Back to Journals » Neuropsychiatric Disease and Treatment » Volume 17
Early Improvement of Psychiatric Symptoms with Long-Acting Injectable Antipsychotic Predicts Subsequent Social Functional Remission in Patients with Schizophrenia
Authors Ohnishi T, Wakamatsu A , Kobayashi H
Received 12 January 2021
Accepted for publication 30 March 2021
Published 16 April 2021 Volume 2021:17 Pages 1095—1104
DOI https://doi.org/10.2147/NDT.S294503
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Taro Kishi
Takashi Ohnishi,1 Akihide Wakamatsu,1 Hisanori Kobayashi2
1Medical Affairs Division, Janssen Pharmaceutical K.K., Tokyo, Japan; 2Research and Development Clinical Science Division, Janssen Pharmaceutical K.K., Tokyo, Japan
Correspondence: Takashi Ohnishi
Medical Affairs Division, Janssen Pharmaceutical K.K., 5-2, Nishi-kanda 3-chome Chiyoda-ku, Tokyo, 101-0065, Japan
Tel +81-3-4411-7700
Fax +81-3-4411– 5031
Email [email protected]
Purpose: The aim of this study was to clarify whether early symptomatic improvement in response to a long-acting injectable antipsychotic (LAI) contributes to subsequent social functional remission in patients with schizophrenia using the previous clinical trial data (EudraCT registration number: 2011-004889-15). Associations between other factors and social functional remission were also explored.
Patients and Methods: We analyzed 428 patients with schizophrenia in which the personal and social performance scale (PSP) and the involvement evaluation questionnaire (IEQ) at the time of the base line were recorded. Social functional remission was defined as participants who scored PSP > 70 at the end of 65 weeks. Logistic regression analyses were done to examine associations between social functional remission and clinical and demographic characteristics including early symptomatic response evaluated by Positive and Negative Syndrome Scale (PANSS) at week one.
Results: One hundred out of 428 patients showed social functional remission at the end of the observation period. Shorter duration of illness, higher baseline score of supervision evaluated by IEQ and higher baseline PSP were significantly associated with the social functional remission. Improvement of positive subscale of PANSS at one week was significantly associated with later social functional remission when baseline PSP scores were excluded from predictive variables.
Conclusion: Shorter duration of illness, residual type of schizophrenia, higher baseline score of supervision and higher baseline social functioning were predictors of subsequent social functional remission. Although its effect seems to be limited, early symptomatic improvement could be also was a predictor of social functional remission.
Keywords: schizophrenia, social function, functional outcome, long-acting injectable antipsychotics; LAI
Introduction
The improvement of social function and consequent functional remission are important outcomes for patients with schizophrenia.1–4 Impaired interpersonal relationships and daily living skills, as well as diminished occupational, social, and community interactions are common features that diminish the quality of life of schizophrenic patients. Patients with schizophrenia being confronted not only with the effects of an erroneous social perception of labeling and avoidance, but also with self-stigmatization, such as loss of self-esteem, difficulties in maintaining social relationships, or serious problems on both educational and professional levels, leading to a low socio-economic status. These could influence not only the severity of the symptoms, but also the response to pharmacological treatment strategies.5
Several factors such as upbringing, premorbid personality and adjustment, social context, and short duration of untreated psychosis have been considered to be contributed to functional outcome in schizophrenia patients.6–10 Antipsychotics are used primarily to control symptoms, especially positive symptoms, and have no direct therapeutic effect on cognition, social functioning, or quality of life related to functional outcomes, however, improvements in functional outcomes, including social functioning, should be mediated by symptomatic control.11 Indeed, several studies have also suggested associations between symptomatic response to antipsychotics and favorable functional outcome, remission, and recovery.1–4,11–18 Some studies suggested association between symptomatic response to antipsychotics and favorable functional outcome, even in patients with chronic schizophrenia.16,17,19 In line with previous studies suggesting association between functional outcomes and early symptomatic responses to treatment, our previous study demonstrated associations between early improvement of positive symptoms and social functional remission.18 However, our previous study on an oral antipsychotic did not evaluate several important factors possibly associated with functional remission,19 therefore, whether early improvement of positive symptoms contribute to functional remission remains to be clarified. Furthermore, type of antipsychotic treatment and the route of administration are also important aspects that can be related to both clinical and functional remission.20 To evaluate factors related to social functional remission, we analyzed data from a previously conducted clinical trial with long-acting injections (EudraCT registration number: 2011-004889-15)21 data evaluating paliperidone palmitate. To clarify the relationship between symptomatic improvement and the social functional remission: the relationship between early symptom responses to treatment with a long-acting injection of paliperidone palmitate and social functional remission and which domain of symptomatic improvements, such as positive and negative symptoms, contributes to social functional outcome.
