also supported by results reported by other studies captured in this SLR that did not employ multivariate analyses: trends for higher costs according to more severe disease were noted in both Italy7,26 and the UK.10,18
Other key drivers identified through multivariate analysis as predictors of economic burden in moderate-to-very severe COPD included the number of previous exacerbations, as well as the presence and types of comorbidities. Comorbidities have frequently been associated with significantly higher costs for patients with COPD in the wider literature.53–56 An SLR of 12 studies from the US and across Europe/Asia that analyzed the excess costs of comorbidities in COPD found that pneumonia, cardiovascular disease and diabetes were associated with the highest excess costs.55 This SLR supports these findings: comorbid diabetes and comorbid cardiovascular disease were found to be potential predictors of annual exacerbation-related costs23 and pneumonia was found to be an independent predictor of the cost of exacerbation-related hospitalization.32 These findings highlight the importance of careful management of comorbidities alongside optimal COPD-specific therapy, to minimize HRU and associated costs.
A limitation of this study was perhaps the paucity of data available on indirect costs. However, among the studies identified by the SLR was a 2016 population-based survey across 12 countries (including the US, the UK, Germany, Italy and Spain), which described how indirect costs were several times greater than direct costs in many countries.30 This highlights the extreme, and often hidden, economic burden carried by loss of productivity and absenteeism. A US-based survey focusing on all COPD severities (not just moderate-to-very severe) observed that COPD-attributable absenteeism accounted for costs of approximately $3.9 billion dollars in 2010, with an estimated 16.4 million work days lost due to COPD; these costs are expected to rise in coming years.57 With their clear impact on economic burden, the indirect costs of COPD certainly warrant further investigation across regions and healthcare systems.
This study employed a pragmatic approach and allowed a flexible definition of moderate-to-very-severe COPD, though this automatically introduced a degree of between-study heterogeneity in the SLR, which made comparing data between studies more challenging. Nonetheless, many of the key outcomes were consistently demonstrated across studies, and, where possible, we qualitatively compared HRU and costs according to disease severity. The trends observed and inferences made through qualitative analysis were supported by multivariate analysis studies captured by this SLR, which were specifically designed to identify independent predictors of COPD’s economic burden.
This study focused on moderate-to-very severe COPD: studies examining only mild COPD were excluded. Although widening the search parameters may have delivered a more comprehensive dataset, COPD is underdiagnosed in its early stages. As many patients do not become known to healthcare systems until their disease has advanced to moderate-to-severe stages,58 it is likely that the parameters employed by this SLR captured the majority of patients with a confirmed COPD diagnosis.
This SLR provides detailed evidence of the considerable real-world economic burden carried by moderate-to-very severe COPD, especially for patients with a history of exacerbations and/or more severe COPD, despite the availability of efficacious treatments and well-defined guidance on their use.
With worldwide healthcare budgets facing increasing financial pressures, understanding the economic burden of diseases such as COPD is essential to ensure that the disease is managed efficiently and that emerging therapies are made available to the patients who need them. Further progress in the management of COPD exacerbations and HRU is required to improve patient care and to reduce the associated strain on healthcare budgets around the world.
Abbreviations
AAAAI, American Academy of Allergy, Asthma, and Immunology; ATS, American Thoracic Society; CAD, Canadian dollar; CONSORT, Consolidated Standards of Reporting Trials; COPD, chronic obstructive pulmonary disease; ED, emergency department; ERS, European Respiratory Society; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; GOLD, Global Initiative for Chronic Obstructive Lung Disease; GP, general practitioner; HRU, healthcare resource utilization; ICS, inhaled corticosteroid; ICU, intensive care unit; ISPOR, International Society for Pharmacoeconomics and Outcomes Research; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist; LCADL, London Chest Activity of Daily Living; LTOT, long-term oxygen therapy; MRC, Medical Research Council; NR, not reported; PPPY, per patient per year; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; SABA, short-acting β2-agonist; SAMA, short-acting muscarinic antagonist; SD, standard deviation; SLR, systematic literature review; UK, United Kingdom; US, United States.
