Back to Journals » Journal of Hepatocellular Carcinoma » Volume 10
Early Tumor Marker Response Predicts Treatment Outcomes in Patients with Unresectable Hepatocellular Carcinoma Receiving Combined Lenvatinib, Immune Checkpoint Inhibitors, and Transcatheter Arterial Chemoembolization Therapy
Authors Luo MC , Wu JY , Wu JY, Lin ZT, Li YN, Zeng ZX , Wei SM, Yan ML
Received 14 June 2023
Accepted for publication 22 September 2023
Published 12 October 2023 Volume 2023:10 Pages 1827—1837
DOI https://doi.org/10.2147/JHC.S425674
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Prof. Dr. Imam Waked
Meng-Chao Luo,1,2,* Jia-Yi Wu,1,3,* Jun-Yi Wu,1,3 Zhong-Tai Lin,1,2 Yi-Nan Li,3 Zhen-Xin Zeng,3 Shao-Ming Wei,1,2 Mao-Lin Yan1,3
1Shengli Clinical Medical College of Fujian Medical University, Fuzhou, Fujian Province, 350001, People’s Republic of China; 2Department of General Surgery, Fujian Provincial Hospital, Fuzhou, Fujian Province, 350001, People’s Republic of China; 3Department of Hepatobiliary Pancreatic Surgery, Fujian Provincial Hospital, Fuzhou, Fujian Province, 350001, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Mao-Lin Yan, The Shengli Clinical Medical College of Fujian Medical University, Dongjie Road 134, Fuzhou, Fujian Province, 350001, People’s Republic of China, Tel +86 591-88217130, Fax +86 591-87557768, Email [email protected] Shao-Ming Wei, Department of General Surgery, Fujian Provincial Hospital, Jinrong South Road 516, Fuzhou, Fujian Province, 350001, People’s Republic of China, Tel +86 591-88618707, Fax +86 591-88618900, Email [email protected]
Purpose: Few reliable biomarkers for predicting the efficacy of triple therapy (lenvatinib + immune checkpoint inhibitors + transarterial chemoembolization) exist for patients with unresectable hepatocellular carcinoma (uHCC). This study explored the prognostic role of alpha-fetoprotein (AFP) and des-gamma-carboxyprothrombin (DCP) levels in patients with uHCC receiving triple therapy.
Patients and Methods: This retrospective study included 93 patients with uHCC who received triple therapy at Fujian Provincial Hospital between August 2020 and November 2022. Depending on the respective baseline levels, the patients were divided into high-AFP and high-DCP groups. An early response was defined as an AFP or DCP concentration > 50% less than the baseline concentration after 6 weeks of triple therapy. The primary endpoint was the objective response rate (ORR). The secondary endpoints were progression-free survival (PFS) and overall survival (OS).
Results: After 6 weeks of triple therapy, 75.3% (58/77) and 78.9% (60/76) of patients in the high-AFP and high-DCP groups achieved an objective response. Early AFP and DCP responses were positively associated with ORR (high-AFP group: odds ratio [OR]: 13.542; 95% confidence interval [CI]: 3.991– 45.950, p< 0.001; high-DCP group: OR: 17.853; 95% CI: 4.478– 71.179, p< 0.001). In the high-AFP group, the 6-month, 12-month, and 18-month PFS and OS rates were higher in the AFP responders than those in the non-responders (PFS: 66.4%, 59.6%, 48.2% vs 42.3%, 19.3%, 0%, p< 0.001; OS: 94.5%, 90.4%, 77.3% vs 75.6%, 66.2%, 49.6%, p=0.006). In the high-DCP group, the 6-month, 12-month, and 18-month PFS and OS rates were higher in the DCP responders than those in the non-responders (PFS: 67.4%, 57.7%, 39.0% vs 38.9%, 8.1%, 0%, p< 0.001; OS: 94.7%, 94.7%, 83.3% vs 77.0%, 53.9%, 36.0%, p< 0.001).
Conclusion: After 6 weeks of triple therapy, an AFP or DCP reduction of > 50% predicts better treatment outcomes in uHCC patients.