Patients and Methods
Subjects
This study is an explanatory post hoc analysis of the previously reported clinical trial.21 Therefore, the subjects of this study are essentially the same as in the previous study.21 Adult schizophrenia patients (men and women, aged 18–70 years) based on the Diagnostic and Statistical Manual of Mental Disorders (4th Edition, DSM-IV), with a total Positive and Negative Syndrome Scale (PANSS) score22 between 70 and 120 at baseline screening and worsening of symptoms were enrolled.21 The following are major exclusion criteria: 1) active DSM-IV diagnosis other than schizophrenia; 2) significant risk of suicidal behavior; 3) history of substance dependence within six months before screening; 4) involuntary status in a psychiatric hospital at screening; or history of neuroleptic malignant syndrome, tardive dyskinesia, any unstable or significant medical or neurological illness; 5) morbid obesity (BMI >40 kg/m2), or other systemic disease; 6) mental retardation; and 7) risk factors for prolonged QT interval, torsade de pointes, or sudden death. Patients with a history of intolerability, hypersensitivity, or lack of response to risperidone or paliperidone were also excluded from the study.21 More details can be found in previous papers.21 An independent ethics committee or institutional review board at each study site approved the study protocol (listed in Supplement File). The study was conducted in accordance with the ethical principles originating in the Declaration of Helsinki and International Conference on Harmonization. All patients or representatives provided written informed consent.
Study Design
The study design is also the same as in the previous study.21 The study consists of four phases: screening (up to three weeks), open-label (OL) stabilization study (17 weeks, flexible dosing), double-blind (DB) study (48 weeks, fixed dosing), and follow-up study.21 During screening, patients underwent a washout of disallowed psychotropic medications and oral tolerability test. In the OL phase, all patients were treated with PP1M for 17 weeks. Patients who were clinically stable at weeks 14 and 17 (defined as a total PANSS score of ≤70 and a PANSS, Clinical Global Impression-Severity [CGI-S] score decrease of ≥1 point from OL baseline) were randomized to receive a fixed dose of PP3M or PP1M and entered a DB phase.21 More details can be found in the previous papers.21
Definition of Social Functional Remission
Personal and social performance scale (PSP) was applied to evaluate social function,23 and social functional remission was defined as participants who scored PSP >7024 at the end of 65 weeks.
Statistical Analyses
Differences of demographic data between participants with social functional remission and participants without remission were tested by Chi-squared test for categorical data or two sample t-test for continues variables. To identify variables that explain social functional remission, logistic regression analysis was performed. Variance inflation factor (VIF) was used to check for multicollinearity. The factors associated with social functional remission were regressed on patient’s demographic characteristics such as gender, duration of illness, and type of schizophrenia (Catatonic, Disorganize, Paranoid, Residual, and Undifferentiated), caregiver involvement at baseline assessed by involvement evaluation questionnaire (IEQ)25 and early response to antipsychotic treatment. Since there was a significant correlation between duration of illness and age, we chose duration of illness rather than age as the variable for logistic regression analysis to avoid multicollinearity. Early response to antipsychotic treatment was defined as percentage-change between baseline and one week after treatment and percentage-change between baseline and one week after treatment in sub-sores of PANSS: PANSS general, PANSS positive, and PANSS negative. Since social function at baseline (baseline PSP scores) was expected to be highly associated with the future social functional remission, the above-mentioned logistic regression models including baseline PSP score were performed separately to evaluate sensitivity and independency of other factors.
