Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 16
Dual Bronchodilator Therapy as First-Line Treatment in Maintenance-Naïve Patients with Symptomatic COPD: A Pre-Specified Analysis of the EMAX Trial
Authors Bjermer L , Boucot IH , Maltais F , Kerwin EM , Naya IP, Tombs L, Jones PW , Compton C, Lipson DA , Vogelmeier CF
Received 15 February 2021
Accepted for publication 20 May 2021
Published 28 June 2021 Volume 2021:16 Pages 1939—1956
DOI https://doi.org/10.2147/COPD.S291751
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Russell
Leif Bjermer,1 Isabelle H Boucot,2 François Maltais,3 Edward M Kerwin,4 Ian P Naya,2 Lee Tombs,5 Paul W Jones,2 Chris Compton,2 David A Lipson,6,7 Claus F Vogelmeier8
1Respiratory Medicine and Allergology, Lund University, Lund, Sweden; 2Global Specialty & Primary Care, GSK, Brentford, Middlesex, UK; 3Centre de Pneumologie, Institut Universitaire de Cardiologie et de Pneumologie de Québec, Université Laval, Québec, Canada; 4Clinical Research Institute of Southern Oregon, Medford, OR, USA; 5Precise Approach Ltd, contingent worker on assignment at GSK, Stockley Park West, Uxbridge, Middlesex, UK; 6Respiratory Clinical Sciences, GSK, Collegeville, PA, USA; 7Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA; 8Department of Medicine, Pulmonary and Critical Care Medicine, University Medical Center Giessen and Marburg, Philipps-Universität Marburg, Member of the German Center for Lung Research (DZL), Marburg, Germany
Correspondence: Leif Bjermer
Respiratory Medicine and Allergology, Skane University Hospital, 221 85 Lund, Sweden
Tel +46 46 17 23 25
Email [email protected]
Introduction: Limited prospective evidence is available to guide selection of first-line maintenance therapy in patients with COPD. This pre-specified analysis of the EMAX trial explored the efficacy and safety of dual- versus mono-bronchodilator therapy in maintenance-naïve and maintenance-treated patients.
Methods: The 24-week EMAX trial evaluated lung function, symptoms (including rescue medication use), exacerbations, and safety with umeclidinium/vilanterol, umeclidinium, and salmeterol in symptomatic patients at low exacerbation risk who were not receiving inhaled corticosteroids. Maintenance-naïve and maintenance-treated subgroups were defined by maintenance bronchodilator use 30 days before screening.
Results: The analysis included 749 (31%) maintenance-naïve and 1676 (69%) maintenance-treated patients. For both subgroups, improvements from baseline in trough FEV1 at Week 24 (primary endpoint) were greater with umeclidinium/vilanterol versus umeclidinium (mean difference [95% CI]; maintenance-naïve: 44 mL [1, 87]; maintenance-treated: 77 mL [50, 104]), and salmeterol (maintenance-naïve: 128 mL [85, 171]; maintenance-treated: 145 mL [118, 172]), and in rescue medication inhalations/day over 24 weeks versus umeclidinium (maintenance-naïve: − 0.44 [− 0.73, − 0.16]; maintenance-treated: − 0.28 [− 0.45, − 0.12]) and salmeterol (maintenance-naïve: − 0.37 [− 0.66, − 0.09]; maintenance-treated: − 0.25 [− 0.41, − 0.08]). In maintenance-naïve patients, umeclidinium/vilanterol numerically improved scores at Week 24 for Transition Dyspnea Index versus umeclidinium (0.37 [− 0.21, 0.96]) and versus salmeterol (0.47 [− 0.10, 1.05]) and Evaluating Respiratory Symptoms–COPD versus umeclidinium (− 0.26 [− 1.04, 0.53]) and versus salmeterol (− 0.58 [− 1.36, 0.20]), with similar improvements seen in maintenance-treated patients. All treatments were well tolerated across both subgroups.
Conclusion: Similar to maintenance-treated patients, maintenance-naïve patients receiving umeclidinium/vilanterol showed greater improvements in lung function and symptoms compared with patients receiving umeclidinium or salmeterol. These findings provide support for the consideration of dual bronchodilator treatment in symptomatic maintenance-naïve patients with COPD.
Keywords: COPD treatment, first-line therapy, maintenance-naïve, umeclidinium/vilanterol, umeclidinium, salmeterol
Plain Language Summary
Why was study done?
