Back to Journals » International Journal of General Medicine » Volume 16
Echocardiographic Evaluation of the Effect on Left Ventricular Function Between Left Bundle Branch Pacing and Right Ventricular Pacing
Authors Mao Y, Xie Y, Tang J, Shen Y, Liu Y, Sun B
Received 18 May 2023
Accepted for publication 19 August 2023
Published 4 September 2023 Volume 2023:16 Pages 4007—4016
DOI https://doi.org/10.2147/IJGM.S418315
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Yu Mao,1 Yuan Xie,1 Jiani Tang,1 Yujing Shen,2 Yang Liu,1 Bing Sun1
1Department of Cardiology, Tongji Hospital, Tongji University School of Medicine, Shanghai, People’s Republic of China; 2Department of Echocardiography, Tongji Hospital, Tongji University School of Medicine, Shanghai, People’s Republic of China
Correspondence: Bing Sun, Department of Cardiology, Tongji Hospital, Tongji University School of Medicine, No. 389, Xincun Road, Putuo District, Shanghai, 200065, People’s Republic of China, Tel +86 21-66111048, Email [email protected]
Purpose: The purpose of this study was to assess the left ventricular function effects of permanent left bundle branch pacing (LBBP) versus traditional right ventricular pacing (RVP).
Patients and Methods: Consecutive patients receiving pacemaker implantation were included and divided into left bundle branch block (LBBB) group and right ventricular pacing (RVP) group. Baseline characteristics were collected, and they received 1-year follow-up. Electrocardiogram (ECG) characteristics and pacing parameters were assessed before and after implantation. Cardiac function parameters such as left ventricular ejection fraction (LVEF) and tricuspid regurgitation (TR) were recorded and compared.
Results: Of 78 patients included, 45 patients received LBBP (mean age, 72.7 ± 12.2 years; male, 55.6%) and 33 patients underwent RVP (mean age 72.9 ± 11.8 years; male, 63.6%). The pacing parameters were satisfactory during the implantation and remained stable during mid-term follow-up. During the follow-up period, LBBP patients had a greater decrease in LVEDD and LVESD. The TR in the LBBP group was significantly improved as compared to the RVP group (P=0.016).
Conclusion: Permanent LBBP achieves favorable cardiac hemodynamic effects with good stability and safety. LBBP may reduce severe TR at 1-year follow-up, and LBBP may be an option for patients with severe TR.
Keywords: left bundle branch pacing, electrical synchrony, mechanical synchrony, left ventricular function, tricuspid regurgitation
Introduction
Right ventricular pacing (RVP) has been the standard treatment for patients requiring permanent ventricular pacing. Several studies have demonstrated that RVP may cause electrical and mechanical dyssynchrony and is associated with an increased risk of heart failure, atrial fibrillation, and death.1,2
His bundle pacing (HBP) has become an extraordinary approach due to its physiological pattern of ventricular electrical activation via the native His‐Purkinje system.3,4 Left bundle branch pacing (LBBP), initially reported by Huang et al,5 is a novel pacing technique that has emerged to maintain physiological ventricular activation by capturing the left bundle branch or its branches, preventing the adverse effects of conventional RVP. Meanwhile, LBBP has no disadvantages of HBP, such as high threshold, low R wave amplitude, and difficult operation. However, little is known about the hemodynamic effects of LBBP, and more studies are needed to evaluate the effect of LBBP on cardiac function.
In this study, electrical and mechanical synchronizations were investigated in patients after LBBP and RVP and the effects on cardiac function were compared between patients receiving LBBP and RVP.
