Back to Journals » Journal of Inflammation Research » Volume 16
Early Cytokine Signatures of Hospitalized Mild and Severe COVID-19 Patients: A Prospective Observational Study
Authors Alfadda AA , Siddiqui K , Rafiullah M , AlKhowaiter M, Alotaibi N, Alzahrani M, Binkhamis K, Youssef AM, Altalhi H, Almaghlouth I, Alarifi M, Albanyan S, Alosaimi MF, Isnani A , Nawaz SS , Alayed K
Received 9 March 2023
Accepted for publication 18 May 2023
Published 22 June 2023 Volume 2023:16 Pages 2631—2643
DOI https://doi.org/10.2147/JIR.S408663
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Assim A Alfadda,1– 3 Khalid Siddiqui,2 Mohamed Rafiullah,2 Mohammad AlKhowaiter,1 Naif Alotaibi,4 Musa Alzahrani,5 Khalifa Binkhamis,6 Amira M Youssef,2 Haifa Altalhi,7 Ibrahim Almaghlouth,8 Mohammed Alarifi,9 Saleh Albanyan,1 Mohammed F Alosaimi,10 Arthur Isnani,3 Shaik S Nawaz,2 Khalid Alayed5
1Department of Internal Medicine, College of Medicine, and King Khalid University Hospital, King Saud University Medical City, King Saud University, Riyadh, Saudi Arabia; 2Strategic Center for Diabetes Research, College of Medicine, King Saud University, Riyadh, Saudi Arabia; 3Obesity Research Center, College of Medicine, King Saud University, Riyadh, Saudi Arabia; 4Department of Medicine, College of Medicine, and King Saud University Medical City, King Saud University, Riyadh, Saudi Arabia; 5Department of Medicine, College of Medicine, King Saud University, Riyadh, Saudi Arabia; 6Department of Pathology, College of Medicine, King Saud University, Riyadh, Saudi Arabia; 7Infection Control Department, King Khalid University Hospital, King Saud University Medical City, King Saud University, Riyadh, Saudi Arabia; 8Rheumatology Unit, Department of Internal Medicine, King Khalid University Hospital, King Saud University Medical City, King Saud University, Riyadh, Saudi Arabia; 9Intensive Care Department, King Khalid University Hospital, King Saud University Medical City, King Saud University, Riyadh, Saudi Arabia; 10Pediatric Department, King Khalid University Hospital, King Saud University Medical City, King Saud University, Riyadh, Saudi Arabia
Correspondence: Assim A Alfadda, Department of Internal Medicine and the Obesity Research Center, College of Medicine, King Saud University, P.O. Box 2925(98), Riyadh, 11461, Saudi Arabia, Tel + 966 11 467 1315, Email [email protected]
Background: The severe manifestation of coronavirus disease 2019 (COVID-19) is known to be mediated by several cytokines and chemokines. The study aimed to compare the early cytokine profile of mild and severe COVID-19 patients to that with COVID-19-like symptoms and tested negative for Severe Acute Respiratory Syndrome Coronavirus-2 in the Reverse-Transcriptase Polymerase Chain Reaction (RT-PCR) test.
Methods: This was a prospective, observational study on COVID-19 patients admitted to King Khalid University Hospital, King Saud University Medical City from June to November 2020. Clinical and biochemical data were collected from hospital charts. Blood samples were collected at the time of hospital admission to measure cytokines. A Cytokine and Growth Factor High-Sensitivity Array was used to quantitatively measure cytokines.
Results: The study included 202 RT-PCR-positive individuals and 61 RT-PCR-negative individuals. C-Reactive protein (CRP) and Interleukin-10 (IL-10) levels were found significantly elevated in the RT-PCR positive group compared to the RT-PCR negative group (p=0.001). Patients with severe COVID-19 had significantly longer median hospital stays than those with mild COVID-19 cases (7 vs 6 days). They also had higher CRP and Vascular Endothelial Growth Factor (VEGF) levels and lower Interleukin-4 (IL-4) levels compared to the mild cases. CRP, interleukin-6, IL-10, VEGF, and Monocyte Chemoattractant Protein-1 (MCP-1) levels were significantly elevated in men and IL-10 was significantly higher and interleukin-8 was significantly lower in women compared to negative controls. Elevated Interferon-ɣ (IFN-γ) and IL-10 levels were seen in mild COVID-19 cases and elevated level of MCP-1 was seen in severe COVID-19 cases when categorized according to the length of stay in the hospital.
Conclusion: CRP and IL-10 levels were elevated in the RT-PCR positive group. People with severe COVID-19 had higher CRP and VEGF levels and lower IL-4 levels. Elevated IFN-γ and IL-10 levels were seen in mild COVID-19 cases and elevated level of MCP-1 was seen in severe COVID-19 cases when categorized according to the length of stay in the hospital.