Results
Baseline Demographics and Clinical Characteristics
We analyzed 428 patients in which the PSP and the IEQ at the time of the OL base line and the final evaluation of PSP at 65 weeks were recorded. Patient characteristics of each analytical group are summarized in Table 1. One hundred out of 428 patients showed social functional remission at the end of the observation period. Age, duration of illness, baseline PSP scores, and baseline PANSS negative scores were significantly different patients with remission from those without remission. The remission group was younger than the no remission group (mean ± SD age of the remission group was 36.08 ± 11.11, meanwhile the no remission group was 38.91 ± 11.97, p=0.03, two sample t test), had a shorter duration of illness (mean ± SD duration of illness of the remission group was 8.53 ± 7.85, meanwhile the no remission group was 11.06 ± 9.33, p=0.03, two sample t test), and had a higher baseline PSP score than the no remission group (mean ± SD baseline PSP score; the remission group was 54.25 ± 11.30 and the no remission group was 48.48 ± 10.38. p<0.0001 tested by two sample t-test). Regarding schizophrenic symptoms evaluated by PANSS, the remission group had significantly lower PANSS negative scores at baseline than the no remission group. There was no significant difference in total and subscale of PANSS score at one week and percentage changes of those at one week, however, the percentage change of PANSS positive scores at one week showed a trend level of difference (p=0.079) (Table 1). At the end of 65 weeks, the remission group had a significantly lower total and subscales of PANSS score than the no remission group (Table 1). Other baseline characteristics and treatment assignment during the randomized phase (PP1M or PP3M) appeared similar in both analytical groups.
 |
Table 1 Demographic Data of Analyzed Subjects |
Factors Associated with Social Functional Remission
The baseline PSP score of patients with subsequent social functional remission was 47.75 points in the first quartile, 55.00 points in the median, and 63.00 points in the third quartile, respectively (Table 2). On the other hand, the baseline PSP score of patients without subsequent social functional remission was 42.00 points in the first quartile, 48.00 points in the median, and 55 points in the third quartile, respectively (Table 2). This suggests that patients with higher baseline PSP scores are more likely to achieve social and functional remission than those with lower baseline PSP scores. To evaluate sensitivity and independency of other factors, we separately performed two logistic regression models including baseline PSP score and without baseline PSP.
 |
Table 2 Distribution of Baseline PSP Scores |
The results of the logistic regression analysis excluding baseline PSP scores are shown in Table 3. None of the VIF values were up to 7, indicating that multicollinearity in the following logistic regression model is not evident. A logistic regression analysis by using percentage changes of PANSS score at one week as early treatment response showed that the duration of illness (p=0.005), baseline score of supervision evaluated by IEQ (p=0.006), type of schizophrenia (residual type, p=0.018), and an improvement positive score of PANSS at one week (p=0.047) were significantly associated with the socially functional remission (Table 3).
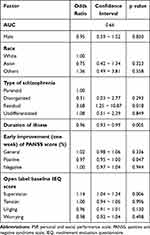 |
Table 3 Results of Logistic Regression Analysis without Baseline PSP Scores |
Results of logistic regression analyses including baseline PSP scores show Table 4. Logistic regression analysis by using percentage changes of PANSS score at one week as early treatment response showed that duration of illness (p=0.010), baseline score of supervision evaluated by IEQ (p=0.006), and baseline PSP score (p<0.001) were significantly associated with the socially functional remission (Table 4). Regarding type of schizophrenia, residual type showed trend level of association with social functional remission (p=0.059). Whereas percentage change of PANSS score at one week were not associated with social functional remission.
 |
Table 4 Results of Logistic Regression Analysis with Baseline PSP Scores |
Discussion
The current post hoc analyses were aimed at determining the factors associated with subsequent remission of social function (PSP >70 at 65w) in patients with schizophrenia treated with long-acting injection of Paliperidone palmitate. The PSP scale evaluates an entire array of socially useful activities, personal and social relationships, self-care, and disturbing and aggressive behavior of patients.23 We identified that shorter duration of illness, higher baseline score of supervision evaluated by IEQ and higher baseline PSP were significantly associated with the social functional remission. Although early improvement of schizophrenia symptoms evaluated with PANSS was not associated with subsequent social functional remission when baseline PSP scores were treated as a predictive variable, improvement of positive subscale of PANSS at one week was significantly associated with subsequent social functional remission when baseline PSP scores were excluded from predictive variables. Regardless of predictive models, shorter duration of illness was significantly associated with social functional remission. Type of schizophrenia also showed relatively robust association with social functional remission. Residual type of schizophrenia showed trend level of association with social functional remission (p=0.059) when baseline PSP scores were included as predictive variables. When baseline PSP scores were excluded from predictive variables, residual type schizophrenia was significantly associated with social functional remission. Regardless of the predictive model, gender and race were not associated with social functional remission.