Acknowledgments
This study was funded by GlaxoSmithKline plc. (study HO-16-16282). The authors thank Juliet Kenny for her assistance in the conception of the study and interpretation of the data. Editorial support (in the form of writing assistance, collating author comments, assembling tables/figures, grammatical editing and referencing) was provided by Fiona Scott, PhD, and Molly Macpherson, BSc, of Gardiner-Caldwell Communications (Macclesfield, UK), and was funded by GlaxoSmithKline plc.
Author Contributions
All authors contributed to the study conceptualization, data sourcing, data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare the following conflicts of interest during the last 3 years in relation to this article: SZ and ASI are employees of, and hold shares in, GlaxoSmithKline plc.; ASI is also a part-time unpaid professor at McMaster University, Canada. Evidera (II) was contracted by GlaxoSmithKline plc. to conduct the systematic literature review but was not paid for the development of this manuscript. MR is an employee of Xcenda UK and was previously employed by Evidera and contracted by GlaxoSmithKline plc. to conduct the systematic literature review but was not paid for the development of this publication. DK is a former GlaxoSmithKline plc. employee and is currently employed by Forest Systematic Reviews Ltd, contracted by GlaxoSmithKline plc. The authors report no other conflicts of interest in this work.
References
1. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease; 2019. Available from: https://goldcopd.org/wp-content/uploads/2018/11/GOLD-2019-v1.7-FINAL-14Nov2018-WMS.pdf.
2. Adeloye D, Chua S, Lee C, et al. Global and regional estimates of COPD prevalence: systematic review and meta-analysis. J Glob Health. 2015;5(2):020415. doi:10.7189/jogh.05.020415
3. Guarascio AJ, Ray SM, Finch CK, Self TH. The clinical and economic burden of chronic obstructive pulmonary disease in the USA. Clinicoecon Outcomes Res. 2013;5:235–245. doi:10.2147/CEOR.S34321
4. Løkke A, Hilberg O, Tønnesen P, Ibsen R, Kjellberg J, Jennum P. Direct and indirect economic and health consequences of COPD in Denmark: a national register-based study: 1998–2010. BMJ Open. 2014;4(1):e004069. doi:10.1136/bmjopen-2013-004069
5. Wacker ME, Jörres RA, Karch A, et al. Assessing health-related quality of life in COPD: comparing generic and disease-specific instruments with focus on comorbidities. BMC Pulm Med. 2016;16(1):70.
6. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi:10.1371/journal.pmed.1000097
7. Koleva D, Motterlini N, Banfi P, Garattini L. Healthcare costs of COPD in Italian referral centres: a prospective study. Respir Med. 2007;101(11):2312–2320. doi:10.1016/j.rmed.2007.06.020
8. Merinopoulou E, Raluy-Callado M, Ramagopalan S, MacLachlan S, Khalid JM. COPD exacerbations by disease severity in England. Int J Chron Obstruct Pulmon Dis. 2016;11:697–709. doi:10.2147/COPD.S100250
9. Mullerova H, Han MK, Baxter R, GOLD DK. 2013 COPD classification: a comparison with GOLD 2011 and COPD outcomes in the UK Clinical Practice Research Datalink (CPRD) COPD cohort. Am J Respir Crit Care Med. 2014;189:A5965. doi:10.1164/rccm.201306-1150OC
10. Punekar YS, Wurst K, Shukla A. Resource use and costs up to two years post diagnosis among newly diagnosed COPD patients in the UK primary care setting: a retrospective cohort study. COPD. 2015;12(3):267–275. doi:10.3109/15412555.2014.933953
11. Thomas M, Radwan A, Stonham C, Marshall S. COPD exacerbation frequency, pharmacotherapy and resource use: an observational study in UK primary care. COPD. 2014;11(3):300–309. doi:10.3109/15412555.2013.841671
12. Bustamante-Fermosel A, De Miguel-yanes JM, Duffort-Falcó M, Munoz J. Mortality-related factors after hospitalization for acute exacerbation of chronic obstructive pulmonary disease: the burden of clinical features. Am J Emerg Med. 2007;25(5):515–522. doi:10.1016/j.ajem.2006.09.014
13. Valido A, Gomez-Bastero A, Almadana V, Luque E, Montemayor T. Clinical and functional differences, exercise capacity and physical activity among frequent and not frequent exacerbators in COPD patients. Eur Respir J. 2014;44(Suppl 58):P4883. doi:10.1183/09031936.00003814