Keywords: SARS-CoV-2, early inflammation, asymptomatic patient, proinflammatory cytokines, regulatory cytokines
Introduction
The coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV2) became a pandemic affecting the population worldwide. While most patients remain asymptomatic and have a mild disease, some require hospitalization. The severity of the disease is largely dependent on age and the presence of comorbidities. The severe manifestation of COVID-19 is known to be mediated by several cytokines and chemokines. When the virus infects the lung cells by attaching to the ACE2 receptors, it activates the macrophages, natural killer cells, chemokines, and cytokines.1 Excessive production of inflammatory cytokines aggravates respiratory distress and causes extensive tissue damage in other organs. COVID-19 can trigger an uncontrolled release of cytokines, known as a “cytokine storm” which can lead to respiratory failure, multiple organ failure, and death.2 This systemic inflammation along with comorbid conditions such as hypertension, diabetes, and heart disease results in the worst prognosis for COVID-19 patients.3
It is interesting to note that the severity of COVID-19 is heterogeneous but the exact mechanisms behind the heterogeneity are not fully understood. Several researchers have attempted to understand the molecular basis of immune response to SARS-CoV2 to identify suitable biomarkers or targets to predict and treat the COVID-19 disease progression.4,5 The excessive inflammatory response has been one of the hallmarks of the development of severe COVID-19 infection. Cytokines and chemokines are in the midst of the pro-inflammatory and anti-inflammatory pathways. The imbalance between these systems will result in tissue damage and lead to severe disease.6 Several studies have reported marked elevation of pro-inflammatory cytokines and chemokines in severe cases of COVID-19. TNF-α, IL-6, IL-8, and IL-10 were found higher in patients with severe COVID-19 when compared to mild cases.7 Another study found levels of cytokines IL-6, IL-1RA, IL-10, and G-CSF to be the difference between patients with and without clinical endpoints. However, the study concluded that the cytokine storm reflected by the increased release of cytokines did not correlate with the standard parameters of disease severity.8 Age, one of the comorbidities of COVID-19 was found to be associated with cytokine signatures in COVID-19 patients. IL-8, IL-10, IL-15, IL-27, and TNF-α levels correlated with older age, longer duration of hospitalization, and severity of the disease in patients with COVID-19.9
Even though the altered levels of cytokines and chemokines have been frequently reported, it is not easy to differentiate the dysfunctional and normal cytokine responses. Cytokines and chemokines are part of the host immune response and are needed to clear infections. The immune response is an important process that could affect the prognosis of COVID-19 infection. Therefore, knowledge of the influence of host response on the severity of the disease will be necessary for the appropriate management of COVID-19. Previous studies have compared the differences in the cytokine levels in patients with COVID-19 to either healthy controls6 or mild cases of COVID-19.10 To better understand the unique cytokine signatures of COVID-19, we decided to compare the cytokine profile of mild and severe COVID-19 patients to that with COVID-19-like symptoms and tested negative for SARS-CoV-2 antigen in the RT-PCR test.
Methods
Study Design
This was a prospective, observational study on patients admitted with suspected COVID-19 to King Khalid University Hospital (KKUH), King Saud University Medical City (KSUMC), Riyadh, Saudi Arabia from June to November 2020, who were included in this COVID-19 cohort. The Saudi Center for Disease Prevention and Control’s recommendations published in April 2020 was used as the basis for the diagnosis of SARS-CoV2 infection.11 Patients with symptoms of COVID-19 swab samples were collected from the patient’s upper respiratory tract (nasopharyngeal and/or oropharyngeal), placed in a viral transport media, and delivered immediately to a biosafety Level 2-facility (BSL-2) in KSUMC, Riyadh, KSA, for reverse transcription-polymerase chain reaction (RT-PCR) analysis. Patients with negative RT-PCR assay tests were assessed again within 48–72 hours to avoid the false-negative test.
This research was reviewed and approved by the Institutional Review Board (IRB) College of Medicine, King Saud University, Saudi Arabia. Written informed consent was obtained from all the patients who participated in this study. Clinical data were extracted from the electronic medical files for the identified patients using a predesigned case report form. Demographic characteristics, clinical, biochemical, and hematological laboratory findings on admission, treatment, and outcomes were retrieved from medical records.
