Back to Journals » Journal of Pain Research » Volume 16
Early Functional Outcomes in Low Back Pain Subjects with a Novel Interspinous Fusion Device: REFINE Study 6-Month Results
Authors Falowski SM , Raso LJ, Mangal V, Nairizi A, Patterson DG , Danko MD, Justiz R, Vogel RS, Koga S , Josephson Y, Pope JE, Raji OR
Received 21 July 2023
Accepted for publication 14 November 2023
Published 1 December 2023 Volume 2023:16 Pages 4113—4126
DOI https://doi.org/10.2147/JPR.S427407
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Krishnan Chakravarthy
Steven M Falowski,1,* Louis J Raso,2 Vipul Mangal,3 Ali Nairizi,4 Denis G Patterson,5 Michael D Danko,6 Rafael Justiz,7 Rainer S Vogel,8 Sebastian Koga,9 Yousseff Josephson,10 Jason E Pope,11,* Oluwatodimu Richard Raji12
1Argires-Marotti Neurosurgical Associates of Lancaster, Lancaster, PA, USA; 2Jupiter Medical Center, Jupiter, FL, USA; 3National Spine and Pain Centers, Oxon Hill, MD, USA; 4Reno Tahoe Pain Associates, Reno, NV, USA; 5Nevada Pain Specialists, Reno, NV, USA; 6Premier Pain Treatment Institute, Loveland, OH, USA; 7Oklahoma Pain Physicians, Oklahoma City, OK, USA; 8Comprehensive and Interventional Pain Management, Henderson, NV, USA; 9Koga Neurosurgery, Covington, LA, USA; 10National Spine and Pain Centers, Voorhees, NJ, USA; 11Evolve Restorative Center, Santa Rosa, CA, USA; 12Medical Device Development, San Francisco, CA, USA
*These authors contributed equally to this work
Correspondence: Oluwatodimu Richard Raji, Medical Device Development, 2390 Mission Street, Suite 8, San Francisco, CA, 94110, USA, Tel +15105707741, Email [email protected]
Purpose: Lumbar interlaminar decompression with interspinous fixation is an established safe and effective treatment for spinal stenosis. Early maintenance of improvements in pain intensity and function are critical for durability of symptom relief. The purpose of this study was to investigate the efficacy of minimally invasive treatments for low back pain during the early period after treatment and their utility in setting the course for longer term success.
Patients and Methods: This study utilized patient evaluations at 3- and 6-months following treatment and is part of an actively enrolling, institutional review board (IRB) approved, single-arm, multicenter, prospective, open-label 12-month study. Clinical efficacy was assessed primarily using the change from baseline in Oswestry Disability Index (ODI), Visual Analog Scale (VAS) of the back and leg pain during walking and standing, and Zurich Claudication Questionnaire (ZCQ), and secondarily using the Patient Global Impression of Change (PGIC) and Patient-Reported Outcomes Measurement Information System (PROMIS) 29 v2.1. The safety endpoints were the adverse events and reoperations or revisions at the index level(s).
Results: At 6-month post-op, 76%, 62%– 64%, and 64% of patients demonstrated clinical meaningful, and statistically significant improvement in their pain as defined by ZCQ, VAS (back and leg), and ODI, respectively. In addition, 78% of patients noted improvement in PGIC. Two procedure-related adverse events were noted which fully resolved without surgical intervention.
Conclusion: This 6-month interim analysis at 42% enrollment of patients was conducted to determine prolonged safety and efficacy of the interspinous fusion device. Our analysis showed a sustained improvement in clinical efficacy, and safety endpoints, when compared to the 3-months evaluations, across both interventional pain and neurosurgery specialties.
