Back to Journals » Infection and Drug Resistance » Volume 16
During the Omicron Pandemic Wave, the Severe Systemic Inflammatory Status of COVID-19 Indicated a Higher Risk of In-Hospital Mortality and Mediated the Clinical Efficacy of Corticosteroids
Authors Cao Y, Han Y , Wu J, Sun J , Dai Y, Qiao G, Li K, Li A, Zhang Y, Ma Y, Song Q
Received 27 July 2023
Accepted for publication 16 November 2023
Published 30 November 2023 Volume 2023:16 Pages 7377—7387
DOI https://doi.org/10.2147/IDR.S432679
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Yu Cao,1 Ying Han,2 Jiangping Wu,1 Jianping Sun,3 Yanchao Dai,3 Guifang Qiao,3 Kang Li,3 Ang Li,3 Yonghong Zhang,4 Yingmin Ma,5,* Qingkun Song1,3,*
1Department of Clinical Epidemiology, Beijing Youan Hospital, Capital Medical University, Beijing, People’s Republic of China; 2Center of Liver Diseases, Beijing Youan Hospital, Capital Medical University, Beijing, People’s Republic of China; 3Center of Biobank, Beijing Youan Hospital, Capital Medical University, Beijing, People’s Republic of China; 4Department of Hepatic Intervention, Beijing Youan Hospital, Capital Medical University, Beijing, People’s Republic of China; 5Department of Respiratory and Infectious Diseases, Beijing Youan Hospital, Capital Medical University, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yingmin Ma, Department of Respiratory and Infectious Diseases, Beijing Youan Hospital, Capital Medical University, Youanmen Wai 8, Fengtai District, Beijing, 100069, People’s Republic of China, Tel/Fax +86-10-83997022, Email [email protected] Qingkun Song, Department of Clinical Epidemiology, Beijing Youan Hospital, Capital Medical University, Youanmen Wai 8, Fengtai District, Beijing, 100069, People’s Republic of China, Tel/Fax +86-10-83997022, Email [email protected]
Background: For the distinct immune/inflammatory responses from Omicron variant infection, this study aimed to investigate the diagnostic efficacy of systemic inflammatory indicators and the clinical efficacy of corticosteroids on the in-hospital mortality among COVID-19 patients.
Methods: Under a retrospective cohort study, 1081 COVID-19 patients were recruited from Beijing Youan Hospital, Capital Medical University between November 16, 2022 and January 30, 2023. We chose neutrophil-to-lymphocyte ratio (NLR), CRP-to-lymphocyte ratio (CLR), and CRP-to-albumin ratio (CAR) as the systemic inflammatory indicators. Receiver operating curve (ROC) and multivariate logistic regression analysis were used to determine the diagnostic efficacy of systemic inflammatory indicators and the association between systemic inflammatory indicators and in-hospital mortality.
Results: Among 684 patients included in analysis, 96 died during hospitalization. NLR, CLR and CAR performed well (with an area under the curve (AUC) greater than 0.75) in discriminating in-hospital mortality among COVID-19 patients. The severe status of systemic inflammation, with optimal cut-off value derived from ROC analysis, significantly associated higher risk of in-hospital mortality (OR = 3.81 for NLR ≥ 6.131; OR = 3.76 for CLR ≥ 45.455; OR = 5.10 for CAR ≥ 1.436). Corticosteroids use within 72 hours of admission increased the in-hospital mortality 2.88-fold for COVID-19 patients. In the subgroup of patients with severe systemic inflammation, corticosteroids increased the risk of in-hospital mortality (OR = 2.11 for NLR, p = 0.055; OR = 2.94 for CLR, p = 0.005; OR = 2.31 for CAR, p = 0.036).
Conclusion: Systemic inflammatory indicators had good diagnostic performance for in-hospital mortality. Patients with severe systemic inflammatory status should not receive corticosteroid treatment and further studies are warranted for confirmation.
Keywords: COVID-19, systemic inflammatory indicators, in-hospital mortality, anti-inflammatory treatment
Introduction
The pandemic of coronavirus disease 2019 (COVID-19) resulted from the infection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and lasted for more than three years. Up to February 7th 2023, 6.8 million deaths occurred from the SARS-CoV-2 infection worldwide.1 Besides wild-type SARS-CoV-2, the variants of concern were termed Alpha, Beta, Delta and Omicron, defined by the mutation on the spike protein.2
The immune and inflammatory responses were activated first to fight against the virus, and then the dysregulated inflammatory responses and cytokine storm were induced, leading the immunopathology to the organs.3 Cytokine storm, characterized as dysregulated change of cytokines, is a major cause of disease severity and death. The systemic immune status was effective to predict the prognosis of COVID-19, but the guidance of immunotherapy was seldom studied.