Materials and Methods
Study Population
This was a non-randomized, prospective, observational study performed in Tongji Hospital, Tongji University. All patients who underwent pacemaker implantation were consecutively enrolled in this study between January 2019 and May 2020. All patients had symptomatic bradycardia and were scheduled for pacemaker implantation according to current guidelines of American College of Cardiology/American Heart Association/Heart Rhythm Society.6 The exclusion criteria were as follows: patients were younger than 18 years; the expected life span was no longer than 1 year; patients had concomitant malignant tumors or diseases that affect prognosis; vascular occlusion, lead cannot be implanted; and indications for cardiac resynchronization therapy. A total of 78 patients were randomized divided into LBBP group and RVP group, including 45 patients in the LBBP group and 33 patients in the RVP group. Single-chamber pacemakers minimize ventricular pacing through hysteresis and sleep function. Dual-chamber pacemakers minimize ventricular pacing through Ventricular Intrinsic Preference (VIP®) (Abbott), Managed Ventricular Pacing (MVPTM) (Medtronic), Vp Suppression (Biotronik).
Patient and Public Involvement
All patients signed an informed consent form and underwent successful treatment at Shanghai Tongji Hospital. This study was approved by the Ethics Committee of Tongji Hospital affiliated with Tongji University School of Medicine and all procedures were conducted according to the Declaration of Helsinki.
LBBP
Each patient underwent LBBP as described previously. Briefly, LBBP was performed using the Select Secure 3830 lead and C315HIS sheath (Medtronic, Inc, Minneapolis, MN). After the delivery sheath was advanced into the right ventricle, the pacing lead was advanced through the sheath in the right anterior oblique 30° projection. Pace mapping through the tip of the lead was used to localize the optimal pacing sites. Once the paced ECG QRS morphology in lead V1 showed a “W” configuration with a mid-notch or paced QRS duration (QRSd) <145 ms, the pacing lead was advanced with approximately 5 to 6 clockwise rotations. During the procedure, the unipolar paced 12-lead ECG, intracardiac electrogram, pacing impedance, and pacing stimulus to left ventricular activation time (Stim‐LVAT) at different outputs (usually at 2.0 V/0.4 ms and 5.0 V/0.4 ms) were recorded. LBBP was confirmed and classified according to previously reported criteria. In the right ventricular pacing (RVP) group, the pacing leads (models 5076, Medtronic Inc, Minneapolis, MN) were positioned in the right ventricular septum.
Electrocardiographic Measurements
The pacing thresholds, R-wave amplitudes, and impedances were measured by unipolar configurations through the programmer (Medtronic 2090) during the procedure and at follow-up. QRS duration was measured at 100 mm/s during intrinsic rhythm (from the onset to the end of QRS wave) and pacing at 3.5 V/0.4 ms (from the stimulus to the end of QRS wave) was recorded in two groups. Procedure-related complications including lead dislocation, lead perforation, device or lead infection, pericardial effusion, thromboembolism, and ventricular tachycardia were also recorded. The intrinsic QRSd, paced QRSd, and Stim‐LVAT were measured in sequence. The paced QRSd was measured from the stimulus to the end of last deflection of the QRS complex on 12 leads. The Stim‐LVAT was measured from the pacing stimulus to the peak of R‐wave in lead V5. During follow-up period, the pacing threshold, R-wave amplitude, and impedance were routinely measured, with a pulse width of 0.40 ms.
Echocardiographic Measurements
Transthoracic echocardiography was performed before procedure and during follow-up period, using the same equipment (EPIQ 7C, Philips Medical Systems). Echocardiographic parameters, including left atrial (LA) dimension, right ventricular (RV) dimension, and right atrial (RA) dimension, left ventricular end‐diastolic diameter (LVEDD); left ventricular end‐systolic diameter (LVESD), left ventricular ejection fraction (LVEF), and LV end-diastolic volume (LVEDV) were measured with biplane Simpson’s method. Tricuspid regurgitation (TR) was estimated using a multiparametric approach, including the vena contracta width and regurgitant jet area on color Doppler ultrasonography, as recommended by the American Society of Echocardiography. TR severity was classified as follows: none or trivial, mild, moderate, and severe. A value of ≤0.1 cm was graded as none to trivial TR, 0.1–0.2 cm as mild TR, and ≥0.2 cm as moderate-to-severe TR.