Biochemical Analysis
Patients were recruited at hospital admission and blood samples were taken for cytokine measurement during the “early phase” within 48 hours of admission. 5 mL of the patient’s blood samples were collected via venipuncture for the Evidence Investigator Cytokine and Growth Factors High-Sensitivity Array, and routine biochemistry laboratory measurements. The blood and serum samples were obtained during the time of hospital admission for COVID-19. Human serum (non-hemolyzed and non-lipemic) are stored frozen in small aliquots at −80° C. The Evidence Investigator Cytokine High-Sensitivity Array is used to Quantitatively measure multiple cytokines immunoassays from a single sample for detection of Epidermal Growth Factor (EGF), Interferon-γ (IFN-γ), Interleukin-1α (IL-1α), Interleukin-1β (IL-1β), Interleukin-2 (IL-2), Interleukin-4 (IL-4), Interleukin-6 (IL-6) Interleukin-8 (IL-8), Interleukin-10 (IL-10), Monocyte Chemotactic Protein-1 (MCP-1), Tumor Necrosis Factor-α (TNF-α), Vascular Endothelial Growth Factor (VEGF) respectively according to the manufacturer’s instructions (catalog No.EV3623) Randox Laboratories Limited, United Kingdom.
The Severity of COVID-19 (Mild and Severe Cases)
The severity of COVID-19 was classified as mild and severe as previously described.12 Severe cases included any patient with shortness of breath, loss of appetite, confusion or persistent pain or pressure in the chest, respiratory distress, respiratory rate ≥30/min or oxygen saturation ≤93% in room air or mechanical ventilation, developed shock, or had other organ failures or admitted to Intensive Care Unit (ICU). All other RT-PCR-positive cases were considered mild. Those who had RT-PCR negative reports and were hospitalized for less than 5 days were included as a comparator.
Statistical Analysis
Data analysis was done using the Statistical Package for Social Sciences (SPSS) version 23.0 (IBM, SPSS, Armonk, New York, USA). Demographic characteristics were presented as numbers (n) and percentages (%) for categorical variables and continuous data were described with median and interquartile range (IQR). Kolmogorov Smirnov Test was performed to determine the normality of the distribution for continuous variables. A Chi-Square test was done to determine differences in the proportions of categorical variables. Non-parametric Mann–Whitney U-test was used to determine the significant difference in the blood biochemistry and inflammatory markers results between RT-PCR positive and negative patients, mild and severe RT-PCR positive patients, and between admission positive and negative RT-PCR results for men and women. P value ≤0.05 was considered statistically significant.
Results
The study included 202 RT-PCR-positive individuals and 61 RT-PCR-negative individuals. The demographic profiles of the study subjects show that RT-PCR-positive patients were younger (51.9 years) compared to the RT-PCR-negative group (60.0 years) (Table 1). Other common comorbidities found higher in RT-PCR positive group were diabetes and hypertension followed by coronary heart disease. While, the clinical presentation of the RT-PCR-positive group was dominated by shortness of breath, cough, and fever. The biochemical characteristics of study participants showed elevated liver enzyme Alanine aminotransferase (ALT), and fibrinogen in RT-PCR positive group compared to the RT-PCR-negative group (Table 2). Conversely, BUN, International normalised ratio (INR), and Prothrombin time (PT) were significantly lower in the RT-PCR-positive group compared to the RT-PCR-negative group (p=0.004). Inflammatory markers C-reactive protein (CRP) and IL-10 levels were found significantly elevated in the RT-PCR positive group compared to the RT-PCR-negative group (p=0.001), while the other markers such as IL-2, IL-4, IL-6, IL-8, VEGF, IFN- ɣ, TNF-α, IL-1α, IL-1β, MCP-1, and EGF were not significantly different between the groups.
 |
Table 1 Demographic Profile and Clinical Presentation of People with COVID-19 RT-PCR Positive Patients and Negative Reports at the Time of Hospitalization |
 |
Table 2 Biochemical Characteristics of COVID-19 RT-PCR Positive Patients and RT-PCR Negative at the Time of Hospitalization |
The RT-PCR-positive patients were categorized into mild and severe symptoms. Out of the two hundred and two RT-PCR-positive patients included in the study, 81 (40.1%) were with mild COVID-19 symptoms and 121 (59.9%) were with severe COVID-19 symptoms at the time of hospitalization (Table 3). Individuals with severe COVID-19 symptoms were significantly older (55.9 years) than those with mild covid-19 symptoms (p<0.001). The frequency of gender was equally distributed among the mild and severe COVID-19 symptom groups (p=0.004). In severe COVID-19 cases, the median hospitalization duration was significantly higher than the mild cases (p<0.001). The respiratory rate was significantly higher among the severe COVID-19 subjects group compared to the mild cases group (p=0.001), while the other vital signs such as pulse rate, body mass index (BMI), systolic and diastolic blood pressure did not differ significantly between the two groups. The frequency of comorbidities such as diabetes, hypertension, coronary heart disease, Chronic Obstructive Pulmonary Disease (COPD), and Chronic Kidney Disease (CKD) was significantly higher in the severe COVID-19 group compared to the mild group (p=0.036, p<0.001, p<0.001, p=0.040, and p=0.048 respectively). COVID-19-related symptoms such as loss of appetite (15.7%), fever (62.0%), cough (62.8%), tiredness (18.3%), shortness of breath (81.0%), and body aches (22.7%) were significantly higher among the severe COVID-19 symptoms group compared to the mild symptoms group.