14. Martín A, Rodríguez-González Moro JM, Izquierdo JL, Gobartt E, de Lucas P. Health-related quality of life in outpatients with COPD in daily practice: the VICE Spanish Study. Int J Chron Obstruct Pulmon Dis. 2008;3(4):683–692. doi:10.2147/COPD.S4791
15. Punekar YS, Landis SH, Bonar K, Le H. Health care utilisation and costs among COPD patients newly prescribed maintenance therapy in the United Kingdom (UK). Thorax. 2015b;70:A142–A143. doi:10.1136/thoraxjnl-2015-207770.269
16. Yeo J, Karimova G, Bansal S. Co-morbidity in older patients with COPD–its impact on health service utilisation and quality of life, a community study. Age Ageing. 2006;35(1):33–37. doi:10.1093/ageing/afj002
17. Mapel DW, Dutro MP, Marton JP, Woodruff K, Make B. Identifying and characterizing COPD patients in US managed care. A retrospective, cross-sectional analysis of administrative claims data. BMC Health Serv Res. 2011;11:43. doi:10.1186/1472-6963-11-43
18. Punekar YS, Shukla A, Müllerova H. COPD management costs according to the frequency of COPD exacerbations in UK primary care. Int J Chron Obstruct Pulmon Dis. 2014;9:65–73. doi:10.2147/COPD
19. Abusaid GH, Barbagelata A, Tuero E, Mahmood A, Sharma G. Diastolic dysfunction and COPD exacerbation. Postgrad Med. 2009;121(4):76–81. doi:10.3810/pgm.2009.07.2033
20. Mittmann N, Kuramoto L, Seung SJ, Haddon JM, Bradley-Kennedy C, Fitzgerald JM. The cost of moderate and severe COPD exacerbations to the Canadian healthcare system. Respir Med. 2008;102(3):413–421. doi:10.1016/j.rmed.2007.10.010
21. Xu X, Knight T, Baik R, Tu X, Parker JM. Patient characteristics, health resource utilization (HRU) and treatment costs of chronic obstructive pulmonary disease (COPD) patients treated in hospital facilities for exacerbations. Am J Respir Crit Care Med. 2012;185:A3047.
22. Dalal AA, Patel J, D’Souza A, Farrelly E, Nagar S, Shah M. Impact of COPD exacerbation frequency on costs for a managed care population. J Manag Care Spec Pharm. 2015;21(7):575–583. doi:10.18553/jmcp.2015.21.7.575
23. Pasquale MK, Sun SX, Song F, Hartnett HJ, Stemkowski SA. Impact of exacerbations on health care cost and resource utilization in chronic obstructive pulmonary disease patients with chronic bronchitis from a predominantly medicare population. Int J Chron Obstruct Pulmon Dis. 2012;7:757–764. doi:10.2147/COPD.S36997
24. Blasi F, Cesana G, Conti S, et al. The clinical and economic impact of exacerbations of chronic obstructive pulmonary disease: a cohort of hospitalized patients. PLoS One. 2014;9(6):e101228. doi:10.1371/journal.pone.0101228
25. Miravitlles M, Calle M, Alvarez-Gutierrez F, Gobartt E, López F, Martin A. Exacerbations, hospital admissions and impaired health status in chronic obstructive pulmonary disease. Qual Life Res. 2006;15(3):471–480. doi:10.1007/s11136-005-3215-y
26. Vitacca M, Bianchi L, Bazza A, Clini EM. Advanced COPD patients under home mechanical ventilation and/or long term oxygen therapy: italian healthcare costs. Monaldi Arch Chest Dis. 2011;75(4):207–214. doi:10.4081/monaldi.2011.208
27. Small M, Holbrook T, Wood R, Mullerova H, Naya I, Punekar YS. Prevalence and burden of dyspnoea among COPD patients in Japan. Int J Clin Pract. 2016;70(8):676–681. doi:10.1111/ijcp.2016.70.issue-8
28. de Miguel-díez J, Carrasco-Garrido P, Rejas-Gutierrez J, et al. The influence of heart disease on characteristics, quality of life, use of health resources, and costs of COPD in primary care settings. BMC Cardiovasc Disord. 2010;10:8. doi:10.1186/1471-2261-10-8
29. Abudagga A, Sun SX, Tan H, Solem CT. Exacerbations among chronic bronchitis patients treated with maintenance medications from a US managed care population: an administrative claims data analysis. Int J Chron Obstruct Pulmon Dis. 2013;8:175–185. doi:10.2147/COPD.S40437
30. Foo J, Landis SH, Maskell J, et al. Continuing to Confront COPD International Patient Survey: economic impact of COPD in 12 countries. PLoS One. 2016;11(4):e0152618. doi:10.1371/journal.pone.0152618
31. Roberts MH, Borrego M, Petersen H, Kharat A, Blanchette C. Estimating the burden and course of severe COPD exacerbations in the U.S. Hispanic population. Am J Respir Crit Care Med. 2011a;183:A1496.