Immunosuppressive treatment was recommended to regulate the inflammatory responses.4 In some clinical trials, corticosteroid medicine was effective at improving the in-hospital and 28-day mortality of COVID-19.5,6 However, the clinical efficacy on mortality was based on wild-type SARS-CoV-2, but not the Omicron variant, which had a distinctive immune reaction status and clinical course.7 The efficacy of corticosteroids treating Omicron infection was unclear, especially the interaction with systemic inflammatory status.
This study aimed to investigate the prognostic value of systemic inflammatory indicators and efficacy for corticosteroid treatment on COVID-19.
Materials and Methods
Study Design
COVID-19 patients admitted to Beijing Youan Hospital, Capital Medical University, between November 16, 2022 and January 31, 2023 were enrolled in this retrospective cohort study.
Participants
The diagnosis and treatment management followed was as per the Diagnosis and Treatment protocol for COVID-19 patients (tentative 10 version), as released by the National Health Commission of China.8 The inclusion criteria included i) positive tests of nucleic acid amplification or antigen for SARS-CoV-2 at admission, ii) systemic inflammatory indicators within 3 days after admission, iii) definite prognosis (recovery and discharging from hospital, or in-hospital mortality) at the end of the study. The exclusion criteria included i) younger than 16 years, ii) pregnancy, iii) died within 2 days of admission.
Primary Outcome
The standards of hospital discharge included more than 3-day normal temperature, remission of respiratory symptoms, improvement of acute lung infiltrates on imaging, and two consecutively (interval of 24 hours at least) negative nucleic acid test or cycle threshold (Ct) value being ≥35. The primary outcome was all-cause in-hospital mortality.
Data Collection
The demographic data, clinical characteristics, diagnosis of comorbidities, laboratory data, and prognosis status were extracted from electronic medical records. The severity of COVID-19 at admission was defined as the guidelines recommended by the National Institute of Health, including asymptomatic infection, mild, moderate, severe and critical illness, based on a range of clinical manifestations.8 The comorbidities included hypertension, diabetes mellitus (DM), cardiovascular disease, malignancies, cerebrovascular disease, chronic obstructive pulmonary disease (COPD), liver cirrhosis and renal dysfunction.
The laboratory data included white blood cell count (WBC), absolute neutrophil count (NEU), absolute lymphocyte count (LYM), absolute monocyte count (MONO), and platelet count (PLT) in complete blood count test, albumin, globulin, aspartate aminotransferase (AST), glutamic-pyruvic transaminase (ALT), creatinine, urea, and estimated glomerular filtration rate (eGFR) in blood biochemistry test, fibrinogen, prothrombin time (PT), prothrombin time international normalization ratio (PTINR), and D-Dimer in coagulation test and tests of C-reactive protein (CRP) and procalcitonin.
Corticosteroid use was categorized as the use within and beyond 72 hours of admission.
Definition of Systemic Inflammatory Indicators
NEU and LYM collected from complete blood count test, albumin from the blood biochemistry test and CRP were used to calculate the systemic inflammatory indicators. We chose neutrophil-to-lymphocyte ratio (NLR), CRP-to-lymphocyte ratio (CLR), and CRP-to-albumin ratio (CAR) as the systemic inflammatory indicators.
Statistical Analysis
The continuous variables were described by mean ± standard deviation (SD) or median with interquartile range (IQR) and the categorical variables were described by N (%). The normally distributed data was compared between two groups with T-test, otherwise Wilcoxon rank-sum test. For categorical variables, differences between groups were assessed with the Chi-square test or Fisher’s exact test.
Receiver operating curve (ROC) analysis was conducted to determine the diagnostic efficacy of systemic inflammation indicators, and the area under the ROC curve (AUC) was estimated. The maximum Youden’s index was used for selecting optimal threshold of systemic inflammation indicators. The sensitivity, specificity, false positive rate, false negative rate, and Youden’s index based on the optimal cutoff point were derived from ROC analysis.