 |
Table 3 Demographic Profile and Clinical Presentation of COVID-19 RT-PCR Positive Patients with Mild and Severe Symptoms at the Time of Hospitalization |
The biochemical characteristics and cytokine profile of RT-PCR-positive patients with mild and severe symptoms at the time of hospitalization are shown in Table 4. Liver enzymes (ALT and Aspartate aminotransferase (AST)), creatinine, Blood Urea Nitrogen (BUN), and fibrinogen were significantly elevated in the severe covid-19 cases group compared to the mild COVID-19 cases group (p=0.044, p=0.004, p= 0.006, and p=0.002 respectively). IL-4 level was found to be significantly lower in the severe COVID-19 symptom group compared to the mild symptoms group (p=0.042). On the other hand, the other cytokines such as IL-2, IL-6, IL-8, IL-10, IFNG, TNF-α, IL-1α, IL-1β, MCP-1, and EGF were not significantly different between the groups. Moreover, FBS CRP and VEGF levels were significantly elevated in the severe COVID-19 group compared to the mild COVID-19 symptoms group (p=0.020, p=0.014, p=0.003) respectively.
 |
Table 4 Biochemical Characteristics and Cytokine Profile of Covid-19 RT-PCR Positive Mild and Severe Patients |
There was a gender difference in the biomarker profile of covid-19 RT-PCR positive compared to RT-PCR negative study participants (Supplementary Tables 1 and 2). CRP, IL-6, IL-10, VEGF, and MCP-1 levels were significantly elevated in the male subjects with COVID-19 positive group at the time of hospitalization compared to COVID-19 negative male subjects (p=0.001, p=0.033, p=0.001, p=0.053, and p=0.048 respectively). Whereas, IL-10 was significantly elevated and IL-8 was significantly decreased in the female subjects with COVID-19 positive group at the time of hospitalization compared to COVID-19 negative female participants (p=0.003 and p=0.028 respectively).
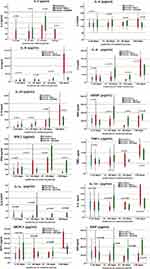
The serum cytokine levels in RT-PCR negative, mild RT-PCR positive subjects, and severe RT-PCR positive subjects were compared according to the length of stay in the hospital (Figure 1). The participants were categorized based on their hospital length of stay. The cytokine levels of mild and severe COVID-19 groups were compared against RT-PCR negative group. However, since the RT-PCR negative group included individuals with less than 5 days, we compared the cytokine levels between mild and severe groups in categories of duration of hospitalization more than 10 days. IL-10 levels were significantly higher in mild and severe cases compared to the RT-PCR negative among people with a length of stay in the hospital of 1–10 days (p<0.001). However, in people with a length of stay in the hospital between 20–30 days IL-10 levels were higher in mild cases compared to severe COVID-19 cases (p=0.021). VEGF levels were significantly higher in severe COVID-19 cases when compared to RT-PCR negative group with 1–10 days of stay in the hospital (p=0.015) and in mild COVID-19 cases with 11–20 and 20–30 days of stay in the hospital (p=0.037 and p=0.018) respectively. IFN-γ was significantly lower in severe cases compared to mild cases in people who had a 20–30 days stay in the hospital (p=0.041). The severe COVID-19 group had a higher MCP-1 level than the mild cases among participants who had a length of stay in the hospital of 11–20 days (p=0.020). The scatter plots show the relationship between cytokines and length of stay in the hospital (Supplementary Figure 1a and b). IL-6 and EGF-1 levels were positively associated with the length of stay in the hospital among mild and severe COVID-19 cases whereas IL-6, IL-8, Il-10, IFN-γ, TNF-α, and MCP-1 levels showed association only in severe cases.
Discussion
The severity of covid-19 is heterogeneous and the exact mechanisms behind the heterogeneity are not fully understood. Cytokines are small proteins that play a role in the immune system. They affect cell growth, differentiation, and death. Cytokines are secreted by various types of cells and bind to specific receptors on other cells.13 In this study, we found that serum CRP and IL-10 levels were significantly higher in COVID-19 patients at the time of hospitalization compared to the RT-PCR-negative individuals. CRP, an inflammatory marker is known to be associated with COVID-19. Elevated serum CRP level was seen in the majority of patients with severe COVID-19.14 CRP levels increase rapidly and reach a peak within 48 h of the illness.15 IL-10 is an anti-inflammatory cytokine that suppresses the production of Th1 cytokines. It is produced in response to the rapid production of pro-inflammatory cytokines.16 Remarkably, in our study, the levels of pro-inflammatory cytokines such as TNF-α and IL-6 were not higher than the RT-PCR negative group. It appears that the RT-PCR-negative individuals had certain clinical conditions, that led to the high level of pro-inflammatory markers resulting in comparable pro-inflammatory cytokine levels between positive and negative groups. Nevertheless, the significantly higher serum CRP levels in the RT-PCR positive group distinguished the positive and negative cases. The demographic and clinical characteristics of individuals with severe COVID-19 in our study are in agreement with previously reported characteristics of severe COVID-19.17 The frequency of symptoms was found more in people with severe COVID-19 compared to those with mild disease.