Keywords: unresectable hepatocellular carcinoma, alpha-fetoprotein, des-gamma-carboxyprothrombin, combination therapy, tumor response
Introduction
Hepatocellular carcinoma (HCC) is a commonly diagnosed cancer with a high social and economic burden, ranking third in cancer-related global mortality.1 Because of its insidious onset, approximately 80% of patients with HCC are diagnosed at intermediate and advanced stages, resulting in a poor prognosis with a 5-year survival rate of 18%.2–4
Although the introduction of tyrosine kinase inhibitors (TKIs) and immune checkpoint inhibitors (ICIs) significantly improved the systemic treatment of advanced HCC, the objective response rate (ORR) remains unsatisfactory.5 Sorafenib, lenvatinib (LEN), and atezolizumab plus bevacizumab have been approved as first-line treatments for advanced HCC, with an ORR of 12.4–40.6%.6,7 Transarterial chemoembolization (TACE) is widely performed as the primary first-line treatment for unresectable HCC (uHCC) in the clinic.3 Surprisingly, patients with uHCC who received a combination of TKIs, ICIs, and TACE achieved promising treatment outcomes, with an ORR of 42.9–80.6%.8–13 Liu et al reported that TACE combined with LEN and camrelizumab resulted in an ORR of 68.2% and a median progression-free survival (PFS) of 11.4 months in a cohort of 22 patients with advanced HCC.8 Another study involving 41 patients with uHCC who received TACE plus LEN and ICIs (sintilimab, tislelizumab, or camrelizumab) reported an ORR of 56.1%.12 Furthermore, we previously reported an ORR of 80.6% in patients with uHCC receiving triple therapy (LEN + ICIs + TACE).13 Triple therapy exhibits a potential synergistic effect to enhance anti-tumor activity and improve patient prognoses.14 Therefore, assessing its anti-tumor effects during the initial treatment period is of great clinical significance.
To date, efficient biomarkers to identify patients who might respond to triple therapy at the treatment initiation do not exist. Alpha-fetoprotein (AFP) and des-gamma-carboxyprothrombin (DCP) are commonly used tumor markers for diagnosing HCC.15 In addition, changes in AFP and DCP levels can serve as prognostic indicators for the recurrence and survival of patients with HCC receiving LEN, ICIs, or TACE.16–18 Nonetheless, the predictive roles of AFP and DCP in patients with uHCC undergoing triple therapy remain unclear.
Therefore, this study explored the clinical utility of early AFP and DCP responses as predictors of treatment outcomes in patients with uHCC receiving triple therapy. The primary endpoint of this study was ORR, assessed according to the modified Response Evaluation Criteria in Solid Tumors (mRECIST); the secondary endpoints included PFS and overall survival (OS).
Materials and Methods
Patients
This retrospective study comprised patients with uHCC receiving triple therapy (LEN + ICIs + TACE) at Fujian Provincial Hospital between August 2020 and November 2022. The inclusion criteria were 1) age >18 years; 2) a clinical or pathological HCC diagnosis based on the European Association for the Study of the Liver guidelines and deemed unresectable after a multidisciplinary team discussion; 3) uHCC treated with triple therapy; 4) at least one measurable target lesion assessable by mRECIST; and 5) an Eastern Cooperative Oncology Group performance status (ECOG PS) score of 1 or less. The exclusion criteria were 1) Child-Pugh class C; 2) Barcelona Clinic Liver Cancer (BCLC) stages A; 3) no elevated baseline AFP and DCP levels (AFP ≤10 ng/mL and DCP ≤40 mAU/mL); 4) missing follow-up imaging or serum tumor marker records after the triple therapy; and 5) combined with other malignant tumors.
This study was conducted in accordance with the Declaration of Helsinki. The study received approval from the Ethics Committee of Fujian Provincial Hospital, and all patients provided informed consent.