Data Collection and Follow-Up
Baseline characteristics of patients were collected on admission. During implantation, intracardiac, surface electrographic parameters and imaging data were collected. Lead parameters, ECG morphology, and echocardiographic data were recorded before the procedure and at each follow‐up visit. Patients were followed up 3 days after the operation and 1, 3, 6, and 12 months after implantation. Possible complications such as infections, pericardial effusion, capture threshold elevation, lead dislodgment, and lead deficiencies were recorded.
Statistical Analysis
Continuous variables are presented as mean ± standard deviation (SD) for those with normal distribution or median with interquartile ranges for those without normal distribution. Means were compared with the 2-tailed Student’s t-test and medians with the Mann–Whitney U-test. Categorical variables are expressed as numbers or percentages and were compared using the chi-square test. A value of 2-tailed P < 0.05 was considered statistically significant. In this nonrandomized study, analyses were not adjusted for covariates. All statistical analyses were performed using R version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline Clinical Characteristics
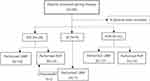
From January 2019 to May 2020, 78 patients were consecutively recruited into present study. Of them, 47 patients received LBBP, 2 patients failed and received RVP instead. Finally, 45 patients underwent LBBP (mean age, 72.7±12.2 years; 55.6% male) and 33 patients underwent RVP (mean age 72.9±11.8 years; 63.6% male) (Figure 1). Cardiac pacing indication included sick sinus syndrome (SSS) in 35.9% of patients, atrioventricular block (AVB) in 37.2% (complete heart block in 15.4%) and atrial fibrillation (Af) with slow heart rate in 11.5%. The baseline characteristics of these patients are summarized in Table 1. Dual-chamber pacemakers were implanted in 36 patients (80.0%) in the LBBP group compared with 30 patients (90.9%) in the RVP group. Compared with patients in the RVP group, patients in the LBBP group were more likely to have longer QRS duration of baseline ECG (106.89 ± 27.1 ms vs 88.6 ± 12.6 ms, P = 0.001) and more likely to present with NYHA functional class ≥2 (62.2% vs 33.3%, P = 0.00). There were no significant differences in age, gender, clinical risk factors, and cardiac pacing indication between two groups. For pacemaker implantation indication including SSS, AVB, and Af with low ventricular rate, there was no significant difference between the percentage of ventricular pacing of two groups (P>0.05).
 |
Table 1 Comparisons of Characteristics Between LBBP and RVP Group |
ECG, Pacing Characteristics, and Outcomes
Lead performance is summarized in Table 2. LBBP capture threshold was similar to the RVP threshold at implantation (0.90 ± 0.21 V/0.4 ms vs 0.84 ± 0.18 V/0.4 ms, P = 0.27). The capture threshold decreased slightly during the follow-up period in the LBBP group (P < 0.05). The lead pacing threshold remained unchanged in the RVP group. Significant lower impedance was observed in the LBBP group (661.60 ± 133.43 Ω vs 532.59 ± 83.88 Ω, P < 0.001) and RVP group (700.20 ± 176.9 Ω vs 587.27 ±119.59 Ω, P < 0.001) at follow-up as compared to those at implantation. In the LBBP group, the paced QRS duration was significantly shorter than in the RVP group (P < 0.001). Mean time to peak LV activation in the LBBP group was 66.2 ± 14.34 ms. There were no pericardial tamponade, valve and tendon injury, aortic and coronary artery injury, and embolic events during the perioperative period in all patients. Lead dislocation was observed in one patient in the LBBP group.
 |
Table 2 Pacing Parameters at Implant and Follow-Up. Comparison Between Implantation and 12 Months Follow-Up Within the Same Group |
Echocardiographic Changes After Pacing
Echocardiographic evaluation was performed in 56 patients before and 1 year after procedures (30 in the LBBP group and 26 in the RVP group). Echocardiographic findings after pacemaker implantation in two groups are summarized in Table 3.