32. Silver H, Blanchette CM, Roberts M, Petersen H, StCharles ME. Prevalence of comorbidities in patients hospitalized for COPD exacerbations and impact on impatient mortality and hospital expenditures. Am J Respir Crit Care Med. 2010;181:A5943. doi:10.1164/rccm.200904-0493OC
33. Garcia-Aymerich J, Serra Pons I, Mannino DM, Maas AK, Miller DP, Davis KJ. Lung function impairment, COPD hospitalisations and subsequent mortality. Thorax. 2011;66(7):585–590. doi:10.1136/thx.2010.152876
34. Gallego M, Pomares X, Capilla S, et al. C-reactive protein in outpatients with acute exacerbation of COPD: its relationship with microbial etiology and severity. Int J Chron Obstruct Pulmon Dis. 2016;11:2633–2640. doi:10.2147/COPD
35. Dhamane AD, Witt EA, Su J. Associations between COPD severity and work productivity, health-related quality of life, and health care resource use: a cross-sectional analysis of national survey data. J Occup Environ Med. 2016;58(6):e191–e197. doi:10.1097/JOM.0000000000000735
36. Stanford RH, Shen Y, McLaughlin T. Cost of chronic obstructive pulmonary disease in the emergency department and hospital: an analysis of administrative data from 218 US hospitals. Treat Respir Med. 2006;5(5):343–349. doi:10.2165/00151829-200605050-00005
37. Mahmud N, McNabb-Balter J, Chan WW. The impact of gastroesophageal reflux on the length of hospital stay for COPD exacerbations: a report of the nationwide inpatient sample. Gastroenterology. 2015;148(4Suppl. 1):S606. doi:10.1016/S0016-5085(15)32044-8
38. Vallabhajosyula S, Haddad TM, Sundaragiri PR, et al. Role of B-type natriuretic peptide in predicting in-hospital outcomes in acute exacerbation of chronic obstructive pulmonary disease: a five-year retrospective analysis. Am J Respir Crit Care Med. 2015;191:A2938.
39. Escarrabill J, Torrente E, Esquinas C, et al. Clinical audit of patients hospitalized due to COPD exacerbation. MAG-1 Study. Arch Bronconeumol. 2015;51(10):483–489. doi:10.1016/j.arbres.2014.06.023
40. McGhan R, Radcliff T, Fish R, Sutherland ER, Welsh C, Make B. Predictors of rehospitalization and death after a severe exacerbation of COPD. Chest. 2007;132(6):1748–1755. doi:10.1378/chest.06-3018
41. Chen Y, Li Q, Johansen H. Age and sex variations in hospital readmissions for COPD associated with overall and cardiac comorbidity. Int J Tuberc Lung Dis. 2009;13(3):394–399.