Treatment Protocol
All patients were administered either 8 mg/day of LEN for those with bodyweight <60 kg or 12 mg/day for bodyweight ≥60 kg, along with one of the following intravenous ICIs administered once every 3 weeks: sintilimab (200 mg), tislelizumab (200 mg), camrelizumab (200 mg), pembrolizumab (200 mg), or toripalimab (240 mg). For TACE, a mixture of iodized oil contrast medium and pirarubicin was infused into the super-selected tumor-feeding artery via a microcatheter. Subsequently, embolization was performed using gelatin sponge particles to achieve tumor-feeding arterial flow stasis. The TACE was repeated on demand. The median numbers of ICIs and TACE were 4 cycles (range: 1–9 cycles) and 2 times (range: 1–7 times), respectively. The median duration of lenvatinib treatment was 5.8 months (range: 1.4–26.2 months). LEN and ICIs were suspended for 3 days before and after the TACE procedure.
Data Collection and Follow-Up
We collected and analyzed clinical baseline characteristics and follow-up data of eligible patients, including age, sex, etiology, ECOG PS score, albumin-bilirubin (ALBI) grade, BCLC stage, tumor number, maximum tumor size, hemogram, serum chemistry, AFP level, DCP level, and presence or absence of macrovascular invasion. The ALBI score was determined using serum albumin and bilirubin levels measured at baseline, employing the following formula: ALBI score = (log 10 bilirubin [µmol/L] × 0.66) + (albumin [g/L] × −0.085). Pretreatment AFP and DCP levels were determined within 7 days before initiating triple therapy. To evaluate the early AFP and DCP responses, data were collected after 6±2 weeks of triple therapy; an early response was defined as a >50% decrease in the concentration from the baseline level. Treatment responses were assessed using contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) based on mRECIST. Tumor assessments were performed every 4–8 weeks by two experienced radiologists. ORR was determined based on the best treatment response, requiring a complete response (CR; arterial-enhancing lesion disappearance) or partial response (PR; an arterial-enhancing lesion diameter reduction of ≥30%) sustained for ≥1 month. Adverse events (AEs) were assessed using the Common Terminology Criteria for Adverse Events v5.0. The patients were treated with triple therapy until the onset of intolerable AEs or disease progression. PFS was defined as the period between the initial treatment and disease progression, death, or last follow-up. OS was defined as the period from the initiation of treatment until death or last follow-up.
Statistical Analyses
Normally distributed descriptive data are presented as means ± standard deviations or percentages; otherwise, they are presented as medians (ranges). Continuous data were analyzed using independent t-tests. Categorical data were compared using the chi-squared test or Fisher’s exact test, and binary logistic regression analysis was performed to identify potential predictors of ORR. Survival curves were calculated using the Kaplan–Meier method and compared using the Log rank test. Predictive factors for PFS and OS were determined using a Cox regression model via univariate and multivariate analyses. Factors with p<0.1 in univariate analysis were included in the multivariate analysis. A value of p<0.05 was considered statistically significant. All statistical analyses were performed using Statistical Package for Social Sciences (SPSS) software (Version 23, IBM Corp., Armonk, NY, USA).
Results
Patient Characteristics
The study included 93 patients with uHCC who received triple therapy (LEN + ICIs + TACE) between August 2020 and November 2022 (Figure 1). Supplementary Table S1 presents the patients’ baseline characteristics. Patient age followed a normal distribution (56.0±11.4 years); most patients were male (89.2%, 83/93). In addition, most patients had hepatitis B virus (HBV) infection (92.5%, 86/93), and 76.3% (71/93) of patients had an ECOG PS score of 0. The median baseline concentration was 696.1 ng/mL for AFP and 6874 mAU/mL for DCP. Based on the BCLC staging system, 33 and 60 patients were classified as BCLC stages B and C, respectively. Among these patients, 59.1% (55/93) exhibited macrovascular invasion. Furthermore, 91.4% (85/93) of patients had multiple tumors, and the largest tumor diameter was >5 cm in 80.6% (75/93) of patients. Grade I ALBI was noted in 52.7% (49/93) of patients, and grade II in 47.3% (44/93). AEs were manageable during follow-up; no toxicity-related deaths were reported.