To select the covariables influencing the relationship between systemic inflammation indicators and in-hospital mortality, LASSO logistic regression models were performed. The variables, including age, history of heart failure, cerebrovascular disease and renal dysfunction, severity of COVID-19 illness at admission, and log-transformed AST, D-Dimer, procalcitonin and WBC selected by LASSO regression model, were adjusted by multivariate logistic regression models to assess the effect of systemic inflammation indicators/corticosteroids on in-hospital mortality, with odds ratio (OR), 95% confidence intervals (CI) and p value calculation. In the multivariate logistic regression model, the systemic inflammatory indicators were treated as binary categorical variables by cut-off value estimated in ROC analysis.
Statistical significance was defined as a 2-sided p ≤ 0.05, and all the statistical analysis was performed using the STATA, version 17.0 (Stata Corp., College Station, TX, USA).
Results
General Characteristics of Study Subjects
684 patients participated in the study, with 95 in-hospital deaths (Figure 1), a mean age of 69.5 years, 42.5% were females and 78.1% had a comorbidity (Table 1). The most common comorbidity was hypertension (48.1%), T2DM (26.8%), cardiovascular disease (21.6%), and renal dysfunction (21.2%) (Table 1). In terms of illness severity, 8.2%, 58.6%, 12.0% and 18.7% of patients were classified as mild, moderate, severe and critical illness at admission, respectively (Table 1).
 |
Table 1 Difference of General Characteristics Between Surviving Cases and In-Hospital Mortality Cases |
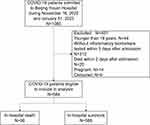 |
Figure 1 The flow chart of subject inclusion in the study. |
Older age, male gender, serious severity of illness categories and more comorbidities, were associated with in-hospital mortality (p<0.05, Table 1). Patients experiencing in-hospital death seemed to have higher levels of WBC, NEU, CRP, and a lower level of LYM (p < 0.05, Table 1). Patients with in-hospital mortality had higher levels of procalcitonin, albumin, globulin, AST, creatinine, urea, D-Dimer, and PT, but lower levels of albumin and eGFR (p < 0.05, Table 1).
Difference of Systemic Inflammatory Indicators Between Groups
Compared with patients of hospital discharge, the patients with in-hospital mortality had NLR increased by 183% (3.84 vs.10.87, p < 0.001), CAR increased by 172% (0.96 vs 2.61, p < 0.001), and CLR increased by 284% (28.31 vs 108.84, p < 0.001) (Figure 2).
 |
Figure 2 The comparison of systemic inflammatory indicators between surviving cases and fatal cases. |
The Diagnostic Efficacy of Systemic Inflammatory Indicators on In-Hospital Mortality
The AUCs of NLR, CLR and CAR were 0.794, 0.789 and 0.788, respectively, indicating good performance in discriminating in-hospital mortality (Figure 3). The cut-off value was 6.131 for NLR with a sensitivity and specificity of 82.3% and 68.9%; 1.436 for CAR with a sensitivity and specificity of 83.3% and 67.3%; and 45.455 for CLR with a sensitivity and specificity of 85.4% and 63.8% (Figure 3).
 |
Figure 3 The performance of systemic inflammatory indicators in diagnosis of in-hospital mortality among COVID-19 patients. |
The Association of Systemic Inflammatory Indicators with the Risk of in-Hospital Mortality
The risk of in-hospital mortality was associated with a 3.81-fold increase with a high level of NLR (OR = 3.81, 95% CI, 1.36–3.74), a 5.10-fold increase with a high level of CAR (OR = 5.10, 95% CI, 1.76−4.69) and a 3.76-fold increase with a high level of CLR (OR = 3.76, 95% CI, 1.32−3.77) (Table 2).