Many patients with chronic obstructive pulmonary disease (COPD) require daily medication, known as maintenance treatment, to help achieve long-term disease control. The Global Initiative for Chronic Obstructive Lung Disease strategy recommends that most of these patients are initially treated with one long-term bronchodilator medication, and are prescribed a second bronchodilator if their symptoms persist or worsen. However, initiating treatment with two bronchodilators may be appropriate for symptomatic patients with COPD, and may lead to better outcomes.
What did researchers do/find?
We analyzed data from a clinical trial (EMAX) that compared the relative benefits of a combination of two bronchodilators, umeclidinium/vilanterol (UMEC/VI), with a single bronchodilator (either UMEC or salmeterol [SAL]) over 6 months. In this analysis, we looked specifically at patients who had not been receiving any other maintenance treatment for COPD in the month before enrollment in the trial (maintenance-naïve), and patients who had received maintenance treatment in the month before the trial (maintenance-treated). We found that maintenance-naïve and maintenance-treated patients receiving UMEC/VI had better lung function and improved symptoms, were less dependent on their reliever inhaler, and were less likely to experience disease worsening than patients receiving UMEC or SAL.
What do these results mean?
A combination of two bronchodilators, such as UMEC/VI, may be better than a single bronchodilator for treating symptomatic patients with COPD, whether or not they have previously received maintenance treatment. Physicians should consider prescribing a combination of two bronchodilators as initial treatment for patients with significant symptom burden who have not previously received long-term maintenance treatment for COPD.
Introduction
Many patients with chronic obstructive pulmonary disease (COPD) remain symptomatic while on one long-acting bronchodilator.1 It is well established that COPD symptoms have a detrimental impact on health status and quality of life, with many symptomatic patients also experiencing anxiety and/or depression.2,3 Furthermore, symptomatic patients are at increased risk of exacerbations and have poorer disease prognosis than those with well-controlled symptoms.4,5
Meta-analyses of clinical trial data have shown that dual long-acting muscarinic antagonist (LAMA)/long-acting β2-agonist (LABA) combination therapies provide greater improvements in lung function, symptoms, and health-related quality of life, as well as a reduced risk of exacerbations compared with LAMA or LABA monotherapy.6–9 Furthermore, dual LAMA/LABA therapies have a similar safety profile to LAMA or LABA monotherapies, with no apparent increase in the incidence of serious adverse events (SAEs).7 As such, dual long-acting bronchodilators may be an appropriate option for initial maintenance therapy in symptomatic patients. In recognition of this, the American Thoracic Society has made a strong recommendation for the use of LAMA/LABA combination therapy over LAMA or LABA monotherapy in patients with COPD and dyspnea or exercise intolerance.10 In addition, some national guidelines, including those provided by the UK National Institute for Health and Care Excellence, recommend dual bronchodilator treatment as first-line therapy in certain patients, such as those without features suggesting steroid responsiveness or asthma, who remain breathless or experience exacerbations despite use of short-acting bronchodilators.11 In contrast, for patients with symptomatic COPD and low risk of exacerbations the Global Initiative for Chronic Obstructive Lung Disease (GOLD) recommends initial maintenance treatment with LAMA or LABA monotherapy, or LAMA/LABA dual therapy for those with severe breathlessness.12 Current recommendations for first-line LAMA/LABA use are therefore inconsistent, and have been formulated based on limited evidence from clinical trials in patients already receiving maintenance therapy, often combination treatments, and usually in the absence of any prospective data on the efficacy of dual bronchodilators as first-line maintenance therapy. Available evidence to guide clinicians in their choice of first-line therapy for patients with COPD is therefore limited.
Previous studies suggest that a considerable population of patients have a significant symptom burden and exacerbation risk and yet do not receive regular maintenance therapy and remain maintenance-naïve (MN).13–15 Post hoc evidence in MN patients indicates that dual bronchodilator therapy may have larger beneficial effects on lung function, symptoms, health status, rescue medication use, and COPD stability compared with monotherapy.13,15–17 Early initiation of LAMA/LABA therapy may therefore improve outcomes for symptomatic patients with COPD; however, prospective studies are required to corroborate this.