Keywords: interspinous fixation, degenerative disc disease, neurogenic claudication, spinal stenosis
Introduction
Lumbar interlaminar decompression with interspinous fixation is an established safe and effective treatment for spinal stenosis. There are many reports on the longer term success of the procedure compared to nonoperative treatments, decompressive laminectomies, and interbody fusion. The landmark SPORT trial established longer term efficacy in spinal stenosis patients with decompressive laminectomy.1 The study also establishes early improvement trajectory as a prognosticator for longer term success. In the SPORT trial, both group’s improvements were significantly related to the initial trajectory after treatment. In other words, if the patient improved substantially immediately after treatment they tended to stay significantly improved. Two other investigations of decompression supplemented with interspinous fixation for spinal stenosis patients exhibited this same trend of early improvement predicting longer term success.2,3
Several important distinctions are appreciated in the longitudinal analysis of these lumbar stenosis patient outcomes after minimally invasive treatments. The first distinction is the treatment’s initial trajectory of success. Greater early improvement forecasts longer term success, starting as early as 3 months.2 The second distinction is the maintenance, diminishment, or continued improvement in the success of the procedure after this early period. For example, the SPORT trial improvements diminished slightly over four years; the Schmidt trial improvements were maintained over two years; and the Kumar trial improvements increased over five years.1–3 Both distinctions are related to an important but unmet need: early maintenance of improvements in pain intensity and function are critical for durability of symptom relief. The long-term efficacy of minimally invasive treatments for low back pain is somewhat understood; however, very little information exists on the early period after surgery and its ability to set the course for long-term success.
In addition to the aforementioned studies on the interspinous spacers, there are biomechanical investigations have been conducted on the stability of both fusion and non-fusion interspinous devices, which indicate increased range of motion and destabilization associated with non-fusion interspinous spacers.4–6 There are, however, few studies which have assessed the safety, clinical efficacy, and fusion results of standalone interspinous fixation and fusion with bone graft 84% to 92%.7,8 These studies showed fusion in 84% to 92% of cases, in addition to 52%, 64%, and 80% reduction in ODI, NRS back and NRS leg in patients with degenerative spondylolisthesis. While the Schmidt study showed a 57.5% reduction in ODI, only 22% of patients presented with degenerative spondylolisthesis.2 Thus, there are no studies which currently report safety and efficacy in similar cohorts between fusion and non-fusion interspinous fixation construct.
This study utilized the ZIPTM MIS Interspinous Fusion System (ZIP) by Aurora Spine (Carlsbad, CA) and bone graft material. The ZIP system provides an alternative to conservative treatment for patients suffering from DDD (defined as back pain of discogenic origin with degeneration of the disc confirmed by history and radiographic studies), spondylolisthesis, spinal stenosis, trauma, and/or tumor.9 The system utilizes a hollow central barrel which serves as a graft chamber, and interlaminar spacer to resist compressive motions such as extension. Superior and inferior spike features on bilateral locking plates connected by the barrel, serve to rigidly engage the spinous processes of the posterior noncervical spine (T1–S1) from an interlaminar approach, thus resisting distraction motions such as flexion. This biomechanical stabilization of the spinal column facilitates indirect decompression, and bony fusion when used in conjunction with autologous bone grafting via surgical decortication of the posterior elements and the use of exogenous allograft.
The current study reports 6-month outcomes from a prospective, non-randomized, multicenter study of patients with chronic low back pain with lower extremity symptoms, which present with degenerative disc disease (DDD) and concurrent neurogenic claudication. The specific aims of the study were to evaluate the effectiveness and safety of the interspinous fusion device and bone graft material in single or two-level DDD patients.
We anticipate the clinical efficacy and safety will be comparable to published studies on the minimally invasive interspinous devices. Future data will evaluate the rate of fusion resulting from rigid interlaminar fixation with the ZIP system, which is expected to be comparable to pedicle screw fixation.
Materials and Methods
Designs and Sites
This prospective, observational, open-label, non-randomized, multicenter study was performed under Western IRB approval (#20211168). The protocol and IRB were each also approved at each of the participating sites by the local governing entity. Clinical follow-up data was collected up to 6-months post operation on patients undergoing interspinous interlaminar fusion with bone graft performed on an ambulatory basis by neurosurgeons, orthopedic surgeons, and interventional pain physicians. Voluntary written informed consent was provided by each enrolled subject. Subjects were allowed to ask questions and were given a copy of the informed consent. Subject data was de-identified for confidentiality and compliance per the Declaration of Helsinki.