 |
Table 3 Echocardiographic Parameters at Baseline and Follow-Up |
During the follow-up period, LVESD decreased significantly in the LBBP group (37.6 ± 11.39 mm vs 34.77 ± 8.82 mm, P = 0.008), but, LVESD increased significantly in the RVP group (29.88 ± 2.97 mm vs 31.29 ± 3.32 mm, P = 0.015). Significant difference in the LVEF between two groups was noted at baseline (55.17 ± 17.25% vs 67.79 ± 5.08%, P = 0.001) and maintained within 1 year after implantation (58.10 ± 11.88% vs 65.38 ± 4.18%, P = 0.005). No significant changes over time were observed in the RV and RA in two groups. There was an increase in TR grade 1 year after implantation in the RVP group (P = 0.016). At baseline, in the RVP group, 17 of 26 (65.4%) patients had none/trivial TR, which was noted in 11 of 26 (42.3%) patients at 12 months; mild TR was noted in 7 (26.9%) patients at baseline and 12 (46.2%) at 12 months; moderate-to-severe TR was observed in 2 (7.7%) patients at baseline and 3 (11.5%) at 12 months. This increase in TR grade was largely explained by the progression from none/trivial TR to mild TR. The TR grade remained unchanged in the LBBP group. At baseline, 10 of 30 (33.3%) patients had none/trivial TR, which was noted in 15 of 30 (50.0%) at 1 year; mild TR was noted in 15 (50.0%) patients at baseline and 10 (33.3%) patients at 1 year. There was no progression to moderate/severe TR in five patients who received LBBP (16.7% vs 16.7%). Compared with baseline, 10 of 30 patients in LBBP group had an improvement, but 1 of 26 patients in RVP group did (P = 0.007) (Figure 2).
 |
Figure 2 Change in tricuspid regurgitation severity from baseline to 1 year after LBBP and RVP. |
Discussion
The main findings of this study were as follows: 1) LBBP achieved satisfactory and stable lead parameters within 1 year after procedure. LBBP achieved higher R-wave amplitude as compared to RVP. 2). LBBP was associated with shorter paced QRS duration, which translated into improved echocardiographic outcome, as compared to RVP. 3) LBBP reduced TR within 1 year in pacemaker-dependent patients with normal cardiac function at baseline.
Cardiac pacing has been a standard therapy for bradycardia and cardiac conduction dysfunction in the absence of reversible causes. The pacing site at the right ventricular has been traditionally determined by considering the ease of transvenous pacing lead placement, stability, and cost-effectiveness. It is well known that RV pacing is non-physiological. This non-physiological electrical activity leads to the redistribution of myocardial strain and unsynchronized left ventricular contraction. Both abnormal electrical and mechanical activities of the ventricles can contribute to ventricular remodeling. RVP is associated with an increased risk for heart failure and atrial fibrillation.7,8
LBBP is a new physiological pacing strategy. Left bundle branch pacing is easy to achieve due to the anatomic characteristics of left conduction system as a wide network.9,10 LBBP is usually defined as a pacing LBB trunk or proximal left anterior or posterior bundle to directly capture the left conduction system and achieve the electrical synchrony of the left ventricle. In 2017, Huang et al5 for the first time reported a heart failure patient with LBBB underwent cardiac resynchronization with LBBP successfully. After implantation, pacing corrected the LBBB; the QRS duration (QRSd) became normal with prolongation of AV delay, indicating that the fusion of LBBP in synchronization with intrinsic RV activation was achieved. Within 1 year after implantation, the pacing threshold was low and stable, the symptoms of heart failure reduced, and LVEF increased. In 2019, Chen et al11 investigated the feasibility of LBBP and characterized the electrocardiogram in comparison with RVP. Their results showed that LBBP could achieve adequate and stable pacing parameters. Moreover, the LBBP capture threshold, R-wave amplitude, and pacing impedance were comparable to those in RVP. Li et al12 reported that patients with AVB had stable pacing threshold and R-wave amplitude of LBBP for 3 months.
