Back to Journals » OncoTargets and Therapy » Volume 9
Dysfunction of microRNA-32 regulates ubiquitin ligase FBXW7 in multiple myeloma disease
Received 6 February 2016
Accepted for publication 1 June 2016
Published 25 October 2016 Volume 2016:9 Pages 6573—6579
DOI https://doi.org/10.2147/OTT.S105945
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr William C. Cho
Jingsheng Hua,1,* Tianling Ding,2,* Linjun Yang3
1Department of Hematology, Taizhou Municipal Hospital, Taizhou, Zhejiang, 2Department of Hematology, Huashan Hospital, Fudan University, Shanghai, 3Department of Surgical Oncology, Taizhou Municipal Hospital, Taizhou, Zhejiang, People’s Republic of China
*These authors contributed equally to this work
Abstract: Dysfunction of microRNA (miRNA) expression has been associated with tumor occurrence, progression, and development. The aim of this work was to study the dysfunction of miR-32 – an miRNA that was abnormally regulated in different tumors – in clinical tissues from patients with multiple myeloma (MM). The tumor tissues in which we assessed miR-32 expression levels were collected during our 5 years of clinical practice. Our study found an increase in miR-32 expression in MM tissues. Assessment of F-box and WD repeat domain-containing 7 (FBXW7) in MM tissues showed an inverse relation between the expression of FBXW7 and miR-32. To further investigate the relation between miR-32 and FBXW7, cells were transfected with miR-32 or anti-miR-32. In vitro studies found that cells transfected with miR-32 showed a lower expression of FBXW7 and a higher expression of cancer-related proteins, c-Jun and c-Myc. In contrast, the cells transfected with anti-miR32 showed a relatively higher expression of FBXW7, but a lower expression of c-Jun and c-Myc. This study may offer perceptive insights into developing new strategies for MM cancer detection and therapy.
Keywords: multiple myeloma, miR-32, F-box and WD repeat domain-containing 7, in vitro
Introduction
Multiple myeloma (MM), also known as plasma cell myeloma, or myelomatosis, is a cancer disease characterized by hindered production of normal plasma blood cells due to clonal expansion and accumulation of monotypic plasma cells in bone marrow.1 MM constitutes 1% of all cancer diseases and has a 5-year survival rate of 45%.2 It is ranked as the second most common hematological malignancy in the US. However, while this disease is aggressive, most of the current cancer therapies of MM are ineffective since the disease has a multiplicity of antiapoptotic signaling mechanisms.3
MicroRNA (miRNA) is a small endogenous, single-stranded, noncoding RNA molecule that usually contains ~22 nucleotides. miRNA can cause mRNAs degradation or translational suppression by directly binding to the 3′-untranslated regions of mRNAs.4,5 In cancer biology, the dysfunction of miRNA expression has been associated with tumor occurrence, progression, and development.6–9 miR-32 is an miRNA that works very differently in several types of cancers – while it is significantly decreased in certain cancers such as gastric cancer and osteosarcoma,10,11 it is upregulated in a variety of other cancers such as colorectal cancer,12 kidney cancer,13 and prostate cancer.14 In particular, miR-32 is overexpressed and associated with pathogenesis in MM diseases.15 By global miRNA expression profiling of cancer cells from MM patients, a variety of miRNAs such as miR-21, miR-106b cluster, miR-181a, miR-181b, miR-32, and miR-17 cluster are found to be upregulated in MM patients.15–17 We, therefore, are interested in investigating the expression of miR-32 in a variety of MM patients from a clinical perspective.
F-box and WD repeat domain-containing 7 (FBXW7) has been considered as an important gene that regulates the stability of oncoprotein substrates such as c-Myc, c-Jun, mammalian target of rapamycin, and MCL.18–20 A variety of miRNAs are potentially regulated by FBXW7 due to their matched alignment in certain domains of gene sequences. Also of note is that recent studies have found that alteration of FBXW7 can result in cancer development. One example showed that loss of FBXW7 was correlated with a poor prognosis in colon cancer.21 Besides, studies also found a similar correlation between the loss of FBXW7 and poor prognoses in glioma22,23 and gastric cancer24 and breast cancer25 with FBXW7. However, very few studies have reported on the expression level of FBXW7 in MM diseases. Considering the importance of FBXW7 in cancer development, we are also interested in studying the role of this gene in MM disease and, in particular, investigating its association with miR-32.