Patient Population
Patients above the age of 18 years were recruited from participating sites, and enrolled in the study, if they met all the inclusion and none of the exclusion criteria. Inclusion criteria included the presence of one or two symptomatic lumbar degenerative disc disease(s) at single or adjacent levels between T1 and S1, mild to moderate spinal stenosis at the index level on MRI, with or without grade I spondylolisthesis, and the presence of neurogenic claudication.10,11 Patients must have completed at least 6 months of non-operative treatment, having a physical function Zurich Claudication Questionnaire (ZCQ) ≥2.0 at baseline as assessment, baseline Visual Analog Scale (VAS) ≥50 mm (100-mm scale). Key exclusion criteria included prior lumbar spine surgery, and ≥ grade II spondylolisthesis on flexion and extension films with 3-mm instability.
Interventions
Upon enrollment, interspinous fixation was performed on the patient in the prone position using the ZIP device as has been previously reported.12 Final device placement, is as shown in Figure 1. Commercial or government payer insurance authorization was received prior to the prior to the procedure.
 |
Figure 1 Image of the Device and its Placement in the Spine. AP Radiograph of the Deployment of the Device. |
Follow-Up
Imaging and history of conservative treatment were reviewed prior to enrollment, to ensure recruited patients met the inclusion criteria as previously described. The goal of enrollment with 60 months of potential follow-up was set at 100 patients. All enrolled patients were approved by the medical monitor. Upon implantation, patients were followed-up immediately postoperatively as per standard of care by site, with scheduled visits occurring at 3 and 6 months.
Endpoints
Baseline demographic information and procedural detail were captured.
Primary
Clinical efficacy was evaluated via functional and neurological outcome measures. Meaningful improvements from baseline were defined as ≥20 mm pain reduction in VAS Back during walking or standing, ≥20 mm pain reduction in VAS Leg during walking or standing, ZCQ improvement ≥0.5 in two or three domains, Oswestry Disability Index (ODI) improvement ≥10 points and no reoperations or revisions at the index level(s). Safety was evaluated via the number of adverse events (infection, bleeding, worsening pain, and hardware malfunction) and their likelihood of relation to the device or procedure, were monitored through the study, as well as reoperation or revisions at the index level.
The ODI questionnaire quantifies the level to which back or leg pain affects the patient’s ability to conduct daily physical activity, as a measure of permanent functional disability.13 This quantification is assessed across 10 sections, each with a total possible score of 5. The ODI is reported as a percentage of the total possible score (Table 1). The ZCQ is self-administered post-operatively, by lumbar spinal stenosis (LSS) patients. It entails 12 questions to evaluate symptom severity (I–VII), and physical function (VIII–XII), and 6 questions to evaluate overall satisfaction, over the prior month.
 |
Table 1 ODI Assessment |
Secondary
Multidimensional pain and function assessment including opioid consumption related to study related pain, health-care consumption, global impression of change relative to baseline at 6 months (PGIC), Patient Reported Outcome Measurement Information System (PROMIS) 29 v2.1, and Pain Impact Score (PIS) (calculated from the PROMIS 29), were utilized as secondary endpoints.
The PROMIS 29 is a validated 29-item profile instrument that assesses eight universal domains. Seven of these non-disease specific domains, ie, anxiety, physical function, fatigue, depression, ability to participate in social roles and activities, sleep disturbance, and pain interference, are assessed with four questions each.14–19 The final domain of pain intensity utilizes a single 11-point numeric rating scale (NRS) from 0 to 10 (no pain to worst imaginable pain). High scores represent more of the domain being measured. Symptom-oriented domains such as anxiety, fatigue, depression, sleep disturbance, and pain interference, are negatively worded, with higher scores representing worse symptomatology. Function-oriented domains such as physical function and social participation are positively worded with higher scores representing better functioning. The PIS is a derivative of the PROMIS 29, calculated by adding the raw scores for pain intensity from 0 to 10 and pain interference from 4 to 20 along with the inverted raw score for physical function from 4 to 20. It ranges from 8 to 50 (low to high impact).