In our analyses, the most important predictive factor for social functional remission were baseline social function. It should be plausible that patients with better social function at the beginning of treatment showed subsequent social functional remission. Indeed, our previous study also showed baseline PSP score was associated with functional outcome.26 Except the social function at the beginning of the treatment, duration of illness was a robust factor related to social functional remission. A previous review documented that duration of illness influences treatment response, suicide risk and loss of social functioning in schizophrenic patients.27 Regarding social functioning, a three-year follow–up study reported that having a shorter duration of illness is significantly associated with remission28,29 and functional outcomes in schizophrenia.30–32 Another study of PP1M also showed an association between shorter duration of illness and better outcomes.26 A meta-analysis of social cognition found that greater deficits in emotion processing were associated with longer duration of illness.33 In line with those previous studies, our analysis also demonstrated an association between duration of illness and social functional outcome. Several magnetic resonance imaging studies demonstrated brain morphological changes, especially reduced grey matter volume in the prefrontal cortex, associated with a long duration of illness.34 A recent meta-analysis reported that that individuals with schizophrenia who had greater whole brain and front-limbic volumes had better functional outcomes.35 These data would explain underlining neurobiological mechanisms of association with duration of illness and social functional outcomes. Another robust factor association with social functional remission was type of schizophrenia. In this study, residual type of schizophrenia was associated with social functional remission. A previous study exploring predictors of relapse and rehospitalization in schizophrenia and schizoaffective disorders revealed that the diagnosis of residual type related to a lower relapse rate.36 However, number of residual type patients was small in the present study, we have no clear explanation for this association at present.
It is not as robust as duration of illness and type of schizophrenia; however early symptomatic improvement was also partially associated with social functional remission. In our analyses, early improvement of positive symptoms (one week after start of the treatment) was associated with subsequent social functional remission. Univariate analysis showed no significance between remission group and no remission group, however, early improvement in PANSS positive scale showed trend level of difference between two groups. Identification of factors related to treatment response influencing subsequent functional outcomes should be important, because it might facilitate informed decision–making for future therapy, especially in ineffective cases. Although antipsychotics have been considered to have no direct effects on social functioning, several studies have demonstrated associations between symptomatic improvement caused by antipsychotic treatment and functional outcomes including social function.1–4,11–19,26 Indeed, the present study showed a social remission group had significantly lower PANSS score than a no remission group at the end of 65 weeks. This suggests that symptomatic improvement and maintenance should be important for functional outcome. A previous study of PP1M showed PANSS reduction at five weeks was a strong factor associated with favorable response in clinical outcomes of symptoms and function.26 In addition to the previous PP1M study, our study demonstrated association between reduced PANSS positive at one week and social functional remission. These results suggested that early symptomatic improvement should contribute to social functional outcomes. Similar to the previous study, our real-world study with oral antipsychotics also showed an association between early improvement of positive symptoms and better social functional outcomes.19 An analysis of 12 studies also identified early symptomatic improvement as one of the predictors of symptomatic remission.37 Although negative symptoms and cognitive function have been considered to be related to social function, a 10-year longitudinal study demonstrated a significant relationship between psychosis and increased impairment of work functioning.38 The author of the 10-year study considered that one of the primary reasons for association between work difficulties and psychosis included “distrust of other people”. Such a feature of psychosis (positive symptoms) should deteriorate social functioning.38 Some studies exploring the association between early improvement and later outcomes have used atypical antipsychotics, such as risperidone, olanzapine, and paliperidone17,19,37 On the other hand, studies using both typical and atypical antipsychotics have reported associations between early symptomatic improvement, regardless of whether the patients were treated by typical or atypical antipsychotics.7,8,10,37 Taken together, we think that early symptomatic improvement including improvement of positive symptoms could be a predictor of symptomatic remission but also be a predictor of functional remission. The American Psychiatric Association suggested that patients receive treatment with a LAI antipsychotic medication if they prefer such treatment or if they have a history of poor or uncertain adherence.39 