The biochemical parameters of severe COVID-19 cases revealed elevated levels of liver enzymes, creatinine, BUN, fibrinogen, and fasting blood sugar. D-dimer levels were raised in both the mild and severe groups. Expectedly the serum CRP levels were higher in severe cases, while the IL-4 level was significantly lower in people with severe COVID-19. IL-4 is an anti-inflammatory cytokine generated from Th2 cells and it interferes with the immune response of Th1 cells.18 Moreover, IL-4 is known to downregulate the ACE2 receptors,19 a target of SARS-CoV-2 virus entry. A lower level of IL-4 in severe COVID-19 cases is therefore comprehensible. The baseline levels of pro-inflammatory markers such as IL-2, IL-6, and TNF-α, could not differentiate between the mild and severe cases of COVID-19 in our study population. VEGF is another molecule that was found higher in people with severe COVID-19 in our study. It is a vascular permeability factor involved in the angiogenesis in various pathological conditions20 and is increased in response to pro-inflammatory cytokines.21 A previous study found elevated VEGF levels in COVID-19 patients on arrival at the hospital and it correlated with inflammatory markers.22 However, in our study, the pro-inflammatory cytokines were not higher in severe COVID-19 compared to mild cases. We collected the blood samples at the time of hospitalization. Therefore, it might have been in an early stage before the levels of the inflammatory cytokine were elevated. When the mild and severe cases were categorized according to the duration of hospitalization, the VEGF levels continued to be higher in severe cases up to 30 days of hospitalization. VEGF is an angiogenic factor reported to be involved in pathological angiogenesis and vasculogenesis.23 It is involved in the pathogenesis of COVID-19 and has been implicated in the severity of the disease. It plays a substantial role in abnormal vascular remodeling and increased vascular permeability following decreased oxygen saturation.24 In addition, it is thought that VEGF may have a role in the movement of the virus from the lungs to blood circulation.25 As none of the inflammatory cytokines were elevated at the time of hospitalization, it seems the VEGF levels increased well before the cytokines were triggered. It is also possible that the role of VEGF in COVID-19 pathophysiology may be independent of the cytokine signaling and inflammatory status of the patients.
IFN-γ is secreted by Th1 cells and it plays an important role in the antiviral defense by controlling the virus replication and activating the cytokine secretion from T cells. It is reported to control SARS-CoV-2 infection.26 However, a persistently high level of IFN-γ exacerbates the inflammation and is implicated in the severe manifestation of COVID-19.27 Our results show that IFN-γ was elevated in people who had mild disease and had a hospital length of stay of 20–30 days when compared to severe cases with the same duration. Interestingly, the Th2 cytokine IL-4 levels were not remarkably different across different categories of the duration of hospitalization. It may be possible that the group of individuals who had 20–30 days of the length of stay in the hospital had a dominant Th1 response as indicated by the elevated IFN-γ levels.
MCP-1 is a chemotactic cytokine that regulates the movement of monocytes and macrophages. It is also linked to the severity of the disease in COVID-19 like cytokines.28 Individuals with severe COVID-19 and 11–20 days of hospitalization were found to have elevated MCP-1 levels than the milder cases of similar duration among our study participants. MCP-1 levels of severe cases with 20–30 days of hospital stay were also high but not significant. On the other hand, the overall MCP-1 levels were found similar between individuals with mild and severe COVID-19. Previous studies found that MCP-1 level was higher among patients admitted to ICU29 and among those who had respiratory failure.30 Another study found that MCP-1 levels were similarly elevated in mild and severe COVID-19 cases compared to healthy controls.31 A rapid early increase in MCP-1 levels was reported in COVID-19 patients during the acute phase of the disease and it declined gradually with the disease progress.32 In our study, the serum MCP-1 levels at the time of hospitalization did not discriminate between the mild and severe cases of COVID-19 except for those who had a length of stay in the hospital of 20–30 days. It could be possible that the immune response had not picked up momentum at the time of hospitalization. The lower level of IL-6 in both mild and severe COVID-19 in our study participants might be actually due to the lower levels of MCP-1 which would have led to the lower mobilization of IL-6-producing macrophages.