Statistical Analysis
Statistical analysis was performed utilizing IBM SPSS statistics (Chicago, Illinois). Sample sizes were calculated assuming a 5% two-sided type 1 error rate and 85% power for a one-sided non-inferiority test. The ZIP MIS group will result in clinically relevant improvements in PROMIS score if the lower bound of the 95% confidence interval does not overlap 3.5. Ninety subjects were required (5% type 1 error rate, 85% power). The sample size was increased by 10% to allow for possible attrition. This gives a sample size of 99 which was rounded up to a total of 100 study subjects. Quantitative primary and secondary outcome measures were compared to baseline using paired t-tests, with analysis of variance (ANOVA) regression utilized to identify and verify reported correlations.
Results
Upon IRB approval, enrollment began on March 21, 2021, and is currently ongoing. Twelve centers participated in this study, spanning interventional pain management and neurosurgery specialties.
Patient Population
Baseline and demographic characteristics are summarized in Table 2. Age (mean ± stdev) was 70.3 ± 11.7 years, with 62% of patients being female. Baseline VAS Back, VAS Leg, and ODI were 77, 72, and 48, respectively.
 |
Table 2 Baseline Characteristics of 42 Subjects Reporting on at 6 Months |
Patient Activity
This is an ongoing study reporting a 6-month interim analysis of safety and efficacy. Currently, there are 100 active patients, 31 are pending implant, 7 withdrew from the study after implant, and 69 are active and implanted. Forty-two are examined here, representing nearly half of the goal enrollment. See Figure 2.
 |
Figure 2 Study-related Activity. |
Surgical Information
Surgical information is shown in Table 3. The neurosurgery specialty accounted for 60% of all cases, with treatment being predominantly single-level, and performed mostly at L3-4 and L4-5. All patients returned home on the same day.
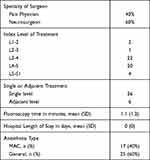 |
Table 3 Surgical Characteristics of 42 Subjects Reporting on at 6 Months |
Clinical Outcomes
Primary clinical outcomes are shown in Table 4. An average improvement of 34 was observed in VAS Back (Figure 3), while an average improvement of 38 was observed for VAS Leg (Figure 4). Mean ODI improvement was 15 (Figure 5), and improvement in mean ZCQ was noted in all 4 measures.
 |
Table 4 Pain and Functional Outcome Measures as Primary Endpoints at 6 Months |
 |
Figure 3 Tornado Plot of Improvement of VAS Score at 6 Months for Back. |
 |
Figure 4 Tornado Plot of Improvement of VAS Score at 6 Months for Leg. |
 |
Figure 5 Tornado Plot of Improvement of ODI Score at 6 Months. |
Secondary endpoints are shown in Table 5 and Table 6. There was statistically significant improvement in all PROMIS 29 v2.1 domains and 78% of patients on PGIC exhibited improvement at 6 months.
 |
Table 5 PROMIS 29 v 2.1 Table of Mean Improvement in Scores at 6 Months from Baseline |
 |
Table 6 PGIC Assessment for Patient Cohort and Interim Analysis at 6 Months from Baseline |
Safety Analysis
Safety Outcomes are shown in Table 7. One patient had post-operative bleeding that resolved after dressing re-application, another had worsening pain after surgery which resolved without surgical intervention, and a third patient had an exacerbation of symptoms of a known comorbidity. The episodes were controlled and completely resolved.
 |
Table 7 Adverse Events |
Discussion
This study presents a 6-month interim analysis, it is active and enrolling, with a goal of 100 patients at 3 and 12 months, and up to 5 years of planned follow-up. This analysis represents the first clinical evaluation of the safety and efficacy of a standalone interspinous fixation device for the treatment of degenerative disc disease in the presence of symptomatic spinal stenosis and neurogenic claudication. Other surgical treatments utilized to treat this condition include epidural injection, decompression with laminectomy, with or without fusion, percutaneous decompression, or interspinous spacer. The interspinous spacer (ISS) is a flexible interspinous device which aims to only decompress indirectly, while the interspinous fusion (ISF) implemented in this study decompresses and stabilizes the spine while facilitating fusion. Both options were presented to the patients during prior to surgical intervention.
