Back to Journals » ClinicoEconomics and Outcomes Research » Volume 13
Economic Analysis of Leak Complications in Anastomoses Performed with Powered versus Manual Circular Staplers in Left-Sided Colorectal Resections: A US-Based Cost Analysis
Authors Pollack E, Johnston S, Petraiuolo WJ, Roy S, Galvain T
Received 24 February 2021
Accepted for publication 10 June 2021
Published 17 June 2021 Volume 2021:13 Pages 531—540
DOI https://doi.org/10.2147/CEOR.S305296
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Giorgio Colombo
Esther Pollack,1 Stephen Johnston,2 William J Petraiuolo,3 Sanjoy Roy,1 Thibaut Galvain4
1Franchise Health Economics and Market Access, Ethicon, Blue Ash, OH, USA; 2Medical Device Epidemiology and Real-World Data Sciences, Johnson & Johnson, New Brunswick, NJ, USA; 3Medical Affairs, Ethicon, Cincinnati, OH, USA; 4Global Health Economics, Johnson and Johnson Medical Devices, New Brunswick, NJ, USA
Correspondence: Esther Pollack
Franchise Health Economics and Market Access, Ethicon, 4545 Creek Road, Blue Ash, OH, USA
Tel +1 513 800 4888
Email [email protected]
Purpose: To estimate the cost impact of using the ECHELON CIRCULAR™ Powered Stapler (ECP) compared with manual circular staplers (standard of care, SOC) among patients undergoing colectomy procedures that involve left-sided anastomosis.
Methods: A US hospital-based budget impact model was developed to estimate the impact of ECP in reducing the surgical complication of anastomotic leak. The incremental acquisition cost of ECP vs SOC was compared to the net potential savings from reduced complication costs. The model was based on complication rates from a recently published matching-adjusted indirect comparison (MAIC) that compared clinical and healthcare utilization outcomes of patients using ECP with those of a propensity score-matched retrospective SOC control cohort from a real-world clinical practice population. The model assessed total cost, average length of stay (LOS), proportion of patients with a non-home discharge, and all-cause readmission. Deterministic (DSA) and probabilistic sensitivity analyses (PSA) were conducted to evaluate the robustness of the model assumptions and inputs.
Results: Despite a higher device cost of $412 for ECP compared with $298 for a manual stapler, annual savings due to avoided complications with ECP was $53,987 for anastomotic leak, assuming 100 procedures per year with each type of circular stapler. ECP also helped to avoid 27 LOS days, 0.38 readmissions and 0.22 non-home discharges. Sensitivity analyses around potential drivers of costs established the robustness of economic savings with the use of ECP – with annual savings being most impacted by the probability of anastomotic leak complication in the DSA.
Conclusion: This model demonstrates that among patients undergoing left-sided colectomy procedures, the incremental cost of using the ECHELON CIRCULAR™ Powered Stapler instead of a manual circular stapler was offset by the savings from lowered incidence and cost of management of anastomotic leaks in the hospital setting.
Keywords: circular stapler, colectomy, costs, outcomes, anastomotic leak
Introduction
Colon resection in its many forms is used in the treatment of diseases such as colorectal cancer, ulcerative colitis, Crohn’s disease, mechanical bowel obstruction and recurrent diverticulitis.1 Successful creation and healing of an anastomosis is the most critical component of any such procedure and depends on sufficient blood supply, lack of tension at the anastomosis site, and disease-free bowel tissue at the site.2,3
Major post-operative complications of colorectal surgery include anastomotic leak, bleeding, ileus and infection,1 and studies report overall complication rates of 6%-39%. Rates are similar in open and minimally invasive surgery (MIS) procedures, with slightly lower infection rates reported in the MIS group.4–6 Anastomotic leaks constitute the most severe complication and occur in 2.5–36% of surgical cases. These complications are associated with increased length of stay (LOS), higher rates of reoperation, as well as increased morbidity and mortality.3,7–12 The economic burden of these complication is substantial, due to increased LOS and readmissions.11,13 Amman et al evaluated total hospital cost data of 7479 patients who underwent a low anterior resection (LAR) for colorectal cancer and found that the mean incremental total hospital cost difference for patients with complication vs without complication was $11,081 for anastomotic leak.14
Colorectal anastomosis using surgical stapling has been shown to be safe and effective.2,15 Circular staplers and the double stapling technique were first used over three decades ago to facilitate left-sided (ie, hemicolectomy, low anterior resection, or sigmoidectomy) and/or low anastomoses16–20 and are now widely used during colorectal resections. Manual circular staplers have been the standard until recently. The force required by the user to form staples through tissue can lead to difficulty in operation of the device and may compromise the stability of the surgical closure.20 The incidence of technical errors with manual circular staplers is high and these devices may also pose problems in operation for surgeons with smaller glove sizes.17,21