However, some studies suggest that long-acting injectable antipsychotics (LAI) should be considered earlier in therapy.20,40 In such a situation, not only maintenance of symptomatic improvement but also early symptomatic improvement should be required for LAI. A previous study of PP1M demonstrated early symptomatic improvement (within a few days).41 Pharmacokinetic studies demonstrated that PP1M achieves therapeutic, steady-state plasma levels rapidly on initiation without oral antipsychotics supplementation.42,43 Such pharmacokinetics may explain early symptomatic improvement observed with PP1M therapy. These data suggested a relevance of earlier use of LAI in therapy. However, our results suggested that predetermined factors such as baseline social functioning, duration illness and type of schizophrenia should contribute more to social functional remission rather than effects of pharmacological intervention. Although current antipsychotics are highly effective against positive symptoms, they are considered to have limited direct effects on functional outcomes including social function. Development of new drugs that are more effective for social functions should be warrant. In this analysis, we also identified that a higher baseline score of supervision evaluated by IEQ was significantly associated with social functional remission. The role of the caregiver is important in chronic diseases; however, of the many studies in patients with schizophrenia, relatively few focus on the influence of caregiver burden on therapy. At present, we have no clear explanation for the association between higher baseline scores of supervision and social functional remission. One possible explanation is that patients having higher scores of supervision could be protected from excessive alcohol consumption and taking illegal drugs (these items are included items for scoring of supervision). A previous study demonstrated that comorbid substance use disorder had negative impacts on both symptomatic and functional remission.7 However, patients with a history of drug dependence within six months prior to screening were excluded in this study, the relationship between high-scoring supervision and protection from drug use does not seem plausible to draw from the present study.
Finally, we should mention some limitations of the analysis. The study consisted of four phases: screening (up to three weeks), OL stabilization (17 weeks, flexible doses), DB (48 weeks, fixed doses), and a follow-up phase. Only clinically stable patients at weeks 14 and17 then entered the DB phase. For analyses exploring relationship between symptomatic improvement and subsequent functional outcomes, such a study design could be a source of selection bias. Because unstable subjects were excluded from analyses. We consider that such a selection bias could be related relatively lesser robust association with small effect size between symptomatic improvement and subsequent social functional remission. In addition, such a selection bias should be interpreted with caution when applying the present results to schizophrenia in general population. For example, the social functional remission rate in the present study may be overestimated due to the survival bias.
Conclusion
We found that shorter duration of illness, residual type of schizophrenia, higher baseline score of supervision, and higher baseline social functioning were predictive variables of subsequent social functional remission. Although its effect seems to be limited, early symptomatic improvement could could be a predictor of social functional remission.
Data Sharing Statement
The data set used is owned by Janssen pharmaceutical company and data sharing is restricted. The authors may not make their study data available at the time of publication, but in accordance with the Neuropsychiatric disease and Treatment Availability Policy, they promise to provide all authors with access to the data they request that form the basis of the findings described in this study. However, in accordance with the Neuropsychiatric Disease and Treatment Availability Policy For further information, please contact https://yoda.yale.edu/.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This clinical trial was funded by Janssen Pharmaceutical K.K. of Johnson & Johnson in Japan.
Disclosure
All authors have disclosed that they are full-time employees of Janssen Pharmaceutical K.K. of Johnson & Johnson in Japan. The authors report no other conflicts of interest in this work.
References
1. Nasrallah HA, Targum SD, Tandon R, et al. Defining and measuring clinical effectiveness in the treatment of schizophrenia. Psychiatr Serv. 2005;56:273–282. doi:10.1176/appi.ps.56.3.273
2. Yeomans D, Taylor M, Currie A, et al. Resolution and remission in schizophrenia: getting well and staying well. Adv Psychiatr Treat. 2010;16:86–95. doi:10.1192/apt.bp.108.006411
3. Lieberman JA, Malaspina D, Jarskog LF. Preventing clinical deterioration in the course of schizophrenia: the potential for neuroprotection. CNS Spectr. 2006;11(Suppl 4):1–13.
4. Larnbert M, Naber D. Current issues in schizophrenia: overview of patient acceptability, functioning capacity and quality of life. CNS Drugs. 2004;18:1–13.
5. Duțescu MM, Popescu RE, Balcu L, et al. Social functioning in schizophrenia clinical correlations. Curr Health Sci J. 2018;44(2):
6. Haro JM, Novick D, Suarez D, et al. Predictors of the course of illness in outpatients with schizophrenia: a prospective three year study. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1287–1292.