42. Suissa S, Dell’Aniello S, Ernst P. Long-term natural history of chronic obstructive pulmonary disease: severe exacerbations and mortality. Thorax. 2012;67(11):957–963. doi:10.1136/thoraxjnl-2011-201518
43. Quintana JM, Esteban C, Garcia-Gutierrez S, et al. Predictors of hospital admission two months after emergency department evaluation of COPD exacerbation. Respiration. 2014;88(4):298–306. doi:10.1159/000365996
44. Nguyen HQ, Chu L, Liu I-L A, et al. Higher level of regular physical activity is associated with lower risk of 30-day readmissions in patients with COPD. Am J Respir Crit Care Med. 2014;189:A2452. doi:10.1164/rccm.201306-1150OC
45. Gatheral T, Kumar N, Sansom B, et al. COPD-related bronchiectasis; independent impact on disease course and outcomes. COPD. 2014;11(6):605–614. doi:10.3109/15412555.2014.922174
46. Nantsupawat T, Limsuwat C, Nugent K. Factors affecting chronic obstructive pulmonary disease early rehospitalization. Chron Respir Dis. 2012;9(2):93–98. doi:10.1177/1479972312438703
47. DuVall S, Brown K, Nici L, et al. Incidence and predictors of hospital readmission among patients with chronic obstructive pulmonary disease in the Department of Veterans Affairs. Chest. 2015;148(4 Suppl):684A. doi:10.1378/chest.2223166
48. Benzo RP, Kirsch JL, Dulohery MM, Abascal-Bolado B. Emotional intelligence: a novel outcome associated with wellbeing and self-management in chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2016;13(1):10–16. doi:10.1513/AnnalsATS.201508-490OC
49. Nishi SP, Zhang W, Kuo YF, Sharma G. Oxygen therapy use in older adults with chronic obstructive pulmonary disease. PLoS One. 2015;10(3):e0120684. doi:10.1371/journal.pone.0120684
50. Garciía-Polo C, Alcázar-Navarrete B, Ruiz-Iturriaga LA, et al. Factors associated with high healthcare resource utilisation among COPD patients. Respir Med. 2012;106(12):1734–1742. doi:10.1016/j.rmed.2012.09.009
51. Tran M, Xiang P, Rascati KL, et al. HEDIS quality measure performance and other factors predictive of health care costs following COPD-related admission. Value Health. 2016;19:A122.
52. Punekar YS, Mullerova H, Small M, et al. Prevalence and burden of dyspnoea among patients with chronic obstructive pulmonary disease in five European countries. Pulmonary Ther 2016;2(1):59–72.
53. Srivastava K, Thakur D, Sharma S, Punekar YS. Systematic review of humanistic and economic burden of symptomatic chronic obstructive pulmonary disease. Pharmacoeconomics. 2015;33(5):467–488. doi:10.1007/s40273-015-0252-4
54. Dalal AA, Shah M, Lunacsek O, Hanania NA. Clinical and economic burden of patients diagnosed with COPD with comorbid cardiovascular disease. Respir Med. 2011;105(10):1516–1522. doi:10.1016/j.rmed.2011.04.005
55. Huber MB, Wacker ME, Vogelmeier CF, Leidl R, Watz H. Excess costs of comorbidities in chronic obstructive pulmonary disease: a systematic review. PLoS One. 2015;10(4):e0123292. doi:10.1371/journal.pone.0123292
56. Mannino DM, Higuchi K, Yu TC, et al. Economic burden of COPD in the presence of comorbidities. Chest. 2015;148(1):138–150. doi:10.1378/chest.14-2434
57. Ford ES, Murphy LB, Khavjou O, Giles WH, Holt JB, Croft JB. Total and state-specific medical and absenteeism costs of COPD among adults aged >/= 18 years in the United States for 2010 and projections through 2020. Chest. 2015;147(1):31–45. doi:10.1378/chest.14-0972
58. Løkke A, Ulrik CS, Dahl R, et al. Detection of previously undiagnosed cases of COPD in a high-risk population identified in general practice. COPD. 2012;9(5):458–465. doi:10.3109/15412555.2012.685118
59. Bu XN, Yang T, Thompson MA, Hutchinson AF, Irving LB. Changes in the BODE index, exacerbation duration and hospitalisation in a cohort of COPD patients. Singapore Med J. 2011;52(12):894–900.
60. Carrasco Garrido P, de Miguel Díez J, Rejas Gutiérrez J, et al. Negative impact of chronic obstructive pulmonary disease on the health-related quality of life of patients. Results of the EPIDEPOC study. Health Qual Life Outcomes. 2006;4:31. doi:10.1186/1477-7525-4-31
61. Collins EG, Halabi S, Langston M, Schnell T, Tobin MJ, Laghi F. Sexual dysfunction in men with COPD: impact on quality of life and survival. Lung. 2012;190(5):545–556. doi:10.1007/s00408-012-9398-4
62. Dalal AA, Shah M, D’Souza AO, Rane P. Costs of inpatient and emergency department care for chronic obstructive pulmonary disease in an elderly Medicare population. J Med Econ. 2010a;13(4):591–598. doi:10.3111/13696998.2010.521734
63. Lindenauer PK, Pekow P, Gao S, Crawford AS, Gutierrez B, Benjamin EM. Quality of care for patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 2006;144(12):894–903. doi:10.7326/0003-4819-144-12-200606200-00006
64. Dalal AA, Shah M, D’Souza AO. Economic burden of health care admissions related to chronic obstructive pulmonary disease exacerbations: data from inpatient administrative database. Am J Respir Crit Care Med. 2010b;181:A1492.