Powered stapling systems are a new option for surgeons. They are easier to operate, requiring a reduced force to fire which allows more stability of the device while creating the anastomosis, possibly improving the reliability of the staple line.20 In March of 2019, the first powered circular stapler, the ECHELON CIRCULAR™ Powered Stapler (ECP, Ethicon Endo-Surgery, Inc., Cincinnati, OH, USA) was launched in the US and European markets.22
A preclinical study of ECP compared to manual staplers found ECP required reduced force to fire with less movement during staple application, and benchtop testing demonstrated less leaking at the staple line with ECP versus manual circular staplers.20 In a clinical study (N=17) of left-sided anastomoses, ECP demonstrated acceptable safety results and anastomotic integrity immediately following surgery.23 In a propensity score matched cohort study (119 cases manual circular stapler [MCS] versus 60 ECP stapler), anastomotic leak was observed in 14 (11.8%) patients in the MCS group and in 1 (1.7%) patient in the ECP group (P = 0.022).24 Most recently, a prospective, post-market, open label, single-arm multicenter clinical study of ECP at 12 sites in the USA and Europe (November 28, 2017 through January 15, 2020) reported adequate performance for creation of anastomoses in left-sided colon resection procedures, few technical issues, a favorable safety profile, and ease of use for creation of left-sided anastomoses as reported by operating surgeons.25 Using data from the ECP trial, a matching-adjusted indirect comparison (MAIC) was recently conducted to compare clinical and healthcare utilization outcomes of patients using ECP with those of a propensity score-matched retrospective standard of care (SOC) control cohort from a real-world clinical practice population.26 Patients treated with ECP had statistically significant lower 30-day incidence proportions of several surgical complications and 30-day readmissions. Given that these surgical complications carry substantial economic ramifications in the hospital setting, the objective of the current study was to estimate the cost impact of using ECP versus manual circular staplers to US hospitals for the complication of anastomotic leak.
Methods
An economic model was developed to evaluate the cost of ECP compared with current practice (SOC) using manual circular staplers in the United States.
Model Structure
A budget impact analysis was performed (US hospital perspective) using data from literature and the previously described MAIC to estimate the economic impact of using ECP in surgical units in the US. The model was developed using Microsoft® Excel following the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) guidelines “Principles of Good Practice for Budget Impact Analysis”.27,28 The model evaluated the impact of using ECP instead of the SOC in reducing the rate of anastomotic leak complications related to colectomy procedures that involved left-sided anastomosis. The incremental acquisition cost of ECP over SOC was compared to the net potential savings due to reduced complication costs among patients receiving ECP. The base case analysis used model inputs from the MAIC (based on previous clinical and retrospective database studies), along with inputs from the literature. The model structure is presented in Figure 1.
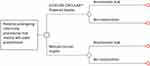 |
Figure 1 Model structure. |
Model Inputs
This model included inputs for the rate and cost of anastomotic leak complications (Table 1). Results from the MAIC described above were used as inputs for the rate of complications with ECP compared with SOC. The MAIC utilized data from a prospective clinical study of ECP (N=168) as well as a retrospective study that analyzed a SOC control cohort using data collected from the Premier Healthcare Database® (N=4544).
 |
Table 1 Model Inputs |
The prospective clinical study of ECP enrolled 168 adult (>18 years) patients in the ECP cohort.25 Patients in this clinical study underwent colectomy procedures that involved left-sided anastomosis performed with ECP and procedures were carried out with a minimally invasive or open procedure. Patients were excluded if they had concurrent enrollment in a different clinical study, any condition (physical or psychological) that would impair study participation or impact endpoints, emergency surgery, American Society of Anesthesiologists (ASA) status ≥ 4, multiple synchronous colon resections, anastomosis creation that was not distal to the splenic flexure, or no anastomosis attempted.