7. Lambert M, Schimmelmann BG, Naber D, et al. Prediction of remission as a combination of symptomatic and functional remission and adequate subjective well-being in 2960 patients with schizophrenia. J Clin Psychiatry. 2006;67(11):
8. Spellmann I, Riedel M, Schennach R, et al. One-year functional outcomes of naturalistically treated patients with schizophrenia. Psychiatry Res. 2012;198(3):378–385. doi:10.1016/j.psychres.2011.12.047
9. Schennach-Wolff R, Jäger M, Seemüller F, et al. Defining and predicting functional outcome in schizophrenia and schizophrenia spectrum disorders. Schizophr Res. 2009;113(2–3):210–217.
10. Lambert M, Naber D, Schacht A, et al. Rates and predictors of remission and recovery during 3 years in 392 never-treated patients with schizophrenia. Acta Psychiatr Scand. 2008;118(3):220–229. doi:10.1111/j.1600-0447.2008.01213.x
11. Mortimer AM. Symptom rating scales and outcome in schizophrenia. Br J Psychiatry Suppl. 2007;50:S7–S14. doi:10.1192/bjp.191.50.s7
12. European Medicines Agency. Guideline on clinical investigation of medicinal products, including depot preparations in the treatment of schizophrenia. Available from:http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/10/WC500133437.pdf.
13. Lehman AF. Developing an outcomes-oriented approach for the treatment of schizophrenia. J Clin Psychiatry. 1999;60(Suppl 19):S30–S35.
14. Andreasen NC, Carpenter WT
15. Lipkovich IA, Deberdt W, Csernansky JG, et al. Defining “good” and “poor” outcomes in patients with schizophrenia or schizoaffective disorder: a multidimensional data-driven approach. Psychiatry Res. 2009;170:161–167. doi:10.1016/j.psychres.2008.09.004
16. Ascher-Svanum H, Nyhuis AW, Faries DE, et al. Clinical, functional and economic ramifications of early nonresponse to antipsychotics in the naturalistic treatment of schizophrenia. Schizophr Bull. 2008;34(6):1163–1171. doi:10.1093/schbul/sbm134
17. Kinon BJ, Chen L, Ascher-Svanum H, et al. Early response to antipsychotic drug therapy as a clinical marker of subsequent response in the treatment of schizophrenia. Neuropsychopharmacology. 2010;35:581–590. doi:10.1038/npp.2009.164
18. Leucht S, Shamsi SA, Busch R, et al. Predicting antipsychotic drug response - replication and extension to six weeks in an international olanzapine study. Schizophr Res. 2008;101(1–3):312–319. doi:10.1016/j.schres.2008.01.018
19. Nakagawa R, Ohnishi T, Kobayashi H, et al. The social functional outcome of being naturalistically treated with paliperidone extended-release in patients with schizophrenia. Neuropsychiatr Dis Treat. 2015;22(11):1511–1521.
20. Gorwood P, Bouju S, Deal C, et al. Predictive factors of functional remission in patients with early to mid-stage schizophrenia treated by long acting antipsychotics and the specific role of clinical remission. Psychiatry Res. 2019;281:112560. doi:10.1016/j.psychres.2019.112560
21. Savitz AJ, Xu H, Gopal S, et al. Efficacy and safety of paliperidone palmitate 3-month formulation for patients with schizophrenia: a randomized, multicenter,double-blind, noninferiority study. Int J Neuropsychopharmacol. 2016;19:7. doi:10.1093/ijnp/pyw018
22. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):
23. PSP Scale. In: Michalos AC, editor. Encyclopedia of Quality of Life and Well-Being Research. Dordrecht: Springer; 2014.
24. Pinna F, Tusconi M, Bosia M, Cavallaro R, Carpiniello B; Cagliari Recovery Group Study. Criteria for symptom remission revisited: a study of patients affected by schizophrenia and schizoaffective disorders. BMC Psychiatry. 2013;26(13):235.