In this work, we first assessed the expression levels of miR-32 in tissues from MM patients collected during our past 5 years of clinical practice and investigated the prognostic significance of miR-32 expression. We also studied the expression level of FBXW7 in the tissues of these patients. Our study found that FBXW7 is a significant downstream target of miR-32. To confirm our findings, we transfected cells in vitro with miR-32 or anti-miR-32 and investigated the expression level of FBXW7 in these cells. We found that the expression of FBXW7 is significantly reduced in cells transfected with miR-32. In addition, our study also found cancer-related proteins in the miR-32-transfected cells. This study may provide new insights into MM cancer treatment and diagnosis.
Materials and methods
Tissue samples
Tissue samples were collected from 132 MM patients diagnosed with the disease between the years 2005 and 2012, and written informed consent was obtained from all patients. Clinical and pathological characteristics of all patients, including age, sex, pathology, and tumor-node metastasis classification, were recorded before and after obtaining tumor tissue samples from patients. Survival information was collected from the day of diagnosing the disease until the day of patient death. All studies were approved by the medical ethics committee board of Taizhou Municipal Hospital.
All studies including human participants were performed in accordance with the ethical standards of The Research Ethical Committee at Taizhou Municipal Hospital. The experiment also in accordance with the 1964 Helsinki declaration as well as its later amendments or comparable ethical standards. Formal informed consent in written form was obtained from each patient involved in this study.
Cell culture
Human MM cell lines RPMI 8226 were obtained from Taizhou Municipal Hospital. RPMI 1640 (Cambrex, East Rutherford, NJ, USA) with 10% fetal bovine serum (Sigma-Aldrich Co., St Louis, MO, USA), 100 units of penicillin per milliliter, and 100 mg of streptomycin per milliliter (Cambrex) were used for cell culture. Cells were cultured at 37°C with 5% CO2.
Isolation of miRNA
miRNAs were extracted using the Recover All Total Nucleic Acid Isolation Kit from the formalin-fixed and paraffin-embedded tissues (Thermo Fisher Scientific, Waltham, MA, USA), following manufacturer’s instructions. NanoDrop ND-1000 (Thermo Fisher Scientific) was used to assess the absorbance of miRNA at 260/280 nm to determine its purity and concentration. All tumor tissues were protected in paraffin and stored at −80°C before usage.
Quantitative real-time reverse transcription PCR
TaqMan quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR) was used to determine the expression of miRNA with TaqMan miRNA assay kits (Thermo Fisher Scientific). The experiment was performed following the manufacturer’s instructions. Primers and probes were designed according to Universal Probe Library (Hoffman-La Roche Ltd., Basel, Switzerland) following the manufacturer’s instructions; the sequence of the primer is as follows: FBXW7 – forward 5′-AAAGAGTTGTTAGCGGTTCTCG-3′, reverse 5′-CCACATGGATACCATCAAACTG-3′, and universal probe #78; 18s rRNA – forward 5′-TGGAGGAGACGTTCCAGTGT-3′, reverse 5′-GATCTGTCCAGGCAGTCCTT-3′, and universal probe #17. The 2−ΔΔCT method was used to quantify the relative amount of miR-32.
Immunohistochemical analysis
Samples from patients were fixed in formalin and embedded in paraffin for immunohistochemical analysis. Briefly, the tissue samples were deparaffinized and soaked in sodium citrate buffer (pH 9.0, 10 mM). The tissues were then treated with 3% H2O2 in methanol for 60 minutes to block the endogenous peroxidase activity. After washing with phosphate buffered saline (PBS) for 2 minutes, the tissues were then incubated with monoclonal anti-FBXW7 (100× dilution; Abnova Corporation, Taipei, Taiwan, Republic of China), mouse monoclonal anti-c-Myc (sc-40, 500× dilution; Santa Cruz Biotechnology Inc., Dallas, TX, USA), or rabbit polyclonal anti-c-Jun (sc-1694, 500× dilution; Santa Cruz Biotechnology) antibody. EnVision reagents (EnVision Dual Link System-HRP, Dako Denmark, Glostrup, Denmark) were then used for the immunohistochemical staining of the tissue sections.