The data represents significant improvement in pain, of 62% and 64% in VAS back and VA leg, respectively. The ZCQ improvement was 76% and at least 2 measures improved by 0.5, while at least 5-point improvement was demonstrated in all the seven domains in the PROMIS 29v 2.1, with 78% of patient demonstrating improvement in PGIC.
Regarding safety, 40% of these cases were done under MAC sedation and none required an overnight stay. Three adverse events were noted, all of which were resolved. One had increased pain at conclusion of the procedure, one had a hospitalization for exacerbation of multiple sclerosis, and had post-operative bleeding that resolved. The mortality event was unrelated to the procedure.
Three previous clinical trials included patient-reported outcomes in a similar population at early postoperative timepoints.1–3 The SPORT trial reported outcomes from decompressive laminectomies as early as 6 weeks. The Schmidt trial and Kumar trial reported results of decompressions accompanied by interspinous stabilization as early as 3 months.2,3 Pain improved in both populations by over 30 points in the early 3- to 6-month timeframe. Function improved by over 20 points in the same period. Afterwards, the improvements either remain consistent or increased slightly in the case of decompression with interspinous stabilization or the improvements diminished slightly in the case of decompression alone.1–3 Our results to date corroborate these findings, providing further evidence that minimally invasive techniques for low back pain are effective and safe. We observed pain intensity improvement of 33 points after 3 months and sustained improvement to 34 points after 6 months. We observed function improvement of 17 points after 3 months and similar improvement of 15 points after 6 months.
Limitations to the study include the relatively short term of follow up as long-term robustness has not yet been evaluated. Anesthesia use was not controlled by the protocol and was decided per patient, based on provider preference. No imaging was conducted during follow-up to evaluate structural changes, stability, or fusion. These will be evaluated at 12-month follow-up. This study was not randomized and only included patients who were approved for the procedure, being a single-arm, prospective study.
Conclusion
This 6-month interim analysis of 42% of the intended patients aimed to provide insight into the procedure’s safety and efficacy profile. Our analysis revealed that in a non-randomized design, and amidst varied anesthesia use, improvements in pain, function, and quality of life remained stable and clinically meaningful when compared to their first 90 days. Thus, suggesting sustained safety and efficacy across the interventional pain and neurosurgery specialties.
Data Sharing Statement
The clinical trial data of this article will not be shared.
Ethics Approval and Informed Consent
IRB approval was provided by Western IRB (IRB #20211168). All patient data collected were de- identified to provide patient data confidentiality and compliance with the Declaration of Helsinki. The protocol and IRB were each approved by the local governing entities of each involved institution. All enrolled subjects provided voluntary written informed consent to participate. Subjects were allowed to ask questions and were given a copy of the informed consent.