The Early MAXimization of bronchodilation for improving COPD stability (EMAX) trial investigated the efficacy and safety of umeclidinium/vilanterol (UMEC/VI; LAMA/LABA), umeclidinium (UMEC; LAMA), and salmeterol (SAL; LABA) in symptomatic patients with COPD at low risk of exacerbation who were not receiving inhaled corticosteroids (ICS).18 In this pre-specified subgroup analysis, clinical benefits and safety of UMEC/VI compared with UMEC and SAL were evaluated in MN patients and in patients who were receiving maintenance treatment prior to the start of the study (maintenance-treated [MT] patients). A secondary aim of this analysis was to observe the mean improvements from baseline with UMEC/VI, UMEC, or SAL in MN and MT patients.
Materials and Methods
Study Design
The multicenter, double-blind, double-dummy, 3-arm parallel-group EMAX trial randomized patients 1:1:1 to 24 weeks of UMEC/VI (62.5/25 µg) once daily via the ELLIPTA inhaler, UMEC (62.5 µg) once daily via ELLIPTA, or SAL (50 µg) twice-daily via the DISKUS inhaler. The trial was conducted between June 2017 and June 2018, and full details of the study design have been published previously.18
This study was performed according to the Declaration of Helsinki and received appropriate ethical approval (Supplementary Table S1). All patients provided written informed consent via a form signed at either the Pre-screening or Screening visit.
Patients
Eligible patients were ≥40 years of age and were current or former smokers with a diagnosis of COPD, pre- and post-albuterol forced expiratory volume in 1 second/forced vital capacity (FEV1/FVC) ratio <0.7, post-albuterol FEV1 of ≥30–≤80% predicted (corresponding to GOLD grade 2 or 3), COPD Assessment Test (CAT) score ≥10, and ≤1 moderate and no severe exacerbations in the previous year. Prior to screening and during the 4-week run-in period, treatment with ≤1 maintenance bronchodilator monotherapy (LAMA or LABA) was permitted, with all eligible patients continuing to be symptomatic (CAT score ≥10) on their run-in treatments. Patients had no ICS or ICS/LABA treatment for ≥6 weeks prior to run-in, and no LAMA/LABA combination therapy for ≥2 weeks prior to run-in. After the run-in period, study treatment was given for 24 weeks.
For this analysis, MN and MT subgroups were defined by maintenance bronchodilator use during the period from 30 days prior to screening until the first dose of study treatment. Over this period, MN patients did not receive any COPD maintenance medications except for short-acting bronchodilators as rescue medication.
Endpoints
This secondary analysis of the EMAX trial included spirometry, patient-reported outcomes (PROs), exacerbations, short-term disease stability (clinically important deteriorations [CID]), and safety outcomes. Spirometry assessments comprised change from baseline in trough FEV1, FVC, and inspiratory capacity (IC). PROs comprised change from baseline for self-administered computerized Transition Dyspnea Index (SAC-TDI) focal score, Evaluating Respiratory Symptoms–COPD (E-RS) total score, rescue medication use (including inhalations/day and the proportion of rescue-free days), global assessment of disease severity (GADS), St George’s Respiratory Questionnaire (SGRQ) total score, and CAT total score. Response rates were calculated based on the proportion of individual patients with a ≥1-unit improvement in SAC-TDI score, ≥2-point reduction in E-RS score from baseline, ≥4-point reduction in SGRQ score from baseline, and ≥2-unit improvement in CAT score from baseline. Short-term disease worsening outcomes included time to first moderate/severe exacerbation and risk of a first CID. For individual patients, risk of a first CID was assessed using three different composite definitions. Definition 1 comprised a first moderate or severe exacerbation, and/or a decrease in trough FEV1 from baseline of ≥100 mL, and/or a deterioration in health status indicated by an increase in SGRQ score from baseline of ≥4 units. Definition 2 was similar to Definition 1, except that a CAT deterioration (≥2 units from baseline) replaced the SGRQ deterioration. Definition 3 comprised a first moderate or severe exacerbation, and/or a SGRQ deterioration, and/or a CAT deterioration, and/or a SAC-TDI deterioration indicated by a decrease of ≥1 unit. Safety was assessed based on the incidence of adverse events (AEs) and SAEs.
Statistical Analysis
The EMAX trial was powered to detect differences between treatments for trough FEV1 and SAC-TDI at Week 24 in the intent-to-treat (ITT) population; however, the trial was not powered to detect treatment differences in the smaller maintenance treatment subgroups. For this reason, treatment differences with 95% confidence intervals (CI) are described without reference to statistical significance within subgroups, although P-values are presented in the figures and tables.