miRNA transfection
Pre-miR miRNA Precursor Molecule pre-32 (pre-miR-32, 20 nM) and anti-miR miRNA inhibitor (anti-miR-32, 100 nM) (Thermo Fisher Scientific) with Lipofectamine 2000 transfection reagent (Thermo Fisher Scientific) were used for miRNA transfection. All the procedures were performed according to the manufacturer’s instructions. Pre-miR miRNA Precursor Molecule Negative Control #1 (control pre-miR) and anti-miR miRNA Inhibitors Negative Control #1 (control anti-miR) (Thermo Fisher Scientific) were used to assess the transfection. Expressions of miR-32 and FBXW7 were assessed 72 hours after transfection.
Western blot analysis
Cells were collected from cell culture medium by centrifugation (500× g, 5 minutes), washed in PBS, and then treated with lysis buffer to isolate proteins. The lysis buffer was composed of the following components: Tris–HCl (pH 7.4, 25 mmol), NaCl (100 mmol), ethylenediaminetetraacetic acid (2 mmol), 1% Triton X, aprotinin (10 mg·mL−1), leupeptin (10 mg·mL−1), Na3CO4 (1 mmol), and phenylmethylsulfonyl fluoride (1 mmol). Protein samples (15 mg) were first resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and then transferred onto the polyvinylidene difluoride membrane. Monoclonal antibodies against c-Myc (sc-40, 500× dilution; Santa Cruz Biotechnology), c-Jun (sc-1694, 500× dilution; Santa Cruz Biotechnology), or β-actin (1,000× dilution; Sigma-Aldrich Co.) were used to incubate the membrane. Secondary antibodies labeled using the ECL Detection System (GE Healthcare UK Ltd, Little Chalfont, UK) were used to detect the signals.
Statistical analysis
Continuous variables involved in the experiments were assessed as means ± standard deviation. Student’s t-test was applied to analyze the relation between the expressions of miR-32, the FBXW7 protein, as well as the clinical and pathological characteristics of the patients. Kaplan–Meier method was used to plot the overall survival curves, and log-rank test was used to compare the survival curves. P-value <0.05 was considered as statistically significant. SPSS (v.13.0) software program (SPSS Inc., Chicago, IL, USA) was used for analysis.
Results
Clinical and pathological significance of the expression of miR-32 and FBXW7 in MM patients
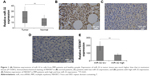
We first used qRT-PCR to assess the relative expression of miR-32 in the samples collected from 132 patients with MM. The expression level of miR-32 was normalized to U6B (miR-32/U6B ratios). Figure 1A shows the expression level of miR-32 in normal and cancer tissues. There was a higher expression level of miR-32 in cancer tissues compared to normal tissues (P<0.001). We also divided the cancer tissues into two groups based on the expression level of miRNA relative to normal tissue. Those with a value of cancer miR-32 expression/normal tissue miR-32 expression ≥1 was defined as high level group; cancer miR-32 expression/normal tissue miRNA expression <1 was considered as low level group. There were 102 cases in the high-level group and 30 cases in the low-level group. We next correlated the high-level and low-level groups with the clinical and pathological characteristics of the patients (Table 1). We did not find any significant difference in sex, age, pathological stage, but there were statistical differences (P<0.05) in tumor depth and tumor size.
  | Table 1 The expression of miR-32 in patients and the tumor’s clinical and pathological characteristics |
Immunohistochemical staining showed an inverse relation between miR-32 expression and FBXW7 protein expression in MM cancer tissues. We next investigated the expression level of FBXW7 in normal tissues and in tissues of patients having high and low miR-32 expression levels. Figure 1B, 1C, and 1D shows the immunohistochemical staining of FBXW7 in patients with normal, low, and high miR-32 expression. The complete H score of FBXW7 expression was used to show the relative expression level of FBXW7. The quantification of complete H score was defined by summing the frequency of cells with a certain fluorescence staining score (0–100) and their staining score (no staining =0; weak staining =1; moderate staining =2; and intense staining =3). We defined the tissue samples with H score ≥85 as FBXW7 high and those with H score <85 as FBXW7 low. Figure 1E shows the complete H score of FBXW7 expression. An inverse relation was found between the expression of miR-32 and FBXW7 in all the samples, where the low-level group had a relatively higher expression of FBXW7 and high-level group had a relatively lower FBXW7 expression.