Elevated IL-10 level is widely reported in COVID-19. It is also linked to severe COVID-19 despite being an anti-inflammatory and immunosuppressive cytokine. Increased IL-10 secreting regulatory T cells were found to be a distinct feature of severe COVID-19.33 It is thought that IL-10 is elevated to counter the hyper-inflammation caused by the pro-inflammatory cytokines in COVID-19.16 Our results show that IL-10 was significantly higher in people with mild and severe COVID-19 compared to RT-PCR negative group when the duration of hospitalization was less than 10 days. However, among people who stayed in the hospital for more than 20 days, IL-10 was predominant in mild COVID-19 cases. It appears having an early elevation of anti-inflammatory cytokine IL-10 could counter the inflammatory pathogenesis of COVID-19 and the individuals who did not have elevated IL-10 at the time of hospitalization had a severe manifestation. But, among patients with more than 30 days of hospitalization, the IL-10 levels were only slightly higher in mild cases than the severe cases. As the duration of hospitalization increases, it is highly likely that patients experienced persistent symptoms, even among mild cases. Therefore, we were not able to observe any significant differences in pro-inflammatory or anti-inflammatory cytokines between mild and severe cases among patients who had a hospitalization duration of more than 30 days.
Cytokine profiles of people with COVID-19 are known to exhibit gender differences. Pro-inflammatory cytokines are reported to be higher in men than in women.34 Our results also show that men had significantly higher CRP, IL-10, VEGF, and IL-1A levels compared to the RT-PCR-negative men. Whereas among women, only CRP level was higher than the RT-PCR negative women. Men were found at more risk of severe COVID-19 compared to women.35 The physiological factors, immune system, and sex hormones are the likely factors that could have contributed to these gender differences. Moreover, women’s immune system responds differently to a viral infection. They usually have a lesser viral load and inflammation than men. The X chromosome has several genes that regulate the immune cells.36
The present study has explored the early cytokine profile of individuals with COVID-19. The cytokine profile of people at the time of hospitalization does not distinguish the mild and severe cases, especially the pro-inflammatory cytokines. While the clinical and biochemical characteristics of RT-PCR-positive individuals exhibited typical COVID-19-induced changes, the pro-inflammatory cytokine profile was equally higher among the positive and negative groups. A similar observation was found when the mild and severe cases were compared. However, the CRP levels distinguished both RT-PCR positive from negative cases and severe from mild cases. CRP is usually secreted in response to cytokines that are released from macrophages and adipocytes. However, in our study population, the CRP levels were proportionately higher than the inflammatory cytokine levels when compared among RT-PCR positive and negative, and mild and severe groups respectively. It seems the induction of CRP production had been higher among severe COVID-19 cases even though their inflammatory cytokines were comparable to the mild cases. There may be other factors involved in the immune response to the SARS-CoV-2 infection. Interestingly, the scatter plots revealed that people with longer duration of hospitalization had higher cytokine levels. However, the changes were smaller to produce a significant difference between the mild and severe groups. This may warrant a periodical assessment of cytokine levels to monitor the disease’s progress. On the other hand, people with RT-PCR-positive had a higher anti-inflammatory cytokine IL-10 compared to the negative group and those with severe COVID-19 had a higher anti-inflammatory cytokine IL-4 compared to mild cases.
Our study participants were in a very early stage of the disease and the samples were collected at the time of admission. Therefore, they were unlikely to have had any COVID-19 treatment at that time. Hence, it may be safely assumed that there was no influence of COVID-19 treatment on cytokine levels. The strength of this study is that we compared the cytokine profiles of people with COVID-19 to those who were negative for SAR-CoV-2 nucleic acid. At the same time, people in the negative group had several comorbidities, and probably had medical conditions that elevated the cytokine levels. Since the blood samples were collected at the time of hospitalization, our study provides insight into the early cytokine profile of people with COVID-19 who later progressed to mild or severe disease. We did not analyze the cytokine levels during the disease progression. Therefore, we do not know the prospective changes in the cytokines which would have played a role in the disease progression. We did not identify the prevalent SARS-CoV-2 strain among the study participants. There could have been differences in the clinical, and biochemical characteristics among patients infected with different strains.
Conclusion
The early cytokine profile of people with COVID-19 has been explored in the study. CRP and IL-10 levels were elevated in the RT-PCR positive group compared to the negative group. People with severe COVID-19 had higher CRP and VEGF levels and lower IL-4 levels compared to the mild cases. Men with COVID-19 had higher cytokine levels compared to those without COVID-19. Elevated IFN-γ and IL-10 levels were seen in mild COVID-19 cases and elevated levels of MCP-1 were seen in severe COVID-19 cases when categorized according to the length of stay in the hospital. Periodical assessment of cytokines may be needed to monitor the progression of the disease.
Ethics Statement
The Institutional Review Board, College of Medicine, King Saud University, Riyadh, Saudi Arabia reviewed and authorized the investigations involving human subjects. The patients gave written informed consent to participate in the study. The study was conducted according to the Declaration of Helsinki.