In our study, there were no significant differences in R-wave amplitude, pacing threshold, and pacing impedance between LBBP group and RVP group during implantation. Compared to the baseline, capture threshold and R-wave amplitude remained stable in LBBP patients 1 year after procedure. The impedance was lower than that at baseline, but still higher than 500 Ω. The capture threshold was comparable between groups (P = 0.14), but LBBP patients had higher R-wave amplitude (P = 0.032), which may be related to myocardial injury, myocardial edema, and inflammatory reaction at implantation. After implantation, inflammation and edema resolved, and the capture threshold changed. Our study suggests that LBBP achieved stable lead parameters similar to those after RVP. Because the cathodal lead helix activates the LBB while the anodal ring electrode activates the RBB, both are embedded in the myocardium and the rich myocardium surrounds the lead. In the present study, LBBP achieved stable low pacing threshold and better R wave amplitude.
QRSd has been accepted as an indicator for cardiac evaluation of electrical synchrony. Shorter QRSd represents a better electrical synchrony.13 Prolonged QRSd is associated with biventricular systolic dyssynchrony and an increased risk for clinical heart failure events. Some studies have reported that QRSd is negatively related with LV function.14,15 In 2019, Hou et al assessed the effect of LBBP, His bundle pacing (HBP), and RVSP on the electrical synchrony.16 Their results showed that LBBP, similar to HBP, preserved better electrical and mechanical synchrony than RVSP. Cai et al employed LBBP and RVSP for the treatment of sick sinus syndrome (SSS) in 78 patients by electrocardiography and echocardiographic examinations.17 Results showed that paced QRSd in the LBBP group was slightly wider than the intrinsic QRSd, while in the LBBP group, the LV mechanical synchrony in LBBP pacing mode was similar to that in native‐conduction mode. The LV synchrony in the LBBP group was significantly better than that in the RVSP group. In our study, the paced QRSd in the LBBP group was significantly shorter than in the RVP group (at 2.5 V/0.4 ms). There was no significant difference in the QRSd between two groups as compared to baseline level within 1 year after procedure.
One possible explanation is that activation transmits quickly anterogradely to the distal left conduction system and retrogradely from His bundle to the RBB or its divisions during LBBP,18 so LBBP results in rapid LV activation and delayed RV activation. The paced QRSd was shorter in LBBP compared to those in RVP. This is an important performance that LBBP achieves electrical synchronization.5,13,15 Our previous study revealed that paced QRSd (QRSd <120 ms) in LBBP capture was only slightly prolonged as compared to the intrinsic QRSd (P>0.05). With LBBP, all patients showed paced RBBB morphology, and the paced QRSd was slightly longer than the intrinsic QRSd. However, LBBP and native‐conduction modes had similar time to peak left ventricular activation (Stim‐LVAT) in lead V5, which indicates that left ventricular electrical conduction synchronization is not damaged.
Stim‐LVAT reflects the depolarization time of left ventricular lateral wall. LBBP can better preserve cardiac electrical and LV mechanical synchrony and achieve shorter Stim‐LVAT. This has been confirmed by phase analysis of single photon emission computed tomography myocardial perfusion imaging (SPECT MPI) and echocardiography.16,17,19 The characteristics of LBB capture are: a) Paced morphology of RBBB, b) LBB potential; c) Stim‐LVAT becomes shorter abruptly with increase in output or remains the shortest and constant at low and high outputs; d) Selective LBBP; and e) Direct evidence of LBB capture by appropriate retrograde conduction to HB. The time from pacing stimulus to the peak of R‐wave, namely LVAT, is usually <80 ms in normal conduction system. This is powerful evidence for LBB capture.10 In the present study, the Stim‐LVAT (66.2±14.34 ms) was less than 70 ms in all patients of LBBP group and was also significantly shorter than that of RVSP (102.5±18.8 ms).