 |
Table 2 The Association Between Systemic Inflammatory Indicators and In-Hospital Mortality Among COVID-19 Patients Under Multivariate Analysis |
The Clinical Efficacy of Corticosteroids with Stratification of Systemic Inflammatory Indicators
Kaplan–Meier plot showed a significantly worse survival rate in patients treated with corticosteroid treatment within 72 hour than others (Figure 4). Adjusting multiple co-variables, corticosteroid treatment within 72 hours of admission increased the in-hospital mortality by 188% for COVID-19 patients (OR = 2.88, 95% CI, 1.51−5.53) (Table 3). ORs of corticosteroid treatment within 72 hours of admission increased to 2.52 for aged patients and 6.24 for patients with more than three comorbidities (p < 0.05, Table 3). Corticosteroid use within 72 hours of admission increased the risk of in-hospital mortality (OR = 5.54, 95% CI, 1.85−16.56) among mild/moderate illness category of COVID-19, but not in the severe/critical illness category (OR = 2.03, 95% CI, 0.92−4.52, Table 3). Additionally, among the patients with severe systemic inflammatory status, corticosteroid treatment within 72 hours of admission increased the risk of in-hospital mortality to 2.11-fold (high level of NLR, p = 0.055), 2.94-fold (high level of CLR, p = 0.005) and 2.31-fold high (high level of CAR, p = 0.036) (Table 3). Patients with regular systemic inflammatory status did not have a significantly increase of in-hospital mortality on corticosteroid use (Table 3).
 |
Table 3 The Clinical Efficacy of Corticosteroid Use on In-Hospital Mortality with Stratification of Systemic Inflammatory Indicators Under Multivariate Analysis |
 |
Figure 4 The Kaplan-Meier plot of patient survival in three groups, based on the use of corticosteroid. |
Discussion
In summary, among 684 COVID-19 patients analyzed in this study, 96 patients with higher levels of NLR, CAR and CLR at admission died during hospitalization. NLR, CAR and CLR had good performances in discriminating COVID-19 mortality with AUC greater than 0.75. Similar results have been arrived upon in previous studies. NLR shows 78.8% (95% CI, 73.5–83.2%) sensitivity and 73.0% (95% CI, 68.4–77.1%) specificity for mortality in the meta-analysis integrating 64 studies,9 and a higher CAR has a good discriminatory‐power to predict COVID‐19 mortality (AUC = 0.81, 95% CI 0.74–0.87, p < 0.001) in the pooled analysis with eight studies.10 But there are variations on the optimal cut-off of these inflammatory indicators for risk stratification under the consideration of potential differences in population-specific characteristics, virus strains, and the normal range reference of laboratory tests.
In line with previous research, our study demonstrated that higher levels of NLR, CAR and CLR were independently associated with a higher risk of COVID-19 in-hospital mortality. The dysregulated immune/inflammatory responses triggered by the infection of SARS-CoV-2 are commonly characterized with the elevation of NEU or CRP and the reduction of LYM or albumin,11–14 and the imbalanced status is pronounced in severe or fatal cases with a cytokine storm.15 A previous study has found that COVID-19 patients admitted to the ICU have high NLR combined with high CLR, while non-ICU individuals have low NLR combined with low CLR.16 In the mechanism, it is reported that lymphocyte apoptosis is due to the substantial cell migration to the site of infection where the immune response is initiated.17 SARS-CoV-2 infection induces the secretion of pro-inflammatory cytokines, such as interleukin (IL)-6, IL-18, IL-15, IL-2, interferon g (IFN-g), and tumor necrosis factor α (TNF-α),18 which activates the hepatocytes to synthesize numerous acute-phase reactants,19 such as CRP.20 Inversely, pro-inflammatory factors negatively affect the hepatocytes to produce albumin, increasing the catabolism of albumin and downregulating the synthesis of albumin.21 Even worse, cytokines are able to increase capillary permeability of albumin into the interstitial space among COVID-19 patients,10,22 exacerbating the low level of albumin.
Regarding the mechanism of corticosteroids therapy in COVID-19, the presence of corticosteroids can inhibit the SARS-CoV-2 induced NF-κB activation, which blocks the elevation of pro-inflammatory cytokines, such as TNFα, IL-6, IL-1β and IL-8, thereby exerting the anti-inflammatory and immunosuppressive activity.23 Based on this, corticosteroids are widely used in severe COVID-19,4 but its clinical efficacy is still inconsistent. In some trials, the prescription of corticosteroids is effective to significantly reduce the in-hospital and 28-day mortality of COVID-19.5,6 However, in a meta-analysis involving only observational studies, corticosteroid is slightly associated with reduced 30-day mortality, but not associated with in-hospital mortality and 120-day mortality.24 Inversely, among patients without respiratory support, corticosteroid has a relationship with a higher risk of death.24 The remarkable difference on efficacy of corticosteroid may be explained by the heterogeneity of participants, and its dose25,26 and type.27 The RECOVRY trial shows that, compared with low-dose corticosteroid, higher dose corticosteroid increases the 28-day mortality with RR of 1.59 (95% CI, 1.20–2.10).26 Moreover, administration of corticosteroids significantly elevates the risk of developing a bacterial/fungal superinfection in COVID-19 patients (HR = 2.80, 95% CI, 1.33–5.9), and then results in unfavorable outcomes, which could counteract the favorable effect of corticosteroids on death.28 Also, the inflammation status could influence the effect of corticosteroid treatment. A retrospective cohort study reports that corticosteroid is associated with a lower risk of 60-day all-cause mortality in patients with an NLR > 6.11, rather than in patients with an NLR ≤ 6.11.29 To be noted, the patients included in the forementioned study were infected with wild-type virus, not the Omicron variant, which partly contributes to the inconsistency with our findings.