65. Patel J, Dalal AA, Nagar S, D’Souza AO, Shah M, Farrelly E. Economic burden of frequently and infrequently exacerbating patients with a diagnosis of COPD. Am J Respir Crit Care Med. 2013;187:A1437.
66. Dhamane A, Witt EA, Hernandez G, Su J. Association between COPD severity and work productivity, quality of life and healthcare resource use. Am J Respir Crit Care Med. 2015;191:A4447.
67. Dushianthan A, Temblett P, Bhatta A. Outcome of a cohort of older population with COPD, admitted with hypercapnaeic respiratory failure and acidosis. Am J Respir Crit Care Med. 2010;181:A1506. doi:10.1164/rccm.200904-0493OC
68. Esteban C, Arostegui I, Aburto M, et al. Chronic obstructive pulmonary disease subtypes. Transitions over time. PLoS One. 2016;11(9):e0161710. doi:10.1371/journal.pone.0161710
69. Gadre S, Duggal A, Guzman J. Epidemiological characteristics and outcomes of patients with severe COPD requiring mechanical ventilation. Chest. 2014;146(4 Suppl 2):58A. doi:10.1378/chest.1991911
70. Garcia-Aymerich J, Félez MA, Escarrabill J, et al. Physical activity and its determinants in severe chronic obstructive pulmonary disease. Med Sci Sports Exerc. 2004;36(10):1667–1673. doi:10.1249/01.MSS.0000142378.98039.58
71. Gavazzi A, De Maria R, Manzoli L, et al. Palliative needs for heart failure or chronic obstructive pulmonary disease: results of a multicenter observational registry. Int J Cardiol. 2015;184:552–558. doi:10.1016/j.ijcard.2015.03.056
72. Huang H, Lin I, Goldstein B, Rosiello R, Menzin J. Rehospitalization rates and mortality among patients with severe chronic obstructive pulmonary disease receiving long-term oxygen therapy in a managed-care setting. Am J Respir Crit Care Med. 2014;189:A3058. doi:10.1164/rccm.201306-1150OC
73. Keilty S, Murphy P, Hart N. 1 year UK survival readmission rate in chronic obstructive pulmonary disease (COPD) survivors following acute non-invasive ventilation (NIV). Thorax. 2013;68(Suppl. 3):A47–A48. doi:10.1136/thoraxjnl-2013-204457.95
74. Lusuardi M, Lucioni C, De Benedetto F, Mazzi S, Sanguinetti CM, Donner CF. GOLD severity stratification and risk of hospitalisation for COPD exacerbations. Monaldi Arch Chest Dis. 2008;69(4):164–169. doi:10.4081/monaldi.2008.378
75. Matkovic Z, Huerta A, Soler N, et al. Predictors of adverse outcome in patients hospitalised for exacerbation of chronic obstructive pulmonary disease. Respiration. 2012;84(1):17–26. doi:10.1159/000335467
76. Punekar YS, Shukla A, Muellerova H. Comparing COPD costs by exacerbation frequency and dyspnoea level in a primary care setting in the United Kingdom. Value Health. 2013;16(7):A723. doi:10.1016/j.jval.2013.08.2259
77. Punekar YS, Wurst K, Shukla A. COPD management costs among newly diagnosed COPD patients in the UK primary care setting. Am J Respir Crit Care Med. 2013;187:A4373.
78. Punekar YS, Shukla A, Muellerova H. Disease management costs associated with COPD patients with infrequent exacerbations in the UK primary care setting. Am J Respir Crit Care Med. 2013;187:A4374.