Full details of the statistical analyses have been reported previously.18 Comparisons of change from baseline in lung function and PROs used mixed model repeated measures (MMRM) analyses. Least squares (LS) mean and LS mean change from baseline with estimated treatment differences are reported with 95% CIs. Response rates were compared using generalized linear mixed model analyses, and odds ratios (OR) with 95% CIs are reported. For time to first CID, hazard ratios (HR) and 95% CIs were calculated using a Cox proportional hazards model. A full description of the covariates included in each model is included in the Supplementary Methods.
To assess improvements from baseline with UMEC/VI, UMEC, and SAL in MN and MT patients, multidimensional plots of LS mean change from baseline in the four clinical outcomes of interest (trough FEV1 at Week 24 [upward axis], SAC-TDI focal score at Week 24 [rightward axis], rescue medication mean inhalations/day over Weeks 1–24 [downward axis], and SGRQ score at Week 24 [leftward axis]) are presented for each subgroup.
Safety endpoints were analyzed descriptively.
Results
Study Population
The ITT population included 2425 patients, of whom 749 (31%) were MN (UMEC/VI: 250, UMEC: 250, SAL: 249) and 1676 (69%) were MT (UMEC/VI: 562, UMEC: 554, SAL: 560). Overall, 619 (83%) MN patients and 1431 (85%) MT patients completed study treatment. In the MN subgroup, 84%, 80%, and 84% of patients receiving UMEC/VI, UMEC, and SAL completed the trial; the corresponding proportions in the MT subgroup were 90%, 81%, and 85%. A post hoc analysis revealed that the risk of early withdrawal in the MT subgroup was lower with UMEC/VI than with UMEC (risk reduction [95% CI]: 48% [28, 62]) or SAL (36% [10, 54]). In the MN subgroup, the difference in risk of early withdrawal was 24% (−15, 50) with UMEC/VI versus UMEC and 5% (−47, 39) versus SAL.
Baseline demographics and clinical characteristics for each subgroup are described in Table 1 and were well balanced between treatment arms (Supplementary Table S2). Compared with the MT subgroup, the MN subgroup was slightly younger and included a greater proportion of females and current smokers. The MN subgroup appeared to have better lung function on average than the MT subgroup, as indicated by a higher mean FEV1 and a larger proportion of individuals who were classified as GOLD grade 2 versus 3. Furthermore, the MN subgroup had more severe symptom burden and worse health status (as indicated by higher mean CAT, E-RS, and SGRQ scores), as well as greater mean daily albuterol use than the MT subgroup. Fewer MN than MT patients had a moderate exacerbation in the previous year.
 |
Table 1 Baseline Demographics and Clinical Characteristics |
Lung Function Outcomes
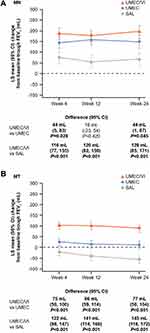
In MN patients, mean (95% CI) change from baseline in trough FEV1 at Week 24 was 44 mL (1, 87) and 128 mL (85, 171) greater with UMEC/VI versus UMEC and SAL, respectively (Table 2; Figure 1). Mean change from baseline in trough FVC and trough IC at Week 24 were also generally greater with UMEC/VI versus UMEC and SAL in MN patients (Table 2).
 |
Table 2 Change from Baseline in Lung Function Outcomes, Rescue Medication Use, and GADS in MN and MT Patients |
In MT patients, mean (95% CI) change from baseline in trough FEV1 at Week 24 was 77 mL (50, 104) and 145 mL (118, 172) greater with UMEC/VI versus UMEC and SAL, respectively (Table 2; Figure 1). Mean changes from baseline in trough FVC and trough IC at Week 24 were also greater with UMEC/VI versus UMEC and SAL (Table 2).
In both MN and MT subgroups, mean changes from baseline in trough FEV1, and trough FVC at Week 24 were greater with UMEC versus SAL (Supplementary Table S3).
Symptom Severity and Health Status Outcomes
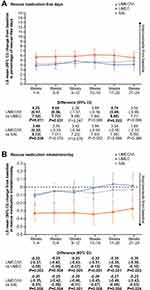
In the MN subgroup, mean improvements from baseline in SAC-TDI focal score and E-RS total score at all time points (Figure 2A–B) and the odds of responding for either outcome (Figure 3A) were greater with UMEC/VI versus UMEC or SAL. Mean improvements in rescue medication inhalations/day and the proportion of rescue-free days were also greater with UMEC/VI versus both monotherapies at all time points (Figure 4A–B) and over Weeks 1–24 (Table 2). A higher proportion of patients receiving UMEC/VI reported GADS to be improved by Week 24 (68%) compared with UMEC (65%) and SAL (63%) (Table 2).