Distinct with wild-type SARS-CoV-2, the Omicron variant has 32 mutations on the spike protein,2 which causes a series of varying features, ranging from inflammatory responses against the infection to the clinical characteristics and course.7 During the six waves of different SARS-CoV-2 pandemic strains, the lowest NLR was observed in the Delta wave, and the lowest CLR was observed in the Omicron wave in critical patients, and a major difference is identified in lymphocyte count dynamic change during hospitalization between wild-type and Omicron waves.30 Based on the different inflammatory responses among virus variants, it was plausible that immunoregulatory therapies of glucocorticoids might fluctuate.
During the Omicron BA.2.2 wave in Hong Kong, the concomitant corticosteroid use is associated with a 1.34-fold increased risk of virus burden rebound.31 Methylprednisolone use results in a higher risk of prolonged virus shedding time of SARS-CoV-2 Omicron BA.2.2 (OR = 2.39, 95% CI 0.71–8.02).32 In a real-world retrospective cohort study,33 dexamethasone increases the risk of death, with a comparison of anti-inflammatory treatment or tocilizumab (TCZ, an inhibitor of IL-6 receptors); and dexamethasone combined with TCZ has a higher risk of death than TCZ alone,34 indicating the adverse effect of corticosteroids on IL-6 increase,34,35 despite that tocilizumab treatment could not reduce the risk of death in COVID-19 (HR = 0.83, 95% CI, 0.38–1.8).36 Further studies should focus on the clinical practice of corticosteroids on Omicron and other pandemic variants and the directive biomarkers of host immune status, particularly longitudinal change of biomarkers and its guidance role to clinical therapies.
The study had some potential limitations. First, this study was under a retrospective cohort design and the inherent limitation of observational study made it unable to assess the causal relationship. Second, we only recruited a fraction of patients with definitive laboratory measurements, which may bring some selection bias and reduce the power of the statistical test. Third, information about vaccination against COVID-19 was not collected which may cause some confounding bias. According to the previous studies, no significant differences of inflammatory indicators were observed between COVID-19 breakthrough cases and unvaccinated cases,37 and among recipients of different vaccine doses as well,38 which may mitigate the confounding bias to some extent. Fourth, due to the similarity of dose, type and duration of corticosteroids use in this cohort, our current study could not answer whether these factors have influence on mortality.
Conclusions
Systemic inflammatory indicators had good diagnostic performance for in-hospital mortality. Patients with severe systemic inflammatory status may not gain any benefit from receiving corticosteroid treatment and further studies were warranted for confirmation.
Ethics Approval
This study complied with the Helsinki Declaration for investigation of human subjects and was approved by the ethics committee of Beijing Youan Hospital, Capital Medical University (LL-2023-092-K). Informed consent was obtained from the participants.
Acknowledgments
This study was supported by Mr. Song Guo and Ms. Likun Yang from the Department of Internet, Beijing Youan Hospital, Capital Medical University.
Funding
This study was supported by Beijing Natural Science Foundation (L222120), High Level Public Health Technical Talents Construction Project from Beijing Municipal Health Commission (2022-2-025) and Bejing Hospital’s Authority (XMLX202114). The supporting organizations had no role in study design, data collection, analysis, and interpretation.
Disclosure
The authors report no conflicts of interest in this work.