The SOC control cohort used in the MAIC was constructed using data collected from the Premier Healthcare Database® (PHD, N=4544).29 The SOC cohort included elective inpatient admission with an International Classification of Diseases, 10th Revision, Procedure Classification System (ICD-10-PCS) code for hemicolectomy, LAR, or sigmoidectomy as a primary procedure between October 1, 2016 and December 31, 2018 and use of manual circular stapler was identified from hospital administrative charge records. Patients in the ECP trial were all enrolled in urban hospitals with 400 beds or more and not transferred from other institutions; accordingly, SOC patients who were transferred from another institution, with index admissions in rural hospitals and/or in hospitals with 399 or fewer beds were excluded.
In the MAIC analysis, ECP patients from the clinical study and SOC patients from the retrospective Premier database study were propensity score matched on baseline patient and hospital characteristics available in both data sources up to a 1:10 ratio. Specifically, the following variables were available for matching from both cohorts: age, sex, Hispanic ethnicity, insurance type (Medicare vs other), diabetes, hypertension, surgical approach (open, laparoscopic, or robotic), indication for surgery (colorectal carcinoma, colorectal polyps or polyposis syndrome, diverticulitis, inflammatory bowel disease, or other), teaching vs non-teaching hospital, and hospital bed size category (400–499 vs 500 +). After matching, anastomotic leak outcomes were compared between the two cohorts and these complication rates were used as inputs in the current model.
The incremental cost of anastomotic leak was obtained from a retrospective study examining surgical complication costs among patients undergoing LAR for colorectal cancer.15 Costs were inflated to 2020 constant US dollars using the Medical Care component of the Consumer Price Index. Incremental increases in the average LOS, proportion of patients with a non-home discharge (eg skilled nursing facility; intermediate care facility), and all-cause readmission were also obtained from this study for anastomotic leak complications. Price of ECP and SOC manual stapler were based on Ethicon sales data through September 2020 (average prices). Costs were not discounted as model time horizon was less than one year.
Sensitivity Analysis
Deterministic and probabilistic sensitivity analyses (PSA) were conducted to evaluate the robustness of the model assumptions and inputs. One-way sensitivity analyses adjusted key parameters (probability of complication, cost of complication and cost of ECP and SOC) using the 95% confidence intervals (CIs) when available or by applying a 25% range of variation. A PSA was also conducted, and key variables were varied simultaneously by sampling from the specified distribution for each parameter for 1000 iterations using Monte-Carlo simulations (Table 2). Cost and LOS parameters used gamma distribution while probability parameters (probability of complication, non-home discharge, and readmission) used beta distribution.
 |
Table 2 Probabilistic Sensitivity Analysis |
Results
Assuming an annual number of 100 procedures with each type of circular stapler per year, there was an annual savings of $53,987 with ECP use compared with the SOC attributed to a reduction in the rate of anastomotic leaks associated with the use of ECP. The ECP was also associated with 27 fewer LOS days, 0.38 fewer readmissions and 0.22 fewer non-home discharges related to anastomotic leaks annually. The incremental cost burden to a hospital that upgrades to ECP for 100 cases a year is estimated to be $11,400—a cost that is easily offset by preventing 1–2 anastomotic leak complications, given the mean incremental cost estimates for this complication.
For the one-way sensitivity analysis, the annual savings associated with use of ECP remained positive across all values in the ranges for each parameter. It also showed that probability of leak for SOC and ECP had the greatest impact on the annual savings, followed by the cost of ECP and manual staplers (Figure 2). The mean cost of complication for anastomotic leak had minimal impact on the overall savings due to ECP use. Results of the PSA for anastomotic leak are presented in Figures 3–5. The PSA simulation resulted in mean annual savings due to avoided complication of anastomotic leak with ECP use of $54,317 (median: $55,444; 95% CI: $16,566, $84,936; Figure 3). Total annual costs were higher for the SOC group compared with ECP (Figure 4). Figure 5 demonstrates that use of ECP was associated with a reduction in readmissions related to anastomotic leak across all simulations; more than half of all simulation run results fell within the range of 0.33 to 0.45 readmissions avoided.
 |
Figure 4 Total annual costs by simulation run for occurrence of anastomotic leaks among 100 patients with each circular stapler type, according to the probabilistic sensitivity analysis. |
Discussion
Complications in colorectal surgeries are common; a recent MAIC using clinical evidence and retrospective claims data suggests that use of ECP over manual stapling is associated with reductions in complications of anastomotic leak, bleeding, and infection.27 The present budget impact analysis builds on this MAIC analysis and demonstrates an annual savings associated with the reduction in anastomotic leak complications observed with use of ECP. In our analysis, the incremental cost of using ECP instead of the SOC was offset by the savings from lowered complication management costs in the hospital setting. Despite a higher device cost of $412 for ECP compared with $298 for a manual stapler, annual savings due to avoided complications was estimated at $53,987 for anastomotic leak.