25. van Wijngaarden B, Schene AH, Koeter M, et al. Caregiving in schizophrenia: development, internal consistency and reliability of the Involvement Evaluation Questionnaire–European Version. EPSILON Study 4. European psychiatric services: inputs linked to outcome domains and needs. Br J Psychiatry Suppl. 2000;(39):
26. Li N, Feng Y, Lu H, et al. Factors related to improvement of symptoms, function, and caregiver burden in Chinese patients with schizophrenia after switching to paliperidone palmitate once-monthly from oral antipsychotics. Neuropsychiatr Dis Treat. 2018;14:
27. Altamura AC, Serati M, Buoli M. Is duration of illness really influencing outcome major psychoses? Nord J Psychiatry. 2015;69(6):
28. Haro JM, Novick D, Suarez D, et al. Remission and relapse in the outpatient care of schizophrenia: three-year results from the Schizophrenia Outpatient Health Outcomes study. J Clin Psychopharmacol. 2006;26(6):
29. Haro JM, Novick D, Suarez D, Ochoa S, Roca M. Predictors of the course of illness in outpatients with schizophrenia: a prospective three year study. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(5):
30. Siegel SJ, Irani F, Brensinger CM, et al. Prognostic variables at intake and long-term level of function in schizophrenia. Am J Psychiatry. 2006;163(3):
31. Schennach-Wolff R, Jäger M, Seemüller F, et al. Defining and predicting functional outcome in schizophrenia and schizophrenia spectrum disorders. Schizophr Res. 2009;113(2–3):
32. Suresh KK, Kumar CN, Thirthalli J, et al. Work functioning of schizophrenia patients in a rural south Indian community: status at 4-year follow-up. Soc Psychiatry Psychiatr Epidemiol. 2012;47(11):
33. Savla GN, Vella L, Armstrong CC, Penn DL, Twamley EW. Deficits in domains of social cognition in schizophrenia: a meta-analysis of the empirical evidence. Schizophr Bull. 2013;39(5):
34. Altamura AC, Buoli M, Serati M. Duration of illness and duration of untreated illness in relation to drug response in psychiatric disorders. Neuropsychiatry. 2011;1(1):81–90. doi:10.2217/npy.10.2
35. Wojtalik JA, Smith MJ, Keshavan MS, Eack SM. A systematic and meta-analytic review of neural correlates of functional outcome in schizophrenia. Schizophr Bull. 2017;43(6):
36. Doering S, Müller E, Köpcke W, et al. Predictors of relapse and rehospitalization in schizophrenia and schizoaffective disorder. Schizophr Bull. 1998;24(1):
37. Lambert M, Karow A, Leucht S, Schimmelmann BG, Naber D. Remission in schizophrenia: validity, frequency, predictors, and patients’ perspective 5 years later. Dialogues Clin Neurosci. 2010;12(3):
38. Racenstein JM, Harrow M, Reed R, Martin E, Herbener E, Penn DL. The relationship between positive symptoms and instrumental work functioning in schizophrenia: a 10 year follow-up study. Schizophr Res. 2002;56(1–2):
39. Keepers GA, Fochtmann LJ, Anzia JM, et al. The American Psychiatric Association Practice guideline for the treatment of patients with schizophrenia (Systematic Review). Am J Psychiatry. 2020;177(9):868–872. doi:10.1176/appi.ajp.2020.177901
40. Llorca PM, Abbar M, Courtet P, Guillaume S, Lancrenon S, Samalin L. Guidelines for the use and management of long-acting injectable antipsychotics in serious mental illness. BMC Psychiatry. 2013;13:340. doi:10.1186/1471-244X-13-340
41. Alphs L, Bossie CA, Sliwa JK, Ma YW, Turner N. Onset of efficacy with acute long-acting injectable paliperidone palmitate treatment in markedly to severely ill patients with schizophrenia: post hoc analysis of a randomized, double-blind clinical trial. Ann Gen Psychiatry. 2011;10(1):12. doi:10.1186/1744-859X-10-12
42. Coppola D, Liu Y, Gopal S, et al. A one-year prospective study of the safety, tolerability and pharmacokinetics of the highest available dose of paliperidone palmitate in patients with schizophrenia. BMC Psychiatry. 2012;12:26. doi:10.1186/1471-244X-12-26
43. Si T, Su Y, Liu Y, et al. Pharmacokinetics and tolerability of paliperidone palmitate injection in Chinese subjects. Hum Psychopharmacol. 2014;29(2):
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