Some studies have indicated that RV pacing produces electrical and mechanical dyssynchrony and is associated with increased risk for left ventricular dysfunction and heart failure hospitalization.20,21 However, LBBP can improve LVEF in heart failure patients with LBBB, which has been confirmed by some studies.22,23 This may be explained as follows: LBBP directly captures the left bundle branch and delivers physiological pacing to achieve electrical synchrony of the left ventricle. Moreover, LBBP promotes left ventricular reverse remodeling, improves exercise endurance and cardiac function, and reduces hospitalization due to heart failure and mortality in patients with heart failure. In our study, LVEF in the LBBP group remained stable within 1 year after the procedure and tended to improve (P=0.07). LVEDD and LVESD were decreased 1 year after the procedure as compared to that at baseline (P=0.018; P=0.008). There are two possible reasons for the result: 1) The sample size was small and the duration of follow-up was short; 2) LBBP is able to improve LVEF of HF patients with BBB, while LBBP and RVP have little effect on LVEF in patients with normal cardiac function and shorter QRSd during short-term follow-up.24 Therefore, more studies with long-term follow-up are needed to confirm our findings in these patients. After placement of an RV lead through the tricuspid valve apparatus, lead-induced TR may be caused by lead-related tricuspid leaflet injury or perforation or lead entanglement, impingement, or adherence to the tricuspid valve.25 Studies have reported that cumulative rate of moderate-to-severe lead-induced TR is 11%.26 Similarly, lead of LBBP traverses the gap of the tricuspid valve leaf and is fixed into the LV sub-endocardial septum near the valve annulus, which may theoretically worsen TR and increase pulmonary artery pressure. However, our findings showed that TR in the LBBP group tended to improve at 1 year as compared to baseline TR (P=0.09). The improvement of TR in LBBP was significantly better than that in RVP (P=0.007). Vaturi et al found that TR severity significantly increased during active pacing in 23 clinically stable patients who were not pacemaker dependent.27 The PROTECT-PACE substudy reported that right ventricular pacing might increase TR by deteriorating LV function with subsequent elevation of LV filling pressures and subsequent back-pressure on the right heart.28 These findings indicate that TR progression may not be entirely lead-induced but may be related to the deterioration of cardiac function.26 Guo et al employed echocardiography to evaluate the safety of LBBP, and their findings revealed LBBP did not deteriorate TR, which was similar to intrinsic conduction.29 Su et al investigated the long-term safety and feasibility of LBBP in patients who received follow-up for a median of 18.6 months. Their results showed severe TR in 31.4% of patients significantly decreased after LBBP lead implantation.30 Our study indicted 20% of patients achieved improvement of TR after LBBP lead implantation. Potential mechanisms for this improvement of TR in the LBBP group may include26,30 1) For a pacemaker-dependent patient, long-term RV pacing causes LBBB and left ventricular dyssynchrony, increases left ventricular filling pressure, and aggravates TR. LBBP can preserve satisfactory left ventricular electrical synchrony and improve left ventricular systolic function, thus improving TR. 2) In patients with AVB, heart failure is related to diastolic MR and impaired LV compliance due to the mechanical AV dyssynchrony associated with the conduction disorder,31 which aggravates TR. However, LBBP restores the atrioventricular conduction sequence and improves the cardiac function.
Several studies have reported the safety and feasibility of LBBP, but LBBP still has some potential clinical risks, such as lead-related tricuspid leaflet injury, left ventricular perforation from advancing the pacemaker through the septum, RBB injury, potential injury to the septal artery, acute lead dislocation, the impact of the distal part of the pacing lead in the septum on local myocardial contractile performance and other factors.32 In our study, acute lead dislocation was observed in one patient after implantation among 36 patients who underwent LBBP, and other complications were not observed in the present study.
Limitations
There were still limitations in this study. This was a single-center study, the sample size was relatively small, and the duration of follow-up was short. Therefore, more multicenter, randomized, controlled trials with large sample size and long-term follow-up are warranted to confirm our findings.
Conclusions
LBBP is a feasible and safe pacing modality. It has satisfactory and stable lead parameters and fewer complications in the intermediate‐term follow-up. LBBP may also decrease severe TR.