 |
Figure 1 Flow diagram of the study selection process. |
ORR Analysis in the High-AFP Group
Most patients in the high-AFP group were male (88.3%, 68/77) and infected with HBV (92.2%, 71/77). Of these, 76.6% (59/77) had an ECOG PS score of 0. The median baseline levels were 1985.2 ng/mL for AFP and 15,774 mAU/mL for DCP. BCLC staging revealed that 25 and 52 patients had stages B and C, respectively, with 62.3% (48/77) of patients having macrovascular invasion. Most patients had multiple tumors (93.5%, 72/77) and tumor diameters >5 cm (83.1%, 64/77). Grade I ALBI was noted in 49.4% (38/77) of patients and grade II in 50.6% (39/77) (Supplementary Table S1).
After 6 weeks of triple therapy, the AFP level of 72.7% (56/77) of patients decreased by >50% from baseline. In total, 20.8% (16/77) of patients achieved CR, and 54.5% (42/77) achieved PR. The ORRs of the AFP responders (89.3%, 50/56) and non-responders (38.1%, 8/21) were significantly different (p<0.001; Supplementary Table S2). After univariate analysis, age, sex, tumor size, number, macrovascular invasion, ALBI grade, BCLC stage, and baseline AFP level did not affect the ORR. An AFP reduction of >50% was the only factor related to a greater ORR (odds ratio [OR]: 13.542; 95% confidence interval [CI]: 3.991–45.950, p<0.001). Table 1 presents the detailed data.
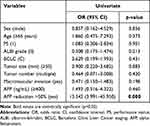 |
Table 1 Univariate Analysis for Objective Response Rates in the High-AFP Group (n=77) |
ORR Analysis in the High-DCP Group
In the high-DCP group, most patients were male (96.1%, 73/76), had an HBV infection (94.7%, 72/76), and had an ECOG PS score of 0 (81.6%, 62/76). The median baseline AFP and DCP concentrations were 999.5 ng/mL and 7337 mAU/mL, respectively. Patients were classified based on the BCLC staging as B (n=24) or C (n=52). Among these, 61.8% (47/76) exhibited macrovascular invasion. Furthermore, 93.4% (71/76) of patients had multiple tumors, and the largest tumor diameter was >5 cm in 81.6% (62/76) of patients. ALBI grades I and II were noted in 56.6% (43/76) and 43.4% (33/76) of patients, respectively (Supplementary Table S1).
After 6 weeks of triple therapy, the DCP concentration decreased by >50% from baseline in 76.3% (58/76) of patients; 18.4% (14/76) achieved CR, and 60.5% (46/76) achieved PR. In total, 91.4% (53/58) of DCP responders achieved a response to triple therapy; only 38.9% (7/18) of DCP non-responders responded to triple therapy (p<0.001; Supplementary Table S2). Univariate analysis revealed that age, sex, tumor size, number, BCLC stage, macrovascular invasion, and baseline DCP level did not affect the ORR. Although the ALBI grades significantly affected ORR in the univariate analysis, a significant correlation was not identified in the multivariate analysis (p=0.109). In the multivariate analysis, only a DCP concentration decrease of >50% was independently associated with ORR (OR: 17.853; 95% CI: 4.478–71.179, p<0.001). Table 2 presents the detailed data.
 |
Table 2 Univariate and Multivariate Analysis for Objective Response Rates in the High-DCP Group (n=76) |
Prognostic Role of the AFP Response for PFS
In the high-AFP group, the median PFS was 10.3 months (95% CI: 4.6–16.0 months; Figure 2A). The PFS of AFP responders was significantly longer than that of non-responders (16.0 months vs 5.0 months, p<0.001). After multivariate analysis, AFP responders had improved PFS compared to non-responders (hazard ratio [HR]: 0.377; 95% CI: 0.195–0.731, p=0.004; Supplementary Table S3).