The one-way sensitivity analysis showed that the annual savings for SOC and ECP were most impacted by the probability of complications. The MAIC analysis that provided these complication probabilities had relatively wide CIs due to the smaller sample size. In contrast, complication costs were used from the burden of complication study by Ammann et al14 which had much smaller CIs given a relatively large sample size, and therefore minimal variation was observed for complication cost within the various scenarios in the present model.
While our model only estimated the cost of anastomotic leak complications, it is important to note that there are other complications not assessed by the current model and that there is evidence that certain complications may be associated with the development of a second related complication.11 Regardless of the potential combined impact of these complications, our economic analysis demonstrates that lower rates of anastomotic leaks with ECP use lead to lowered costs and reduced clinical burden in the hospital setting.
The present budget impact analysis is relevant to hospital decision-makers in the United States as it provides a robust evaluation of the economic impact of shifting to routine use of ECP in colectomy procedures that involve left-sided anastomosis. Since the budget impact analysis was based on several sources of real-world data, the economic inputs for the cost of complications represent current costs and practice patterns for complication management in US hospital settings. The inclusion of a one-way sensitivity analysis and PSA also increases the strength of the model findings and demonstrates that even under the range of inputs tested, ECP is associated with savings due to avoided complications.
Several model limitations should be noted, however. Although real-world evidence was leveraged for the model where available, the inputs for the rates of anastomotic leak complication with ECP were from an MAIC analysis26 which used data inputs from both a prospective clinical study (ECP group)25 and retrospective hospital billing data analysis (SOC). It is possible that patients in a clinical trial setting may experience fewer complications due to trial protocols that require increased monitoring and patient management. However, it is also possible that complication rates for the SOC group were underestimated in the MAIC, which included inputs from a retrospective claims data source to ascertain the SOC complication outcomes by presence of International Classification of Diseases, 10th Revision, Clinical modification (ICD-10-CM) codes, which may only capture the more severe complications warranting a code.14 As the MAIC was not a randomized trial but rather a retrospective indirect comparison, there is potential for unmeasured differences between the ECP and SOC cohorts, which could influence the observed difference in anastomotic leak between the two groups. Furthermore, differences between MAIC study groups and patients within the study from which economic data were obtained mean that the base-case point estimates used in the model are subject to uncertainty with respect to their fungibility across the various data sources. Although we used sensitivity analyses to address this, the present study’s findings must be interpreted in the common limitation of all economic models that the results may not generalize or particularize to all settings in which the ECP may be used. Future modeling efforts that incorporate real-world inputs for both ECP and SOC complication rates will be needed to provide further information to hospital decision makers. Next, the incremental cost of anastomotic leak that we adapted from the literature corresponded only to the index admission and ongoing costs incurred during after the index admission were not included; therefore, our model may be viewed as conservative from a cost saving perspective. Finally, it was not possible to aggregate the cost impacts of anastomotic leak with other complication costs such as bleeding and infection in this model, since the studies that assessed these complications examined each as an independent outcome. However in reality these outcomes may not be independent of one another and may occur at different rates depending on the presence of other complications.11,14 To assess the overall economic impact of routine use of ECP, future studies are necessary to evaluate the combined outcomes of anastomotic leak, infection, and bleeding.
The adoption of a new surgical device is based not only on the clinical efficacy and safety or equivalency of the device, but the economic feasibility of incorporating the device into routine practice within the hospital setting. The current budget impact model is based on a previously completed MAIC analysis that demonstrates the superior comparative effectiveness of ECP vs manual circular staplers with respect to fewer anastomotic leaks as well as lower rates of 30-day readmissions.26 The present model demonstrates the potential savings associated with use of ECP, which offset the higher price of the device, and provides an economic rationale for the adoption of the ECHELON CIRCULAR™ Powered Stapler in patients undergoing left-sided colorectal reconstructions.