Patients with low miR-32 or high FBXW7 expression had a higher 5-year survival rate. We also assessed the association of 5-year survival rate of MM patients with miR-32 and FBXW7 expressions (Figure 2A and B). Our study found that the low-miR-32 group tended to have a higher 5-year survival rate compared to high-miR-32 group (P<0.05) (Figure 2B). Similarly, the patients with lower FBXW7 expression tended to have a higher survival rate compared to patients with higher FBXW7 expression (Figure 2B).
In vitro inverse association between miR-32 expression and FBXW7 expression
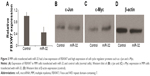
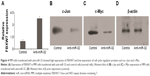
We next investigated the association between miR-32 and FBXW7 mRNA expressions in vitro by transfecting cells with miR-32. qRT-PCR analysis showed that the relative expression of FBXW7 was significantly reduced in cells transfected with miR-32 (Figure 3A). This trend was confirmed by Western blot analysis, which showed blots of two downstream oncoproteins, c-Jun and c-Myc (Figure 3B and C), with β-actin expression used as a control (Figure 3D). To further confirm the association of miR-32 expression with FBXW7 mRNA expression, the cells were transfected with anti-miR-32 to inhibit miR-32 expression. An enhanced expression of FBXW7 was observed in cells transfected with anti-miR-32 compared to the control samples (Figure 4A). These studies indicate an inverse association between miR-32 expression and FBXW7 mRNA expression.
In vitro dysfunction of c-Myc and c-Jun in miR-32 and anti-miR-32 transfected cells
We next analyzed the expression of regulatory proteins, that is, c-Myc and c-Jun, in the cells transfected with miR-32 and anti-miR-32 (Figure 4B–D). These proteins were assessed because they played significant roles in cell cycle progression, apoptosis, and cellular transformation. Compared to the control samples, the cells transfected with miR-32 showed an enhanced c-Myc and c-Jun expression. In contrast, the cells transfected with anti-miR-32 showed a reduced c-Myc and c-Jun expression (Figure 4B and D).
Discussion
This work studied the expression of miR-32 in human MM disease tissues that were collected during our 5 years of clinical practice. The dysfunction of miR-32 has been reported in several types of cancers. For example, miR-32 was reported to be upregulated in colorectal cancer,12 kidney cancer,13 and prostate cancer.14 We started our study by assessing the expression of miR-32 in our clinical MM disease samples. Our study found that miR-32 was upregulated in the disease tissues compared to normal tissues (Figure 1). Importantly, a 5-year clinical record showed that patients who had a higher expression of miR-32 had a lower survival rate. These data indicate the importance of miR-32 in regulating MM diseases.
FBXW7 plays an important role in regulating the stability of oncoprotein substrates including c-Myc and c-Jun.18–20 Of note was that recent studies have discovered the association of FBXW7 dysfunction with cancer development. One example showed that FBXW7 loss was correlated with a poor prognosis in colon cancer.21 Other studies reported the correlation between FBXW7 dissociation with glioma22,23 and gastric cancer24 and breast cancer.25 We, therefore, assessed the expression of FBXW7 in MM tissues (Figure 1C–E). Our study found that there was a lower expression of FBXW7 in cancer tissues compared to normal tissues (Figure 1B and C). We also assessed the relative expression of FBXW7 in groups with both low and high miR-32 and found that low-miR-32 group had a high expression of FBXW7. These results showed that in tumor tissues miR-32 was found to be upregulated, and the upregulated expression of miR-32 may associate with a reduced expression of FBXW7. To further confirm the inverse association between the expressions of miR-32 and FBXW7, we transfected the cells in vitro with miR-32 or anti-miR-32. Our study found that cells transfected with miR-32 showed a lower expression of FBXW7 (Figure 3A). At the same time, cells transfected with anti-miR-32 showed a higher expression of FBXW7 (Figure 4A). These two studies, therefore, suggested the inverse association between miR-32 and FBXW7 expressions.