Prognostic Role of the DCP Response for PFS
In the high-DCP group, the median PFS was 10.3 months (95% CI: 5.9–14.6 months; Figure 2B). The PFS of DCP responders was significantly longer than that of non-responders (13.2 months vs 3.3 months, p<0.001). Multivariate analysis showed that a greater ECOG PS score significantly and adversely affected PFS (HR: 2.133; 95% CI: 1.017–4.476, p=0.045). Patients with an early response to DCP had improved PFS compared to those without an early response (HR: 0.307; 95% CI: 0.154–0.609, p=0.001; Supplementary Table S4).
Prognostic Role of the AFP and DCP Responses for OS
The median OS was not reached in the high-AFP or high-DCP group; Figure 3A and B present the OS survival curves. Patients with an AFP or DCP concentration decrease of >50% had a significantly longer OS than those without (p=0.006 and p < 0.001, respectively). Multivariate analysis of the high-AFP group showed that the OS of patients with an AFP level decrease of >50% was significantly longer than that of patients without (HR: 0.327; 95% CI: 0.116–0.922, p=0.034; Table 3). Multivariate analysis of the high-DCP group showed that OS was adversely affected by high ECOG PS scores (HR: 6.310; 95% CI: 1.984–20.069, p=0.002) and ALBI grades (HR: 3.794; 95% CI: 1.096–13.135, p=0.035). The OS of patients with a DCP level decrease of >50% was significantly longer than that of patients without (HR: 0.221; 95% CI: 0.074–0.664, p=0.007; Table 4).
 |
Table 3 Univariate and Multivariate Analysis for Overall Survival in the High-AFP Group (n=77) |
 |
Table 4 Univariate and Multivariate Analysis for Overall Survival in the High-DCP Group (n=76) |
Discussion
This study demonstrated that a decrease in the AFP or DCP concentration of >50% from baseline after 6 weeks of triple therapy was associated with better ORR, PFS, and OS. In the high-AFP and high-DCP groups, early AFP or DCP responses were identified as independent predictors of ORR. These findings can help identify patients with uHCC who may not benefit from triple therapy, and their treatment strategies can be promptly changed. This study found that greater ECOG PS scores were associated with worse PFS and OS rates in the high-DCP group, and higher ALBI grades adversely affected OS.19 ALBI score is an objective indicator of liver function in HCC patients and predicts patient survival. The ECOG PS score is used to assess the effects of tumor disease on daily life and predict a prognosis.20 However, the prognostic roles of the ALBI grade and ECOG PS score in patients with uHCC receiving triple therapy remain uncertain and must be addressed.
The survival rate of patients with uHCC is poor. Therefore, efficient therapeutic strategies are urgently required to improve patient prognoses. Without a potent systemic treatment, TACE is the primary method for conversion therapy in patients with uHCC. Repeated TACE is often required for residual tumors. However, the response rate of tumor tissues to TACE treatment declines as treatment time increases, known as TACE refractoriness or failure.21 Currently, atezolizumab plus bevacizumab, durvalumab plus tremelimumab, sorafenib, and LEN are the first-line treatments for patients with advanced HCC.3 Following the publication of the IMbrave150 trial,7 combination therapies with TKIs and ICIs have become mainstream for treating uHCC, with an ORR of 33.2%. Moreover, IMbrave150 brings attention to a more aggressive treatment: the triple modality of transarterial therapy, ICIs, and TKIs. During the last 3 years, several clinical trials have been performed to prove the safety and efficacy of triple therapy with promising ORR and survival outcomes.
The mechanisms underlying the synergistic effect of triple therapy could be attributed to the following reasons: ICIs block signals that impede immune attacks on tumors, allowing the immune system to attack and kill tumor cells.22,23 TACE can exacerbate the hypoxic and pro-angiogenic environment of HCC through transarterial embolization, leading to the subsequent upregulation of vascular endothelial growth factor (VEGF) and other pro-angiogenic cytokines. Chronic hypoxia and VEGF overexpression contribute to the immunosuppressive tumor microenvironment (TME).24 Antiangiogenic therapies, including TKIs, can alleviate hypoxia and immunosuppression in the TME by normalizing tumor blood vessels and enhancing the efficacy of ICIs.14 In addition, ICIs treatment may potentially induce tumor vascular normalization.25 Therefore, triple therapy may further improve tumor response and survival outcomes in patients with uHCC. However, potential AEs and the cost-effectiveness of triple therapy should be considered with caution because of its undesirable effects on systemic treatment efficacy and quality of life. Therefore, methods for identifying patients with uHCC who might benefit from triple therapy are urgently needed.