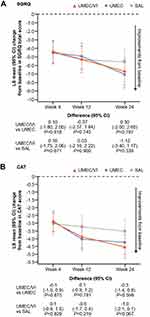
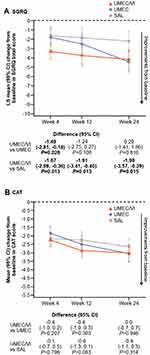
For SGRQ and CAT score, treatment differences favoring UMEC/VI were not consistently seen across all time points, although mean improvements from baseline in CAT score at Week 24 were numerically greater with UMEC/VI compared with monotherapy (Figure 5A–B). The odds of responding for either outcome were similar with UMEC/VI and UMEC, but were greater with UMEC/VI versus SAL (Figure 3A).
For MT patients, mean improvements from baseline in SAC-TDI focal score and E-RS total score were greater with UMEC/VI versus UMEC or SAL at all time points (Figure 6A–B), and the odds of responding for each outcome were greater with UMEC/VI compared with UMEC or SAL (Figure 3B). Mean improvements from baseline in the percentage of rescue-free days were greater with UMEC/VI versus monotherapy at all time points, although this was less clear for mean rescue medication inhalations/day (Figure 7A–B); mean change from baseline for both endpoints over Weeks 1–24 was greater with UMEC/VI versus UMEC or SAL (Table 2). A greater proportion of patients reported the overall severity of their COPD (GADS) improved from baseline to Week 24 with UMEC/VI (66%) versus UMEC (60%) and SAL (61%) (Table 2).
Mean improvements from baseline in SGRQ and CAT scores were consistently greater with UMEC/VI over SAL, but not UMEC, at all time points (Figure 8A–B). The odds of being a responder for either SGRQ or CAT were numerically greater with UMEC/VI than with UMEC or SAL (Figure 3B).
For both subgroups, SAC-TDI scores at Week 24 were similar with UMEC and SAL; mean improvements from baseline in E-RS total score at Weeks 21–24 were slightly greater with UMEC versus SAL (Supplementary Table S3). Improvements from baseline in rescue medication use were generally similar with UMEC and SAL, although improvement from baseline in the percentage of rescue-free days was slightly larger with SAL than UMEC in the MN subgroup (Supplementary Table S3). In both subgroups, a similar proportion of patients reported improvement in the overall severity of their COPD from baseline to Week 24 with UMEC compared with SAL (Supplementary Table S3).
Exacerbations and Disease Stability Outcomes
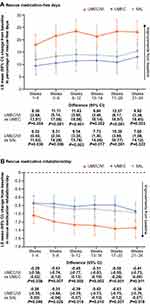
In the MN subgroup, the risk of a first moderate/severe exacerbation with UMEC/VI was similar versus UMEC, with a risk reduction of 8% (HR: 0.92; 95% CI: −68, 49), and lower versus SAL with a risk reduction of 42% (HR: 0.58; 95% CI: 0, 66). There was a reduced risk of a first CID with UMEC/VI versus UMEC for two of the three CID definitions, and versus SAL for all three definitions (Figure 9A).
In MT patients, the risk of a first moderate/severe exacerbation was reduced by 22% with UMEC/VI versus UMEC (HR: 0.78; 95% CI: −5, 42) and 33% versus SAL (HR: 0.67; 95% CI: 11, 50). The risk of a first CID was reduced with UMEC/VI versus UMEC and versus SAL for all three CID definitions (Figure 9B).