79. Merinopoulou E, Raluy-Callado M, Ramagopalan S, MacLachlan S, Khalid JM. Resource use and exacerbations of chronic obstructive pulmonary disease (COPD) by GOLD categories. Value Health. 2015;18(7):A506–A507. doi:10.1016/j.jval.2015.09.1449
80. FitzGerald JM, Haddon JM, Bradley-Kennedy C, Kuramoto L, Ford GT, RUSIC Study Group T. The RUSIC Study Group. Resource Use Study in COPD (RUSIC): a prospective study to quantify the effects of COPD exacerbations on health care resource use among COPD patients. Can Respir J. 2007;14(3):145–152. doi:10.1155/2007/921914
81. Molinari N, Briand C, Vachier I, et al. Hospitalizations for COPD exacerbations: trends and determinants of death. COPD. 2015;12(6):621–627. doi:10.3109/15412555.2015.1007931
82. Philip J, Lowe A, Gold M, et al. Using administrative datasets to assist prognostication in chronic obstructive pulmonary disease (COPD). Palliat Med. 2010;24:S25.
83. Pitassi M, Vitale C, D’Amato M, Stanziola A, Mormile M, Molino A. Impact of bronchiectasis on duration of hospitalization in patients with exacerbations of COPD. Eur Respir J. 2015;46:PA4701.
84. Pretto JJ, McDonald VM, Wark PA, Hensley MJ. Multicentre audit of inpatient management of acute exacerbations of chronic obstructive pulmonary disease: comparison with clinical guidelines. Intern Med J. 2012;42(4):380–387. doi:10.1111/j.1445-5994.2011.02475.x
85. Short PM, Williamson PA, Singanayagam A, Akram A, Chalmers JD, Schembri S. Guideline adherent therapy and reduced mortality and length of stay in adults hospitalised with exacerbations of COPD. Thorax. 2013;68(Suppl. 3):A136. doi:10.1136/thoraxjnl-2013-204457.283
86. Roberts CM, Stone RA, Lowe D, Pursey NA, Buckingham RJ. Co-morbidities and 90-day outcomes in hospitalized COPD exacerbations. COPD. 2011b;8(5):354–361. doi:10.3109/15412555.2011.600362
87. Sharafkhaneh A, Altan AE, Colice GL, et al. A simple rule to identify patients with chronic obstructive pulmonary disease who may need treatment reevaluation. Respir Med. 2014;108(9):1310–1320. doi:10.1016/j.rmed.2014.07.002
88. Steer J, Gibson GJ, Bourke SC. Longitudinal change in quality of life following hospitalisation for acute exacerbations of COPD. BMJ Open Respir Res. 2015;2(1):e000069. doi:10.1136/bmjresp-2014-000069
89. Stefan MS, Nathanson B, Lindenauer P, Higgins T, Steingrub J. Characteristics, ventilation strategies, and outcomes of patients hospitalized in intensive care units with an acute exacerbation of COPD. Am J Respir Crit Care Med. 2014;189:A1632. doi:10.1164/rccm.201306-1150OC
90. Stefan MS, Shieh MS, Pekow PS, Hill N, Rothberg MB, Lindenauer PK. Trends in mechanical ventilation among patients hospitalized with acute exacerbations of COPD in the United States, 2001 to 2011. Chest. 2015;147(4):959–968. doi:10.1378/chest.14-1216
91. Thomas M, Radwan A, Stonham C, Marshall S. Exacerbation frequency and maintenance treatment of COPD in UK clinical practice. Thorax. 2011;66(Suppl 4):A153. doi:10.1136/thoraxjnl-2011-201054c.211
92. Vitacca M, Escarrabill J, Galavotti G, et al. Home mechanical ventilation patients: a retrospective survey to identify level of burden in real life. Monaldi Arch Chest Dis. 2007;67(3):142–147. doi:10.4081/monaldi.2007.485
93. Wang Q, Bourbeau J. Outcomes and health-related quality of life following hospitalization for an acute exacerbation of COPD. Respirology. 2005;10(3):334–340. doi:10.1111/res.2005.10.issue-3
94. Yu AP, Yang H, Wu EQ, Setyawan J, Mocarski M, Blum S. Incremental third-party costs associated with COPD exacerbations: a retrospective claims analysis. J Med Econ. 2011;14(3):315–323. doi:10.3111/13696998.2011.576295
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.