To date, reliable predictive tumor markers for assessing the early response of patients with uHCC to triple therapy are lacking. AFP and DCP are well-established biomarkers for HCC diagnosis and prognosis and correlate positively with the tumor burden; the predictive values of AFP and DCP have been confirmed in patients with HCC who received LEN, ICIs, or TACE. Ichikawa et al reported that a decline of >50% in the DCP level 1 month after TACE predicted a better prognosis in patients with uHCC (OS, 67.0 months vs 19.8 months).18 Furthermore, a recent report indicated that an early decrease in the AFP level (≥40%) after 1 month of LEN treatment was related to treatment efficacy for HCC patients with elevated AFP levels (68.4% ORR vs 7.1% ORR).16 Sun et al also found that early reductions in AFP and DCP levels in patients with HCC were associated with a positive response to ICIs therapy and prolonged PFS and OS.17 Therefore, early decreases in AFP and DCP levels might be associated with the efficiency of triple therapy and the prognoses of patients with uHCC. However, few studies have evaluated the predictive value of AFP and DCP levels in patients with HCC undergoing triple therapy.
Tumor response evaluations using CT or MRI after systemic or locoregional treatment are widely used to decide whether to continue treatment or change strategies. However, imaging methods have limitations, including limited sensitivity, poor accessibility, long waiting times, and the influence of subjective variability among observers. Thus, alternative evaluation modalities should be utilized to supplement imaging methods for a more precise assessment of anti-tumor efficacy. This study found that early decreases in AFP or DCP levels in response to triple therapy predict better outcomes for patients with uHCC and correlate well with imaging responses.
This study has some limitations. First, this study was retrospective with a limited cohort size, resulting in an inevitable selection bias. Second, the follow-up time was short and, therefore, inadequate for evaluating the effects on OS. Third, the ICIs categories varied in this study, possibly affecting the uniformity of the treatment process. Fourth, this study included many patients with HBV-related HCC, limiting its generalizability.
Conclusion
In patients with uHCC treated with triple therapy, an AFP or DCP reduction of >50% after 6 weeks correlated with better ORR, PFS, and OS. Therefore, monitoring AFP and DCP serum levels could help assess and predict the efficacy of triple therapy in patients with uHCC.
Funding
This study was funded by the Natural Science Foundation of Fujian Province (2022J011021, 2020J011105) and the Medical Innovation Project of the Health and Family Planning Commission of Fujian Province (2022CXA002).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Benson AB, D’Angelica MI, Abbott DE, et al. Hepatobiliary cancers, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19(5):541–565. doi:10.6004/jnccn.2021.0022
3. Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76(3):681–693. doi:10.1016/j.jhep.2021.11.018
4. Villanueva A, Longo DL. Hepatocellular carcinoma. N Engl J Med. 2019;380(15):1450–1462. doi:10.1056/NEJMra1713263
5. Pinter M, Jain RK, Duda DG. The current landscape of immune checkpoint blockade in hepatocellular carcinoma: a review. JAMA Oncol. 2021;7(1):113–123. doi:10.1001/jamaoncol.2020.3381
6. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised Phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–1173. doi:10.1016/s0140-6736(18)30207-1
7. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–1905. doi:10.1056/NEJMoa1915745