Effect of Maintenance Therapy Status on Mean Improvements from Baseline
The overall impact of each treatment was evaluated using multidimensional plots of the four clinical outcomes of interest (trough FEV1 at Week 24 [upward axis], SAC-TDI focal score at Week 24 [rightward axis], rescue medication mean inhalations/day over Weeks 1–24 [downward axis], and SGRQ score at Week 24 [leftward axis]). For both MN (Figure 10A) and MT (Figure 10B) subgroups, greater LS mean changes from baseline were observed with UMEC/VI compared with UMEC and SAL for all outcomes except SGRQ. For both subgroups, a greater total area inside the plots was seen for UMEC/VI versus either monotherapy, indicating greater benefits across the assessed clinical outcomes. For all four endpoints, improvements from baseline were numerically greater in the MN subgroup than in the MT subgroup for all treatment arms. For improvements from baseline in trough FEV1 and rescue medication use, there was no overlap in CIs between the MN and MT subgroups for either outcome in any treatment arm, suggesting consistently greater improvements for MN versus MT patients irrespective of treatment (Figure 10). A similar pattern of improvements from baseline was observed when these outcomes were assessed at Week 4 for MN and MT patients (Supplementary Figure S1). Absolute mean values at Week 24 (or over Weeks 1–24 for rescue medication use) for all four measures were similar between the MN and MT subgroups (Supplementary Figure S2).
Safety
The incidence of patients reporting on-treatment AEs and SAEs was similar in all treatment arms in both subgroups (Table 3). In the MN subgroup, no SAEs were considered drug-related and no fatal SAEs were reported. In the MT subgroup, eight fatal SAEs were reported (four each in the UMEC/VI and UMEC treatment arms); however, no fatal or non-fatal SAEs were considered related to the study medication. As expected based on the known safety profile of long-acting bronchodilators, and consistent with the ITT population,18 the most common AE was nasopharyngitis in both subgroups (Table 3).
 |
Table 3 Adverse Events |
Discussion
This pre-specified analysis of the EMAX trial by patient maintenance therapy status prior to randomization found larger improvements in lung function and reductions in rescue medication use, which may be an indirect marker of reduced symptom burden, in patients receiving UMEC/VI compared with either monotherapy from Week 4 to Week 24, in both the MN and MT subgroups. Rescue medication benefits of UMEC/VI included both a reduced number of uses per day and increased rescue-free days versus UMEC and SAL, suggesting that patients’ symptoms improved as they were less dependent on their rescue inhaler.19 For SAC-TDI and E-RS at Week 24, UMEC/VI also showed numerically larger improvements from baseline versus monotherapy. In addition, risk of exacerbations and risk of CID were generally reduced with UMEC/VI versus monotherapy for both subgroups. Patients in both subgroups also showed generally greater improvements with UMEC versus SAL for lung function (trough FEV1, trough FVC, and trough IC), but not for symptoms outcomes (SAC-TDI, E-RS, and rescue medication use) or COPD severity (GADS). All treatments were equally well tolerated in both subgroups. Taken together, these data support an incremental efficacy benefit on lung function, rescue medication use and greater disease stability when using dual bronchodilator treatment as initial maintenance therapy.
In the GOLD strategy report, maintenance bronchodilator therapy is recommended for patients with symptomatic COPD,12 yet 31% of patients in this study were MN. This is consistent with prior estimates of the proportion of patients with COPD who do not receive any maintenance treatment despite being symptomatic.14 The lack of previous maintenance treatment suggests that the symptoms of these patients had been neglected and not treated appropriately, as these patients had been diagnosed with COPD for the same length of time as the MT patients, and had similar CAT scores. One potential reason for this could be that patients do not seek appropriate assessment and maintenance treatment due to a disparity between patient perceived and actual COPD severity.5 Furthermore, a greater proportion of MN than MT patients were current smokers; in the former group, it is possible that COPD symptoms such as dyspnea have been misattributed to smoking, further contributing to underdiagnosis and undertreatment.20,21 This highlights the importance of thorough assessment and treatment (including smoking cessation) in MN patients with COPD symptoms, to prevent further deterioration.12
A secondary objective of this analysis of the EMAX trial was to explore whether MN patients demonstrated greater improvements from baseline with dual- or mono-bronchodilator therapy compared with MT patients. There was a consistent trend for greater mean improvements from baseline in MN versus MT patients across all three treatment regimens for FEV1, SAC-TDI, rescue medication use, and SGRQ. This suggests that MN patients, who were receiving no maintenance treatment at the start of the trial, gained greater benefit from bronchodilator therapy than MT patients, who were already receiving bronchodilator therapy. MN patients had a greater mean rescue medication use at baseline compared with the MT subgroup, indicating that these patients were dependent on short-acting bronchodilators to control their COPD symptoms in the absence of adequate maintenance treatment.22,23 Accordingly, the MN subgroup had more severe symptom burden and worse health status at baseline (as indicated by E-RS, CAT, and