According to our 5-year record, we correlated the survival rate of our patients with the expression level of miR-32 and FBXW7 in those patient tissues. Our record showed that tissues of patients with a lower expression level of miR-32 showed a higher viability than that of patients with a higher expression level of miR-32 (Figure 2A). Similar results were also found for FBXW7 – high-FBXW7 group had a higher viability compared to low-FBXW7 group (Figure 2B). These results further suggested the association of miR-32 and FBXW7 in the development of MM disease.
FBXW7 dysfunctions usually lead to the overexpression of cell cycle regulator genes, such as c-Myc and c-Jun, which are involved in the development of malignant cells.26,27 In most cases, these regulators are usually associated with cancer development by amplification and overexpression.2 We, therefore, assessed the expression of c-Myc and c-Jun in cancer samples in vitro. The study found that miR-32-transfected group showed an increased expression of these two proteins (Figure 3B–D). In contrast, the anti-miR-32 transfected group (low-FBXW7 group) showed a reduced expression of these two cancer-related proteins (Figure 4B and D). These results indicated that the transfection of miR-32 or anti-miR-32 led to a change in the expression of cell cycle genes (ie, c-Myc and c-Jun), where overexpression of these regulator genes usually resulted in cancer development. Importantly, our results are consistent with other clinical and biological studies that have reported an increased expression of c-Myc in MM diseases.28–30 However, further studies are needed to illustrate the association between miR-32 and FBXW7 at the molecular level.
Conclusion
In conclusion, this study analyzed the expression of miR-32 and FBXW7 in MM tumor tissues that were collected during our 5 years of clinical practice. An inverse relation was found between the expressions of miR-32 and FBXW7. This study also found an association between the viability of MM patients and expressions of miR-32 and FBXW7. To further confirm the relation between the expressions of miR-32 and FBXW7, we transfected the cells with miR-32 and anti-miR-32 and assessed the expression of FBXW7 in those cells. The in vitro studies confirmed the inverse relation between FBXW7 and miR-32 expressions, and an overexpression of cell cycle regulator genes (ie, c-Jun and c-Myc) in miR-32-transfected cells was reported, indicating the association of miR-32 overexpression with the amplification of c-Jun and c-Myc. This study may provide important implications for developing new strategies for MM cancer detection and therapy.
Disclosure
The authors report no conflicts of interest in this work.
References
Zhou YH, Alexanian R, Wang M. Targeted therapy in multiple myeloma. Curr Clin Oncol. 2008:213–236. | ||
Raab MS, Podar K, Breitkreutz I, Richardson PG, Anderson KC. Multiple myeloma. Lancet. 2009;374(9686):324–339. | ||
Oancea M, Mani A, Hussein MA, Almasan A. Apoptosis of multiple myeloma. Int J Hematol. 2004;80(3):224–231. | ||
Shukla GC, Singh J, Barik S. MicroRNAs: processing, maturation, target recognition and regulatory functions. Mol Cell Pharmacol. 2011;3(3):83–92. | ||
Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–233. | ||
Calin GA, Liu CG, Sevignani C, et al. MicroRNA profiling reveals distinct signatures in B cell chronic lymphocytic leukemias. Proc Natl Acad Sci U S A. 2004;101(32):11755–11760. | ||
Croce CM, Calin GA. miRNAs, cancer, and stem cell division. Cell. 2005;122(1):6–7. | ||
Shang C, Lu YM, Meng LR. MicroRNA-125b down-regulation mediates endometrial cancer invasion by targeting ERBB2. Med Sci Monitor. 2012;18(4):BR149–BR155. | ||