Acknowledgments
The authors would like to thank the study participants, as well as Eric Bruntlett, Kamren Murrell for their support in the project management and statistical analysis for this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
SMF serves as a consultant for Abbott, Medtronic, Saluda, VertiFlex, Vertos, Surgentec, CornerLoc, Mainstay and Relievant, has received grant for research funding from Mainstay, Relievant, Medtronic, Abbott, VertiFlex, Saluda, Nalu, CornerLoc, Aurora, Biotronik, and Stimgenics, and has an equity position in SynerFuse, Aurora Spine, Thermaquil. SPR Therapeutics, Saluda, CornerLoc, PainTEQ, Stimgenics, Anesthetic Gas Reclamation, Neural Integrative Solutions, SpineThera, and Celeri Health. LJR serves on the speakers Bureau for Boston Scientific, Aurora Spine, SurGen Tec, Vertiflex, and FloSpine. He is a Stockholder for Aurora Spine and receives Royalties from Flospine and SurGen Tec. VM serves as a consultant for Medtronic and Aurora and has received educational support from Medtronic, Abbott, Boston Scientific, Nevro, Vertos, Stimwave, Nalu, Aurora, Spinal Simplicity, SI Fix. AN serves as a consultant for Nevro, Flowonix, Boston Scientific, Surgentec, Aurora Pain and Spine; has received research support from Nevro, Corner Loc, Cardio Surgical Partners, Flowonix and Reno Tahoe Pain Associates. DP serves as a consultant for Abbott, AbbVie, AIS, Allergan, Amgen, Aurora Spine, CornerLoc, Flowonix, Lundbeck, Pajunk Medical, Saluda, Spark Biomedical, Vertos, has received grant and research support from Abbott, Flowonix, Nevro, Saluda and Vertiflex, serve on the Speaker Bureau or Honoraria for Abbott, Allergan, Amgen, CornerLoc, Lundbeck, Saluda, Vertos, and has equity in CornerLoc. MDD is a consultant for Medtronic, Abbott, and Aurora spine and has Stock Options at Aurora Spine. RJ serves as a consultant for Abbott and Medtronic and has research or grant support from Medtronic and Stimgenics. RSV serves as a consultant for Medtronic, Nevro, BSCI and Aurora Spine; teaching agreements with Medtronic, Nevro, BSCI, and Aurora Spine; research associated funding with Medtronic, Nevro, BSCI, PainTEQ, Aurora Spine, Vertos, and Vertiflex; Investments with Medtronic, Nevro and Aurora Spine. SK is a consultant for Aurora Spine Corp., Synaptive Medical Inc., and Osseus Spine; and a shareholder in Aurora Spine Corp. and Synaptive Medical Inc. YJ serves as a consultant for Medtronic, Aurora, Abbott, NALU, Sprint, and PainTEQ; research associated funding with Aurora, Abbott, Sprint, and PainTEQ. JEP serves as a consultant for Abbott, Medtronic, Saluda, Flowonix, SpineThera, Painteq, Vertos, Vertiflex, SPR Therapeutics, Tersera, Aurora, Spark, Ethos, Biotronik, Mainstay, WISE, Boston Scientific, Thermaquil; has received grant and research support from: Abbott, Flowonix, Saluda, Aurora, Painteq, Ethos, Muse, Boston Scientific, SPR Therapeutics, Mainstay, Vertos, AIS, Thermaquil; and is a shareholder of: PainTEQ, Vertos, SPR Therapeutics, Aurora, Spark, Celeri Health, Neural Integrative Solutions, Pacific Research Institute, Thermaquil and Anesthetic Gas Reclamation. He also reports a patent “DRG Neuromonitoring” licensed to NIS. ORR serves as a consultant for Aurora, PainTEQ, Alevio, Captiva, Synergy, Facet Dynamics, Zavation, 3Spine, Vyrsa, Spinal Simplicity, AlloSource, Olympus Terumo Biomaterials, OsteoCentric Technologies. The authors report no other conflicts of interest in this work.
References
1. Weinstein JN, Tosteson TD, Lurie JD., et al. Surgical versus non-operative treatment for lumbar spinal stenosis four-year results of the Spine Patient Outcomes: research Trial (SPORT). Spine. 2010;35(14):1329–1338. doi:10.1097/BRS.0b013e3181e0f04d
2. Schmidt S, Franke J, Rauschmann M, Adelt D, Bonsanto MM, Sola S. Prospective, randomized, multicenter study with 2-year follow-up to compare the performance of decompression with and without interlaminar stabilization. J Neurosurg Spine. 2018;28(4):406–415. doi:10.3171/2017.11.SPINE17643