SGRQ scores), but both subgroups had a similar mean lung function, symptoms, and health status at the end of the study. These numerically larger mean improvements from baseline in the MN versus MT subgroup suggest a significant unmet need for effective maintenance treatment among this population. However, it should be noted that in the responder analyses for SAC-TDI, E-RS, SGRQ, and CAT, point estimates for the odds of responding were generally higher in MT then in MN patients. Furthermore, comparisons between the MN and MT subgroups should be made with caution, since the study was not powered for this analysis and there were potentially confounding differences between the subgroups at baseline. For example, a previous analysis of the EMAX trial has indicated that high SABA use may be a confounding factor when analyzing symptomatic treatment benefits between maintenance bronchodilators.19
The findings of this prospective analysis are generally consistent with previous post hoc analyses. A pooled analysis of 1056 MN patients from two randomized controlled trials found statistically significant improvements in lung function and symptoms with the LAMA/LABA combination aclidinium bromide/formoterol fumarate compared with its monocomponents.15 Another pooled analysis of 533 MN patients showed a significant improvement in trough FEV1 with UMEC/VI versus tiotropium that was numerically larger in the MN subgroup (146 mL) than in the ITT population (95 mL), as well as significant improvements in rescue medication use and CID risk.13 In a subgroup analysis of 678 MN patients in the OTEMTO studies (powered to detect changes in the primary outcomes of SGRQ score and FEV1), greater improvements in lung function, SGRQ score, and TDI score were seen with tiotropium/olodaterol versus tiotropium monotherapy in MN patients.16 Furthermore, a pooled analysis of the Phase III PINNACLE studies showed significant lung function improvements with glycopyrrolate/formoterol fumarate versus its monocomponents in MN patients.17 Overall, the available data, including the results of this analysis, indicate that dual bronchodilator therapy provides incremental benefits with regard to lung function and symptoms when used as initial maintenance therapy.
A key strength of this study was the prospective, well-balanced randomization of a well-characterized MN population. Patients in both subgroups were not receiving concurrent ICS, which can influence the efficacy of add-on therapy, particularly LABA, in patients with COPD.22,24 Certain study limitations should be considered; most importantly, the MN population represented only 31% of the overall study population and the study was not powered to detect treatment differences in the MN or MT subgroups. This is reflected in the inconsistency of the CID results, as in the MN population the reduction in risk of a CID favored UMEC/VI versus UMEC in two of the three definitions of a CID. A greater proportion of patients receiving monotherapy withdrew from the trial compared with patients receiving UMEC/VI, particularly in the UMEC arm, which may have reduced the observed treatment differences between dual and monotherapy due to a “healthy survivor” effect.25 UMEC/VI was compared with SAL rather than VI (the LABA component of UMEC/VI) in this post-registration trial because VI was not licensed as a monotherapy in any country. However, an indirect treatment comparison has suggested that SAL and VI are likely to have broadly similar effects on trough FEV1 and PROs including TDI.26 Additional studies that are powered to detect treatment differences for lung function and symptoms outcomes in MN patients are needed to further evaluate the benefits of initial maintenance therapy with dual versus mono bronchodilator initial maintenance therapy in symptomatic patients with COPD at low risk of exacerbations.
Conclusions
In this pre-specified subgroup analysis of patients with COPD in the EMAX trial, UMEC/VI provided clinically important additional improvements in lung function and symptoms and was well tolerated compared with UMEC and SAL monotherapy in MN patients, as well as in MT patients. There was an expected trend for greater improvement from baseline in lung function and rescue medication use in MN patients than in MT patients, for all treatments. Despite the small sample size in each subgroup, these findings provide support for the consideration of dual bronchodilator treatment early in symptomatic maintenance-naïve patients with COPD.
Data Sharing Statement
Anonymized individual participant data and study documents can be requested for further research from www.clinicalstudydatarequest.com.
Ethics Approval and Informed Consent
This study was performed according to the Declaration of Helsinki and received appropriate ethical approval (Supplementary Table S1). All patients provided written informed consent via a form signed at either the Pre-screening or Screening visit.
Acknowledgments
Editorial support (in the form of writing assistance during development of the initial draft, assembling tables and figures, collating authors comments, grammatical editing, and referencing) was provided by Mark Condon, DPhil, and Meghan Betts, PhD, of Fishawack Indicia Ltd, UK, part of Fishawack Health, and was funded by GSK. ELLIPTA and DISKUS are owned by/licensed to the GSK group of companies.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. LB, FM, and EMK were involved in acquisition of data and data analysis and interpretation. IHB and CFV were involved in data analysis and interpretation. IPN, LT, PWJ, CC, and DAL were involved in study conception and design, and data analysis and interpretation.