Zhang HH, Qi F, Cao YH, Zu XB, Chen MF. Expression and clinical significance of microRNA-21, maspin and vascular endothelial growth factor-C in bladder cancer. Oncol Lett. 2015;10(4):2610–2616. | ||
Xu JQ, Zhang WB, Wan R, Yang YQ. MicroRNA-32 inhibits osteosarcoma cell proliferation and invasion by targeting Sox9. Tumor Biol. 2014;35(10):9847–9853. | ||
Ambs S, Prueitt RL, Yi M, et al. Genomic profiling of microRNA and messenger RNA reveals deregulated microRNA expression in prostate cancer. Cancer Res. 2008;68(15):6162–6170. | ||
Wu W, Yang J, Feng X, et al. MicroRNA-32 (miR-32) regulates phosphatase and tensin homologue (PTEN) expression and promotes growth, migration, and invasion in colorectal carcinoma cells. Mol Cancer. 2013;12:30. | ||
Petillo D, Kort EJ, Anema J, Furge KA, Yang XJ, Teh BT. MicroRNA profiling of human kidney cancer subtypes. Int J Oncol. 2009;35(1):109–114. | ||
Ambs S, Prueitt RL, Yi M, et al. Genomic profiling of microRNA and messenger RNA reveals deregulated microRNA expression in prostate cancer. Cancer Res. 2008;68(15):6162–6170. | ||
Pichiorri F, Suh SS, Ladetto M, et al. MicroRNAs regulate critical genes associated with multiple myeloma pathogenesis. Proc Natl Acad Sci U S A. 2008;105(35):12885–12890. | ||
He L, Thomson JM, Hemann MT, et al. A microRNA polycistron as a potential human oncogene. Nature. 2005;435(7043):828–833. | ||
Zhou YM, Chen LJ, Barlogie B, et al. High-risk myeloma is associated with global elevation of miRNAs and overexpression of EIF2C2/AGO2. Proc Natl Acad Sci U S A. 2010;107(17):7904–7909. | ||
Welcker M, Clurman BE. FBW7 ubiquitin ligase: a tumour suppressor at the crossroads of cell division, growth and differentiation. Nat Rev Cancer. 2008;8(2):83–93. | ||
Mao JH, Kim IJ, Wu D, et al. FBXW7 targets mTOR for degradation and cooperates with PTEN in tumor suppression. Science. 2008;321(5895):1499–1502. | ||
Wertz IE, Kusam S, Lam C, et al. Sensitivity to antitubulin chemotherapeutics is regulated by MCL1 and FBW7. Nature. 2011;471(7336):110–114. | ||
Iwatsuki M, Mimori K, Ishii H, et al. Loss of FBXW7, a cell cycle regulating gene, in colorectal cancer: clinical significance. Int J Cancer. 2010;126(8):1828–1837. | ||
Bredel M, Bredel C, Juric D, et al. Functional network analysis reveals extended gliomagenesis pathway maps and three novel MYC-interacting genes in human gliomas. Cancer Res. 2005;65(19):8679–8689. | ||
Hagedorn M, Delugin M, Abraldes I, et al. FBXW7/hCDC4 controls glioma cell proliferation in vitro and is a prognostic marker for survival in glioblastoma patients. Cell Div. 2007;2:9. | ||
Yokobori T, Mimori K, Iwatsuki M, et al. p53-Altered FBXW7 expression determines poor prognosis in gastric cancer cases. Cancer Res. 2009;69(9):3788–3794. | ||
Ibusuki M, Yamamoto Y, Shinriki S, Ando Y, Iwase H. Reduced expression of ubiquitin ligase FBXW7 mRNA is associated with poor prognosis in breast cancer patients. Cancer Sci. 2011;102(2):439–445. | ||
Nesbit CE, Tersak JM, Prochownik EV. MYC oncogenes and human neoplastic disease. Oncogene. 1999;18(19):3004–3016. | ||
Welcker M, Singer J, Loeb KR, et al. Multisite phosphorylation by Cdk2 and GSK3 controls cyclin E degradation. Mol Cell. 2003;12(2):381–392. | ||
Anguiano A. Gene expression profiles of tumor biology provide a novel approach to prognosis and may guide the selection of therapeutic targets in multiple myeloma. J Clin Oncol. 2012;30(12):1398–1398. | ||
Chng WJ, Huang GF, Chung TH, et al. Clinical and biological implications of MYC activation: a common difference between MGUS and newly diagnosed multiple myeloma. Leukemia. 2011;25(6):1026–1035. | ||
Bai Y, Wang YL, Yao WJ, et al. Expression of miR-32 in human non-small cell lung cancer and its correlation with tumor progression and patient survival. Int J Clin Exp Pathol. 2015;8(1):824–829. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.