Acknowledgment
We would like to acknowledge the services, of Ms. Heba Mohammed, Ms. Salini Scaria Joy, Ms. Teena George Puthiyaparampil, Ms. Nourhan Mohamed, Ms. Tahany Mossa Edrees, and Ms. Faiza Abood from the Strategic Center for Diabetes Research, College of Medicine, King Saud University, Riyadh, Saudi Arabia for data collection and encoding respectively. Ms. Deema Althagabi and Dr. Mohammad Al Wetidy from the College of Medicine Research Center, King Saud University, Riyadh, Saudi Arabia for patient consenting, clinical data collection, and coordination with the clinical laboratory and the services of Mr. Saud Alanazi, and Mr. Hossam Ayed Algohani from the Strategic Center for Diabetes Research, and Ms. Amina Fallata, and Mr. Kenneth Domero from the Obesity Research Center, College of Medicine, King Saud University, Riyadh, Saudi Arabia for assisting in phlebotomy and sample collection are appreciated.
Author Contributions
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit it to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
This Work was funded by the National Plan for Science, Technology and Innovation (MAARIFAH), King Abdulaziz City for Science and Technology, Kingdom of Saudi Arabia, grant to the Strategic Center for Diabetes Research.
Disclosure
The authors declare that they have no competing interests.
References
1. Keam S, Megawati D, Patel SK, Tiwari R, Dhama K, Harapan H. Immunopathology and immunotherapeutic strategies in severe acute respiratory syndrome coronavirus 2 infection. Rev Med Virol. 2020;30(5):e2123. doi:10.1002/rmv.2123
2. Ragab D, Salah Eldin H, Taeimah M, Khattab R, Salem R. The COVID-19 cytokine storm; what we know so far. Front Immunol. 2020;11:1446. doi:10.3389/fimmu.2020.01446
3. Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q. Prevalence of comorbidities in the novel Wuhan coronavirus (COVID-19) infection: a systematic review and meta-analysis. Intern J Infectious Dis. 2020:56.
4. Castillo-Olivares J, Wells DA, Ferrari M, et al. Analysis of Serological Biomarkers of SARS-CoV-2 Infection in Convalescent Samples From Severe, Moderate and Mild COVID-19 Cases. Front Immunol. 2021;12:748291. doi:10.3389/fimmu.2021.748291
5. Ponti G, Maccaferri M, Ruini C, Tomasi A, Ozben T. Biomarkers associated with COVID-19 disease progression. Crit Rev Clin Lab Sci. 2020;57(6):389–399. doi:10.1080/10408363.2020.1770685
6. Ghazavi A, Ganji A, Keshavarzian N, Rabiemajd S, Mosayebi G. Cytokine profile and disease severity in patients with COVID-19. Cytokine. 2021;137:155323. doi:10.1016/j.cyto.2020.155323
7. Dorgham K, Quentric P, Gökkaya M, et al. Distinct cytokine profiles associated with COVID-19 severity and mortality. J Allergy Clin Immunol. 2021;147(6):2098–2107. doi:10.1016/j.jaci.2021.03.047
8. Lebedeva A, Molodtsov I, Anisimova A, et al. Comprehensive Cytokine Profiling of Patients with COVID-19 Receiving Tocilizumab Therapy. Int J Mol Sci. 2022;23(14):1–26. doi:10.3390/ijms23147937
9. Angioni R, Sánchez-Rodríguez R, Munari F, et al. Age-severity matched cytokine profiling reveals specific signatures in Covid-19 patients. Cell Death Dis. 2020;11(11):957. doi:10.1038/s41419-020-03151-z
10. Wang J, Jiang M, Xin Chen X, Montaner LJ. Cytokine storm and leukocyte changes in mild versus severe SARS-CoV-2 infection: review of 3939 COVID-19 patients in China and emerging pathogenesis and therapy concepts. J Leukoc Biol. 2020;108(1):1741. doi:10.1002/JLB.3COVR0520-272R