8. Liu J, Li Z, Zhang W, et al. Comprehensive treatment of trans-arterial chemoembolization plus lenvatinib followed by camrelizumab for advanced hepatocellular carcinoma patients. Front Pharmacol. 2021;12:709060. doi:10.3389/fphar.2021.709060
9. Cao F, Yang Y, Si T, et al. The efficacy of TACE combined with lenvatinib plus sintilimab in unresectable hepatocellular carcinoma: a multicenter retrospective study. Front Oncol. 2021;11:783480. doi:10.3389/fonc.2021.783480
10. Zheng L, Fang S, Wu F, et al. Efficacy and safety of TACE combined with sorafenib plus immune checkpoint inhibitors for the treatment of intermediate and advanced TACE-refractory hepatocellular carcinoma: a retrospective study. Front Mol Biosci. 2020;7:609322. doi:10.3389/fmolb.2020.609322
11. Ju S, Zhou C, Yang C, et al. Apatinib plus camrelizumab with/without chemoembolization for hepatocellular carcinoma: a real-world experience of a single center. Front Oncol. 2021;11:835889. doi:10.3389/fonc.2021.835889
12. Cai M, Huang W, Huang J, et al. Transarterial chemoembolization combined with lenvatinib plus PD-1 inhibitor for advanced hepatocellular carcinoma: a retrospective cohort study. Front Immunol. 2022;13:848387. doi:10.3389/fimmu.2022.848387
13. Wu JY, Yin ZY, Bai YN, et al. Lenvatinib combined with anti-PD-1 antibodies plus transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma: a multicenter retrospective study. J Hepatocell Carcinoma. 2021;8:1233–1240. doi:10.2147/jhc.S332420
14. Li SJ, Chen JX, Sun ZJ. Improving antitumor immunity using antiangiogenic agents: mechanistic insights, current progress, and clinical challenges. Cancer Commun. 2021;41(9):830–850. doi:10.1002/cac2.12183
15. Choi J, Kim GA, Han S, Lee W, Chun S, Lim YS. Longitudinal assessment of three serum biomarkers to detect very early-Stage hepatocellular carcinoma. Hepatology. 2019;69(5):1983–1994. doi:10.1002/hep.30233
16. Saeki I, Yamasaki T, Yamashita S, et al. Early predictors of objective response in patients with hepatocellular carcinoma undergoing lenvatinib treatment. Cancers. 2020;12(4):779. doi:10.3390/cancers12040779
17. Sun X, Mei J, Lin W, et al. Reductions in AFP and PIVKA-II can predict the efficiency of anti-PD-1 immunotherapy in HCC patients. BMC Cancer. 2021;21(1):775. doi:10.1186/s12885-021-08428-w
18. Ichikawa T, Machida N, Sasaki H, et al. Early prediction of the outcome using tumor markers and mRECIST in unresectable hepatocellular carcinoma patients who underwent transarterial chemoembolization. Oncology. 2016;91(6):317–330. doi:10.1159/000448999
19. Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-The ALBI grade. J Clin Oncol. 2015;33(6):550–558. doi:10.1200/jco.2014.57.9151
20. Nishikawa H, Kita R, Kimura T, et al. Clinical implication of performance status in patients with hepatocellular carcinoma complicating with cirrhosis. J Cancer. 2015;6(4):394–402. doi:10.7150/jca.11212
21. He Q, Yang J, Jin Y. Development and validation of TACE refractoriness-related diagnostic and prognostic scores and characterization of tumor microenvironment infiltration in hepatocellular carcinoma. Front Immunol. 2022;13:869993. doi:10.3389/fimmu.2022.869993
22. El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, Phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492–2502. doi:10.1016/s0140-6736(17)31046-2
23. Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label Phase 2 trial. Lancet Oncol. 2018;19(7):940–952. doi:10.1016/s1470-2045(18)30351-6
24. Hato T, Zhu AX, Duda DG. Rationally combining anti-VEGF therapy with checkpoint inhibitors in hepatocellular carcinoma. Immunotherapy. 2016;8(3):299–313. doi:10.2217/imt.15.126
25. Tian L, Goldstein A, Wang H, et al. Mutual regulation of tumour vessel normalization and immunostimulatory reprogramming. Nature. 2017;544(7649):250–254. doi:10.1038/nature21724
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