References
1. World Health Organization. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
2. Tian D, Sun Y, Xu H, Ye Q. The emergence and epidemic characteristics of the highly mutated SARS-CoV-2 Omicron variant. J Med Virol. 2022;94(6):2376–2383. doi:10.1002/jmv.27643
3. Ye Q, Wang B, Mao J. The pathogenesis and treatment of the `Cytokine Storm’ in COVID-19. J Infect. 2020;80(6):607–613. doi:10.1016/j.jinf.2020.03.037
4. Cao X. COVID-19: immunopathology and its implications for therapy. Nat Rev Immunol. 2020;20(5):269–270. doi:10.1038/s41577-020-0308-3
5. Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704.
6. Sterne JAC, Murthy S, Diaz JV, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324(13):1330–1341. doi:10.1001/jama.2020.17023
7. Wang Y, Wang B, Zhao Z, et al. Effects of SARS-CoV-2 omicron BA.1 spike mutations on T-cell epitopes in mice. Viruses. 2023;3:15.
8. Diagnosis and Treatment protocol for COVID-19 Available from: http://www.nhc.gov.cn/.
9. Parthasarathi A, Padukudru S, Arunachal S, et al. The Role of neutrophil-to-lymphocyte ratio in risk stratification and prognostication of COVID-19: a systematic review and meta-analysis. Vaccines. 2022;2022:10.
10. Rathore SS, Oberoi S, Iqbal K, et al. Prognostic value of novel serum biomarkers, including C-reactive protein to albumin ratio and fibrinogen to albumin ratio, in COVID-19 disease: a meta-analysis. Rev Med Virol. 2022;32:e2390.
11. Shen Y, Cheng C, Zheng X, et al. Elevated procalcitonin is positively associated with the severity of COVID-19: a Meta-analysis based on 10 cohort studies. Medicina. 2021;257.
12. Moisa E, Corneci D, Negoita S, et al. Dynamic changes of the neutrophil-to-lymphocyte ratio, systemic inflammation index, and derived neutrophil-to-lymphocyte ratio independently predict invasive mechanical ventilation need and death in critically ill COVID-19 patients. Biomedicines. 2021;9(11):1656. doi:10.3390/biomedicines9111656
13. Li Y, Deng Y, Ye L, et al. Clinical significance of plasma D-dimer in COVID-19 mortality. Front Med. 2021;8:638097. doi:10.3389/fmed.2021.638097
14. Ghahramani S, Tabrizi R, Lankarani KB, et al. Laboratory features of severe vs. non-severe COVID-19 patients in Asian populations: a systematic review and meta-analysis. Eur J Med Res. 2020;25(1):30. doi:10.1186/s40001-020-00432-3
15. Chen R, Lan Z, Ye J, et al. Cytokine storm: the primary determinant for the pathophysiological evolution of COVID-19 deterioration. Front Immunol. 2021;12:589095. doi:10.3389/fimmu.2021.589095
16. Ben Jemaa A, Salhi N, Ben Othmen M, et al. Evaluation of individual and combined NLR, LMR and CLR ratio for prognosis disease severity and outcomes in patients with COVID-19. Int Immunopharmacol. 2022;109:108781. doi:10.1016/j.intimp.2022.108781
17. Belaid B, Lamara Mahammad L, Mihi B, et al. T cell counts and IL-6 concentration in blood of North African COVID-19 patients are two independent prognostic factors for severe disease and death. J Leukoc Biol. 2022;111(1):269–281. doi:10.1002/JLB.4COVA1020-703R
18. García LF. Immune response, inflammation, and the clinical spectrum of COVID-19. Front Immunol. 2020;11:1441. doi:10.3389/fimmu.2020.01441
19. Hu H, Pan H, Li R, He K, Zhang H, Liu L. Increased circulating cytokines have a role in COVID-19 severity and death with a more pronounced effect in males: a systematic review and meta-analysis. Front Pharmacol. 2022;13:802228. doi:10.3389/fphar.2022.802228
20. Eissa M, Shaarawy S, Abdellateif MS. The Role of Different Inflammatory Indices in the Diagnosis of COVID-19. Int J Gen Med. 2021;14:7843–7853. doi:10.2147/IJGM.S337488
21. Karakoyun I, Colak A, Turken M, et al. Diagnostic utility of C-reactive protein to albumin ratio as an early warning sign in hospitalized severe COVID-19 patients. Int Immunopharmacol. 2021;91:107285. doi:10.1016/j.intimp.2020.107285
22. Soeters PB, Wolfe RR, Shenkin A. Hypoalbuminemia: pathogenesis and clinical significance. J Parenter Enteral Nutr. 2019;43(2):181–193. doi:10.1002/jpen.1451
23. Olajide OA, Iwuanyanwu VU, Lepiarz-Raba I, Al-Hindawi AA. Induction of exaggerated cytokine production in human peripheral blood mononuclear cells by a recombinant SARS-CoV-2 spike glycoprotein S1 and its inhibition by dexamethasone. Inflammation. 2021;44(5):1865–1877. doi:10.1007/s10753-021-01464-5
24. Wagner C, Griesel M, Mikolajewska A, et al. Systemic corticosteroids for the treatment of COVID-19: equity-related analyses and update on evidence. Cochrane Database Syst Rev. 2022;11:Cd014963.