Conclusion
This model demonstrates that among patients undergoing left-sided colectomy procedures, the incremental cost of using the ECHELON CIRCULAR™ Powered Stapler instead of a manual circular stapler was offset by the savings from lowered incidence and cost of management of anastomotic leaks in the hospital setting.
Abbreviations
SOC, standard of care; ECP, ECHELON CIRCULAR™ Powered Stapler; MAIC, matching-adjusted indirect comparison; LOS, length of stay; DSA, deterministic sensitivity analyses; PSA, probabilistic sensitivity analyses; MIS, minimally invasive surgery; LAR, low anterior resection; MCS, manual circular stapler; ASA, American Society of Anesthesiologists; PHD, Premier Healthcare Database®; CI, confidence interval; ICD-10-CM, International Classification of Diseases, 10th Revision, Clinical modification.
Data Sharing Statement
This study used only published information from prior manuscripts. The data that support the findings of this study are partially available upon request. Restrictions apply to the availability of some data, which were used under license for this study.
Ethics Approval and Informed Consent
The present study involved retrospective analysis of published information and did not involve analysis of any individual patient data. For the original analysis of the ECP trial, each of the 12 site/investigator’s Institutional Review Board (IRB) or Independent Ethics Committee (IEC) approved the protocol and consent form. Informed consent was obtained for all patients. For the original analyses of the SOC control cohort, analysis was conducted under an exemption from Institutional Review Board oversight for US-based studies using de-identified healthcare records, as dictated by Title 45 Code of Federal Regulations (45 CFR 46.101(b)(4)).
Acknowledgments
We acknowledge Karina Berenson for medical writing support.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work.
Funding
This study was funded by Johnson and Johnson.
Disclosure
Esther Pollack, Stephen Johnston, William J. Petraiuolo, Sanjoy Roy, and Thibaut Galvain are employees of Johnson and Johnson. The authors report no other conflicts of interest in this work.
References
1. Kirchhoff P, Clavien P-A, Hahnloser D. Complications in colorectal surgery: risk factors and preventive strategies. Patient Saf Surg. 2010;4(1):5. doi:10.1186/1754-9493-4-5
2. Docherty JG, McGregor JR, Akyol AM, et al. Comparison of manually constructed and stapled anastomoses in colorectal surgery. West of Scotland and Highland Anastomosis Study Group. Ann Surg. 1995;221(2):176–184. doi:10.1097/00000658-199502000-00008
3. Braunschmid T, Hartig N, Baumann L, et al. Influence of multiple stapler firings used for rectal division on colorectal anastomotic leak rate. Surg Endosc. 2017;31(12):5318–5326. doi:10.1007/s00464-017-5611-0
4. Scheidbach H, Schneider C, Konradt J, et al. Laparoscopic abdominoperineal resection and anterior resection with curative intent for carcinoma of the rectum. Surg Endosc. 2002;16(1):7–13. doi:10.1007/s00464-001-8314-4
5. Manilich E, Vogel JD, Kiran RP, et al. Key factors associated with postoperative complications in patients undergoing colorectal surgery. Dis Colon Rectum. 2013;56(1):64–71. doi:10.1097/DCR.0b013e31827175f6
6. Li YS, Meng FC, Lin JK. Procedural and post-operative complications associated with laparoscopic versus open abdominal surgery for right-sided colonic cancer resection: a systematic review and meta-analysis. Medicine. 2020;99(40):e22431. doi:10.1097/MD.0000000000022431
7. Schiff A, Roy S, Pignot M, et al. Diagnosis and management of intraoperative colorectal anastomotic leaks: a Global Retrospective Patient Chart Review Study. Surg Res Pract. 2017;2017:3852731. doi:10.1155/2017/3852731
8. Schiff A, Brady BL, Ghosh SK, et al. Estimated rate of post-operative anastomotic leak following colorectal resection surgery: a systematic review. J Surg Surgical Res. 2016;2:60–67.
9. Schiff A, Brady BL, Ghosh SK, et al. Intra-operative anastomotic leak rates and testing methodology in colorectal resection surgery. J Surg Surgical Res. 2016;2(1):48–54.