Funding
This study was supported by the Natural Science Foundation of Shanghai (No: 21Y11909300 and 18ZR1434600).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Nielsen JC, Kristensen L, Andersen HR, et al. A randomized comparison of atrial and dual-chamber pacing in 177 consecutive patients with sick sinus syndrome: echocardiographic and clinical outcome. J Am Coll Cardiol. 2003;42:614–623. doi:10.1016/S0735-1097(03)00757-5
2. Liu J, Yang P, Tian H, et al. Right ventricle remodeling in chronic thromboembolic pulmonary hypertension. J Transl Int Med. 2022;10:125–133. doi:10.2478/jtim-2022-0027
3. Shimony A, Eisenberg MJ, Filion KB, et al. Beneficial effects of right ventricular non-apical vs. apical pacing: a systematic review and meta-analysis of randomized-controlled trials. Europace. 2012;14(1):81–91. doi:10.1093/europace/eur240
4. Zografos TA, Siontis KC, Jastrzebski M, et al. Apical vs. non-apical right ventricular pacing in cardiac resynchronization therapy: a meta-analysis. Europace. 2015;17(8):1259–1266. doi:10.1093/europace/euv048
5. Huang W, Su L, Wu S, et al. A novel pacing strategy with low and stable output: pacing the left bundle branch immediately beyond the conduction block. Can J Cardiol. 2017;33(12):1736.e1–1736.e3. doi:10.1016/j.cjca.2017.09.013
6. Huang WJ, Su L, Wu SJ, et al. Long-term outcomes of his bundle pacing in patients with heart failure with left bundle branch block. Heart. 2019;105(2):137–143. doi:10.1136/heartjnl-2018-313415
7. Xie JM, Fang F, Zhang Q, et al. Left atrial remodeling and reduced atrial pump function after chronic right ventricular apical pacing in patients with preserved ejection fraction. Int J Cardiol. 2012;157(3):364–369. doi:10.1016/j.ijcard.2010.12.075
8. Khurshid S, Epstein AE, Verdino RJ, et al. Incidence and predictors of right ventricular pacing-induced cardiomyopathy. Heart Rhythm. 2014;11(9):1619–1625. doi:10.1016/j.hrthm.2014.05.040
9. Saini H, Ellenbogen KA, Koneru JN. Future developments in his bundle pacing. Card Electrophysiol Clin. 2018;10:543–548. doi:10.1016/j.ccep.2018.05.013
10. Chen X, Jin Q, Bai J, et al. The feasibility and safety of left bundle branch pacing vs. right ventricular pacing after mid-long-term follow-up: a single-centre experience. Europace. 2020;22:ii36–ii44. doi:10.1093/europace/euaa294
11. Chen KP, Li YQ, Dai Y, et al. Comparison of electrocardiogram characteristics and pacing parameters between left bundle branch pacing and right ventricular pacing in patients receiving pacemaker therapy. Europace. 2019;21(4):673–680. doi:10.1093/europace/euy252
12. Li X, Li H, Ma W, et al. Permanent left bundle branch area pacing for atrioventricular block: feasibility, safety, and acute effect. Heart Rhythm. 2019;16(12):1766–1773. doi:10.1016/j.hrthm.2019.04.043
13. Liu Q, Yang J, Bolun Z, et al. Comparison of cardiac function between left bundle branch pacing and right ventricular outflow tract septal pacing in the short-term: a registered controlled clinical trial. Int J Cardiol. 2021;322:70–76. doi:10.1016/j.ijcard.2020.08.048
14. Chen S, Yin Y, Lan X, et al. Paced QRS duration as a predictor for clinical heart failure events during right ventricular apical pacing in patients with idiopathic complete atrioventricular block: results from an observational cohort study (PREDICT-HF). Eur J Heart Fail. 2013;15(3):352–359. doi:10.1093/eurjhf/hfs199
15. Kim JH, Kang K-W, Chin JY, et al. Major determinant of the occurrence of pacing-induced cardiomyopathy in complete atrioventricular block: a multicentre, retrospective analysis over a 15-year period in South Korea. BMJ Open. 2018;8(2):e019048. doi:10.1136/bmjopen-2017-019048