25. Toroghi N, Abbasian L, Nourian A, et al. Comparing efficacy and safety of different doses of dexamethasone in the treatment of COVID-19: a three-arm randomized clinical trial. Pharmacol Rep. 2022;74(1):229–240. doi:10.1007/s43440-021-00341-0
26. Group RC. Higher dose corticosteroids in patients admitted to hospital with COVID-19 who are hypoxic but not requiring ventilatory support (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2023;401(10387):1499–1507. doi:10.1016/S0140-6736(23)00510-X
27. Kellogg D, Gutierrez GC, Small CE, et al. Safety and efficacy of methylprednisolone versus dexamethasone in critically ill patients with COVID-19 acute respiratory distress syndrome: a retrospective study. Ther Adv Infect Dis. 2023;10:20499361231153546. doi:10.1177/20499361231153546
28. Novacescu AN, Buzzi B, Bedreag O, et al. Bacterial and fungal superinfections in COVID-19 patients hospitalized in an intensive care unit from timișoara, Romania. Infect Drug Resist. 2022;15:7001–7014. doi:10.2147/IDR.S390681
29. Cai J, Li H, Zhang C, et al. The neutrophil-to-lymphocyte ratio determines clinical efficacy of corticosteroid therapy in patients with COVID-19. Cell Metab. 2021;33(2):258–69.e3. doi:10.1016/j.cmet.2021.01.002
30. Jemaa AB, Oueslati R, Guissouma J, et al. Differences in leucocytes and inflammation-based indices among critically ill patients owing to SARS-CoV-2 variants during several successive waves of COVID-19 pandemic. Int Immunopharmacol. 2023;124(Pt A):110836. doi:10.1016/j.intimp.2023.110836
31. Wong CKH, Lau KTK, Ich A, et al. Viral burden rebound in hospitalised patients with COVID-19 receiving oral antivirals in Hong Kong: a population-wide retrospective cohort study. Lancet Infect Dis. 2023;23(6):683–695. doi:10.1016/S1473-3099(22)00873-8
32. Zhong W, Yang X, Jiang X, et al. Factors associated with prolonged viral shedding in older patients infected with Omicron BA.2.2. Front Public Health. 2022;10:1087800. doi:10.3389/fpubh.2022.1087800
33. Zarębska-Michaluk D, Jaroszewicz J, Rogalska M, et al. Effectiveness of tocilizumab with and without dexamethasone in patients with severe COVID-19: a retrospective study. J Inflamm Res. 2021;14:3359–3366.
34. Shankar-Hari M, Vale CL, Godolphin PJ, et al. Association between administration of IL-6 antagonists and mortality among patients hospitalized for COVID-19: a meta-analysis. JAMA. 2021;326:499–518.
35. Awasthi S, Wagner T, Venkatakrishnan AJ, et al. Plasma IL-6 levels following corticosteroid therapy as an indicator of ICU length of stay in critically ill COVID-19 patients. Cell Death Discov. 2021;7:55.
36. Stone JH, Frigault MJ, Serling-Boyd NJ, et al. Efficacy of tocilizumab in patients hospitalized with Covid-19. N Engl J Med. 2020;383(24):2333–2344.
37. Tian D, Song Y, Zhang M, et al. Genomic, immunological, and clinical analysis of COVID-19 vaccine breakthrough infections in Beijing, China. J Med Virol. 2022;94(5):2237–2249. doi:10.1002/jmv.27636
38. Rzymski P, Pazgan-Simon M, Simon K, et al. Clinical characteristics of hospitalized COVID-19 patients who received at least one dose of COVID-19 vaccine. Vaccines. 2021;9(7):781. doi:10.3390/vaccines9070781
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