11. Saudi Center for Disease Prevention and Control. Coronavirus Disease COVID-19 Guidelines; 2020.
12. National Institutes of Health. Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19). Nih. 2021;2019:1–243.
13. Liu C, Chu D, Kalantar-Zadeh K, George J, Young HA, Liu G. Cytokines: from clinical significance to quantification. Adv.Sci. 2021;8:2004433. doi:10.1002/advs.202004433
14. Smilowitz NR, Kunichoff D, Garshick M, et al. C-reactive protein and clinical outcomes in patients with COVID-19. Eur Heart J. 2021;42(23):2270–2279. doi:10.1093/eurheartj/ehaa1103
15. Ali N. Elevated level of C-reactive protein may be an early marker to predict risk for severity of COVID-19. J Med Virol. 2020;92(11):2409–2411. doi:10.1002/jmv.26097
16. Lu L, Zhang H, Dauphars DJ, He YW. A Potential Role of Interleukin 10 in COVID-19 Pathogenesis. Trends Immunol. 2021;42(1):3–5. doi:10.1016/j.it.2020.10.012
17. Aleanizy FS, Alqahtani FY, Alanazi MS, et al. Clinical characteristics and risk factors of patients with severe COVID-19 in Riyadh, Saudi Arabia: a retrospective study. J Infect Public Health. 2021;14(9):1133–1138. doi:10.1016/j.jiph.2021.07.014
18. Renu K, Subramaniam MD, Chakraborty R, et al. The role of Interleukin-4 in COVID-19 associated male infertility - A hypothesis. J Reprod Immunol. 2020;142:103213. doi:10.1016/j.jri.2020.103213
19. de Lang A, Osterhaus ADME, Haagmans BL. Interferon-gamma and interleukin-4 downregulate expression of the SARS coronavirus receptor ACE2 in Vero E6 cells. Virology. 2006;353(2):474–481. doi:10.1016/j.virol.2006.06.011
20. Shibuya M. Vascular endothelial growth factor and its receptor system: physiological functions in angiogenesis and pathological roles in various diseases. J Biochem. 2013;153(1):13–19. doi:10.1093/jb/mvs136
21. Maloney JP, Gao L. Proinflammatory cytokines increase vascular endothelial growth factor expression in alveolar epithelial cells. Mediators Inflamm. 2015;2015:387842. doi:10.1155/2015/387842
22. Guerra-López JA, Amezcua-Castillo LM, González-Pacheco H, Amezcua-Guerra LM. Levels of Vascular Endothelial Growth Factor and Its Association with Pulmonary Embolism in COVID-19. J Interf Cytokine Res. 2022;42(8):444–448. doi:10.1089/jir.2022.0034
23. Shibuya M. VEGF-VEGFR Signals in Health and Disease. Biomol Ther (Seoul). 2014;22(1):1–9. doi:10.4062/biomolther.2013.113
24. Cao Y. The impact of the hypoxia-VEGF-vascular permeability on COVID-19-infected patients. Exploration. 2021;1(2):20210051. doi:10.1002/EXP.20210051
25. Solimando AG, Marziliano D, Ribatti D. SARS-CoV-2 and Endothelial Cells: vascular Changes, Intussusceptive Microvascular Growth and Novel Therapeutic Windows. Biomedicines. 2022;10(9). doi:10.3390/biomedicines10092242
26. Galbraith MD, Kinning KT, Sullivan KD, et al. Specialized interferon action in COVID-19. Proc Natl Acad Sci. 2022;119(11):e2116730119. doi:10.1073/pnas.2116730119
27. Gadotti AC, de Castro Deus M, Telles JP, et al. IFN-γ is an independent risk factor associated with mortality in patients with moderate and severe COVID-19 infection. Virus Res. 2020;289:198171. doi:10.1016/j.virusres.2020.198171
28. Chen Y, Wang J, Liu C, et al. IP-10 and MCP-1 as biomarkers associated with disease severity of COVID-19. Mol Med. 2020;26(1):97. doi:10.1186/s10020-020-00230-x
29. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506.
30. Jøntvedt Jørgensen M, Holter JC, Christensen EE, et al. Increased interleukin-6 and macrophage chemoattractant protein-1 are associated with respiratory failure in COVID-19. Sci Rep. 2020;10(1):1–11. doi:10.1038/s41598-020-78710-7
31. Kalinina O, Golovkin A, Zaikova E, et al. Cytokine Storm Signature in Patients with Moderate and Severe COVID-19. Int J Mol Sci. 2022;23(16). doi:10.3390/ijms23168879
32. Costela-Ruiz VJ, Illescas-Montes R, Puerta-Puerta JM, Ruiz C, Melguizo-Rodríguez L. SARS-CoV-2 infection: the role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 2020;54:62–75. doi:10.1016/j.cytogfr.2020.06.001
33. Neumann J, Prezzemolo T, Vanderbeke L, et al. Increased IL-10-producing regulatory T cells are characteristic of severe cases of COVID-19. Clin Transl Immunol. 2020;9(11):e1204. doi:10.1002/cti2.1204
34. Qi S, Ngwa C, Morales Scheihing DA, et al. Sex differences in the immune response to acute COVID-19 respiratory tract infection. Biol Sex Differ. 2021;12(1):66. doi:10.1186/s13293-021-00410-2
35. Jin JM, Bai P, He W, et al. Gender Differences in Patients With COVID-19: focus on Severity and Mortality. Front Public Heal. 2020;8:152. doi:10.3389/fpubh.2020.00152
36. Conti P, Younes A. Coronavirus COV-19/SARS-CoV-2 affects women less than men: clinical response to viral infection. J Biol Regul Homeost Agents. 2020;34(2):339–343. doi:10.23812/Editorial-Conti-3
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.