10. Cauchy F, Abdalla S, Penna C, et al. The small height of an anastomotic colonic doughnut is an independent risk factor of anastomotic leakage following colorectal resection: results of a prospective study on 154 consecutive cases. Int J Colorectal Dis. 2017;32(5):699–707. doi:10.1007/s00384-017-2769-9
11. Hammond J, Lim S, Wan Y, et al. The burden of gastrointestinal anastomotic leaks: an evaluation of clinical and economic outcomes. J Gastrointest Surg. 2014;18(6):1176–1185. doi:10.1007/s11605-014-2506-4
12. Jung SH, Yu CS, Choi PW, et al. Risk factors and oncologic impact of anastomotic leakage after rectal cancer surgery. Dis Colon Rectum. 2008;51(6):902–908. doi:10.1007/s10350-008-9272-x
13. Gantz O, Zagadailov P, Merchant AM. The cost of surgical site infections after colorectal surgery in the United States from 2001 to 2012: a longitudinal analysis. Am Surg. 2019;85(2):142–149. doi:10.1177/000313481908500219
14. Ammann EM, Goldstein LJ, Nagle D, et al. A dual-perspective analysis of the hospital and payer-borne burdens of selected in-hospital surgical complications in low anterior resection for colorectal cancer. Hosp Pract. 2019;47(2):80–87. doi:10.1080/21548331.2019.1568718
15. Ricciardi R, Roberts PL, Marcello PW, et al. Anastomotic leak testing after colorectal resection: what are the data? Arch Surg. 2009;144(5):
16. Cohen Z, Myers E, Langer B, et al. Double stapling technique for low anterior resection. Dis Colon Rectum. 1983;26(4):231–235. doi:10.1007/BF02562484
17. Kono E, Tomizawa Y, Matsuo T, et al. Rating and issues of mechanical anastomotic staplers in surgical practice: a survey of 241 Japanese gastroenterological surgeons. Surg Today. 2012;42(10):962–972. doi:10.1007/s00595-012-0303-9
18. Heald RJ. Towards fewer colostomies--the impact of circular stapling devices on the surgery of rectal cancer in a district hospital. Br J Surg. 1980;67(3):198–200. doi:10.1002/bjs.1800670311
19. Ikeda T, Kumashiro R, Taketani K, et al. Endoscopic evaluation of clinical colorectal anastomotic leakage. J Surg Res. 2015;193(1):126–134. doi:10.1016/j.jss.2014.07.009
20. Rojatkar P, Henderson CE, Hall S, et al. A novel powered circular stapler designed for creating secure anastomoses. Med Device Diagn Eng. 2017;2(2):94–100. doi:10.15761/MDDE.1000123
21. Offodile AC
22. Ethicon. ECHELON CIRCULAR™ powered stapler product information. 2020 [cited September 10, 2020]. Available from: https://www.jnjmedicaldevices.com/en-US/product/echelon-circular-powered-stapler.
23. Atallah S, Kural S, Banda N, et al. Initial clinical experience with a powered circular stapler for colorectal anastomosis. Tech Coloproctol. 2020;24(5):479–486. doi:10.1007/s10151-020-02162-4
24. Pla-Marti V, Martin-Arevalo J, Moro-Valdezate D, et al. Impact of the novel powered circular stapler on risk of anastomotic leakage in colorectal anastomosis: a propensity score-matched study. Tech Coloproctol. 2021;25(3):279–284.
25. Herzig DO, Ogilvie JW, Chudzinski A, et al. Assessment of a circular powered stapler for creation of anastomosis in left-sided colorectal surgery: a prospective cohort study. Int J Sury. 2020;84:140–146. doi:10.1016/j.ijsu.2020.11.001
26. Sylla P, Sagar P, Johnston SS, et al. Outcomes associated with the use of a new powered circular stapler for left-sided colorectal reconstructions: a propensity score matching-adjusted indirect comparison with manual circular staplers. Surg Endosc. 2021. doi:10.1007/s00464-021-08542-7
27. Mauskopf JA, Sullivan SD, Annemans L, et al. Principles of good practice for budget impact analysis: report of the ISPOR task force on good research practices--budget impact analysis. Value Health. 2007;10(5):336–347. doi:10.1111/j.1524-4733.2007.00187.x
28. Sullivan SD, Mauskopf JA, Augustovski F, et al. Budget impact analysis-principles of good practice: report of the ISPOR 2012 budget impact analysis good practice II task force. Value Health. 2014;17(1):5–14. doi:10.1016/j.jval.2013.08.2291
29. Premier healthcare database white paper: data that informs and performs. Premier Applied Sciences, Premier Inc.. March 2, 2020. Available from: https://products.premierinc.com/downloads/PremierHealthcareDatabaseWhitepaper.pdf.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.