16. Hou XF, Qian ZY, Wang Y, et al. Feasibility and cardiac synchrony of permanent left bundle branch pacing through the interventricular septum. Europace. 2019;21(11):1694–1702. doi:10.1093/europace/euz188
17. Cai B, Huang X, Li L, et al. Evaluation of cardiac synchrony in left bundle branch pacing: insights from echocardiographic research. J Cardiovasc Electrophysiol. 2020;31(2):560–569. doi:10.1111/jce.14342
18. Wu S, Chen X, Wang S, et al. Evaluation of the criteria to distinguish left bundle branch pacing from left ventricular septal pacing. JACC Clin Electrophysiol. 2021;7(9):1166–1177. doi:10.1016/j.jacep.2021.02.018
19. Chan JYS, Huang WJ, Yan B. Non-invasive electrocardiographic imaging of his-bundle and peri-left bundle pacing in left bundle branch block. Europace. 2019;21:837. doi:10.1093/europace/euy293
20. Cicchitti V, Radico F, Bianco F, et al. Heart failure due to right ventricular apical pacing: the importance of flow patterns. Europace. 2016;18(11):1679–1688. doi:10.1093/europace/euw024
21. Pastore G, Zanon F, Baracca E, et al. The risk of atrial fibrillation during right ventricular pacing. Europace. 2016;18(3):353–358. doi:10.1093/europace/euv268
22. Zhang W, Huang J, Qi Y, et al. Cardiac resynchronization therapy by left bundle branch area pacing in patients with heart failure and left bundle branch block. Heart Rhythm. 2019;16(12):1783–1790. doi:10.1016/j.hrthm.2019.09.006
23. Huang W, Wu S, Vijayaraman P, et al. Cardiac resynchronization therapy in patients with nonischemic cardiomyopathy using left bundle branch pacing. JACC Clin Electrophysiol. 2020;6(7):849–858. doi:10.1016/j.jacep.2020.04.011
24. Liu X, Li W, Wang L, et al. Safety and efficacy of left bundle branch pacing in comparison with conventional right ventricular pacing: a systematic review and meta-analysis. Medicine. 2021;100(27):e26560. doi:10.1097/MD.0000000000026560
25. Lin G, Nishimura RA, Connolly HM, et al. Severe symptomatic tricuspid valve regurgitation due to permanent pacemaker or implantable cardioverter-defibrillator leads. J Am Coll Cardiol. 2005;45(10):1672–1675. doi:10.1016/j.jacc.2005.02.037
26. Van De Heyning CM, Elbarasi E, Masiero S, et al. Prospective study of tricuspid regurgitation associated with permanent leads after cardiac rhythm device implantation. Can J Cardiol. 2019;35(4):389–395. doi:10.1016/j.cjca.2018.11.014
27. Vaturi M, Kusniec J, Shapira Y, et al. Right ventricular pacing increases tricuspid regurgitation grade regardless of the mechanical interference to the valve by the electrode. Eur J Echocardiogr. 2010;11(6):550–553. doi:10.1093/ejechocard/jeq018
28. Saito M, Iannaccone A, Kaye G, et al. Effect of right ventricular pacing on right ventricular mechanics and tricuspid regurgitation in patients with high-grade atrioventricular block and sinus rhythm (from the Protection of Left Ventricular Function During Right Ventricular Pacing Study). Am J Cardiol. 2015;116(12):1875–1882. doi:10.1016/j.amjcard.2015.09.041
29. Guo J, Li L, Meng F, et al. Short-term and intermediate-term performance and safety of left bundle branch pacing. J Cardiovasc Electrophysiol. 2020;31(6):1472–1481. doi:10.1111/jce.14463
30. Su L, Wang S, Wu S, et al. Long-term safety and feasibility of left bundle branch pacing in a Large Single-Center Study. Circ Arrhythm Electrophysiol. 2021;14(2):e009261. doi:10.1161/CIRCEP.120.009261
31. Viskin D, Halkin A, Sherez J, et al. Heart failure due to high-degree atrioventricular block: how frequent is it and what is the cause? Heart Fail. 2021;37:1562–1568.
32. Vijayaraman P, Huang W. Atrioventricular block at the distal his bundle: electrophysiological insights from left bundle branch pacing. Heart Rhythm Case Rep. 2019;5(4):233–236. doi:10.1016/j.hrcr.2019.01.006
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.