3. Kumar N, Thomas AC, Rajoo MS, et al. Evaluating 5-year outcomes of interlaminar devices as an adjunct to decompression for symptomatic lumbar spinal stenosis. Eur Spine J. 2023;32(4):1367–1374. doi:10.1007/s00586-023-07610-x
4. Smith ZA, Vastardis GA, Carandang G, et al. Biomechanical effects of a unilateral approach to minimally invasive lumbar decompression. PLoS One. 2014;9(3):e92611. doi:10.1371/journal.pone.0092611
5. Hedman TP, Ohnmeiss DD, Leasure J, Raji OR, Hochschuler SH. Interspinous-interbody fusion via a strictly lateral surgical approach: a biomechanical stabilization comparison to constructs requiring both lateral and posterior approaches. Cureus. 2023;15(7):e41918. doi:10.7759/cureus.41918
6. Pradhan BB, Turner AW, Zatushevsky MA, Cornwall GB, Rajaee SS, Bae HW. Biomechanical analysis in a human cadaveric model of spinous process fixation with an interlaminar allograft spacer for lumbar spinal stenosis: laboratory investigation. J Neurosurg Spine. 2012;16(6):585–593. doi:10.3171/2012.3.SPINE11631
7. Postacchini F, Postacchini R, Menchetti PP, Sessa P, Paolino M, Cinotti G. Lumbar interspinous process fixation and fusion with stand-alone interlaminar lumbar instrumented fusion implant in patients with degenerative spondylolisthesis undergoing decompression for spinal stenosis. Asian Spine J. 2016;10(1):27–37. doi:10.4184/asj.2016.10.1.27
8. Skoblar M, Hedman T, Rogers AJ, Jasper GP, Beall DP. Instrumented posterior arthrodesis of the lumbar spine: prospective study evaluating fusion outcomes in patients receiving an interspinous fixation device for the treatment of degenerative spine diseases. J Pain Res. 2023;16:2909–2918. doi:10.2147/JPR.S417319
9. Falowski SM, Raso LJ, Mangal V, et al. A prospective, observational, open-label, non-randomized, multicenter study measuring functional outcomes in a novel interspinous fusion device in subjects with low back pain: REFINE study. Pain Ther. 2023;12(1):187–199. doi:10.1007/s40122-022-00447-0
10. Lee GY, Lee JW, Choi HS, Oh KJ, Kang HS. A new grading system of lumbar central canal stenosis on MRI: an easy and reliable method. Skeletal Radiol. 2011;40(8):1033–1039. doi:10.1007/s00256-011-1153-z
11. Lee S, Lee JW, Yeom JS, et al. A practical MRI grading system for lumbar foraminal stenosis. AJR Am J Roentgenol. 2010;194(4):1095–1098. doi:10.2214/AJR.09.2772
12. Falowski SM, Mangal V, Pope J, et al. Multicenter retrospective review of safety and efficacy of a novel minimally invasive lumbar interspinous fusion device. Pain Res. 2021;14(14):1525–1531. doi:10.2147/JPR.S304957
13. Fairbank JCT, Pynsent PB. The Oswestry disability index. Spine. 2000;25(22):2940–2953. doi:10.1097/00007632-200011150-00017
14. Cella D, Riley W, Stone A, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol. 2010;63(11):1179–1194. doi:10.1016/j.jclinepi.2010.04.011
15. Buckley DI, Eckstrom E, Morris C, et al. Performance of a patient reported outcomes measurement information system (PROMIS) short form in older adults with chronic musculoskeletal pain. Pain Med. 2015; 17(2):314–324.
16. Amtmann D, Kim J, Chung H, Askew R, Park R, Cook K. Minimally important differences for PROMIS pain interference for individuals with back pain. J Pain Res. 2016;9:251–255. doi:10.2147/JPR.S93391
17. Chen CX, Kroenke K, Stump TE, et al. Estimating minimally important differences for the PROMIS pain interference scales: results from 3 randomized clinical trials. Pain. 2018;159(4):775–782. doi:10.1097/j.pain.0000000000001121
18. Lee AC, Driban JB, Price LL, Harvey WF, Rodday AM, Wang C. Responsiveness and minimally important differences for 4 patient-reported outcomes measurement information system short forms: physical function, pain interference, depression, and anxiety in knee osteoarthritis. J Pain. 2017;18(9):1096–1110. doi:10.1016/j.jpain.2017.05.001
19. Norman GR, Sloan JA, Wyrwich KW. The truly remarkable universality of half a standard deviation: confirmation through another look. Expert Rev Pharmacoecon Outcomes Res. 2004;4(5):581–585. doi:10.1586/14737167.4.5.581
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