Funding
This study was funded by GlaxoSmithKline (GSK study number: 201749 [NCT03034915]). GSK-affiliated authors had a role in study design, data analysis, data interpretation, and writing of the report and GSK funded the article processing charges and open access fee.
Disclosure
IHB, DAL, CC, and PWJ are employees of GSK and hold stocks and shares in GSK. IHB’s current affiliation is Medical Emerging Markets, GSK, Brentford, Middlesex, UK. IPN was an employee of GSK at the time of the study, holds stocks and shares in GSK, and was a contingent worker on assignment at AstraZeneca. IPN’s current affiliation is RAMAX Ltd, Bramhall, Cheshire, UK. LT is a contingent worker on assignment at GSK. FM has received research grants for participating in multicenter trials for AstraZeneca, Boehringer Ingelheim, GSK, Sanofi, and Novartis, and has received unrestricted research grants and personal fees from Boehringer Ingelheim, Grifols, and Novartis. LB has received honoraria for giving a lecture or attending an advisory board for Airsonett, ALK-Abello, AstraZeneca, Boehringer Ingelheim, Chiesi, GSK, Meda, Novartis and Teva. EMK has served on advisory boards, speaker panels or received travel reimbursement from for Amphastar, AstraZeneca, Boehringer Ingelheim, Connect Biopharma, GSK, Mylan, Novartis, Pearl, Sunovion, Teva, and Theravance and has received consulting fees from Cipla and GSK. CFV has received grants from AstraZeneca, Boehringer Ingelheim, Chiesi, GSK, Grifols, Novartis, and the German Federal Ministry of Education and Research (BMBF) Competence Network Asthma and COPD (ASCONET), and has received personal fees from AstraZeneca, Boehringer Ingelheim, Berlin Chemie/Menarini, Chiesi, CSL Behring, GSK, Grifols, MedUpdate, Novartis, Nuvaira, and Teva. The authors report no other conflicts of interest in this work.
References
1. Dransfield MT, Bailey W, Crater G, Emmett A, O’Dell DM, Yawn B. Disease severity and symptoms among patients receiving monotherapy for COPD. Primary Care Respir j. 2011;20(1):46–53. doi:10.4104/pcrj.2010.00059
2. Doyle T, Palmer S, Johnson J, et al. Association of anxiety and depression with pulmonary-specific symptoms in chronic obstructive pulmonary disease. Int J Psychiatry Med. 2013;45(2):189–202. doi:10.2190/PM.45.2.g
3. Tsiligianni I, Kocks J, Tzanakis N, Siafakas N, van der Molen T. Factors that influence disease-specific quality of life or health status in patients with COPD: a review and meta-analysis of Pearson correlations. Primary Care Respir j. 2011;20(3):257–268. doi:10.4104/pcrj.2011.00029
4. Roche N, Chavannes NH, Miravitlles M. COPD symptoms in the morning: impact, evaluation and management. Respir Res. 2013;14(1):112. doi:10.1186/1465-9921-14-112
5. Miravitlles M, Ribera A. Understanding the impact of symptoms on the burden of COPD. Respir Res. 2017;18(1):67. doi:10.1186/s12931-017-0548-3
6. Mammen MJ, Pai V, Aaron SD, Nici L, Alhazzani W, Alexander PE. Dual LABA/LAMA Therapy versus LABA or LAMA Monotherapy for COPD: a Systematic Review and Meta-analysis in Support of the American Thoracic Society Clinical Practice Guideline. Ann Am Thorac Soc. 2020;17(9):1133–1143. doi:10.1513/AnnalsATS.201912-915OC
7. Oba Y, Sarva ST, Dias S. Efficacy and safety of long-acting beta-agonist/long-acting muscarinic antagonist combinations in COPD: a network meta-analysis. Thorax. 2016;71(1):15–25. doi:10.1136/thoraxjnl-2014-206732
8. Rodrigo GJ, Price D, Anzueto A, et al. LABA/LAMA combinations versus LAMA monotherapy or LABA/ICS in COPD: a systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2017;12:907–922. doi:10.2147/COPD.S130482
9. Calzetta L, Rogliani P, Matera MG, Cazzola M, Systematic Review A. With Meta-Analysis of Dual Bronchodilation With LAMA/LABA for the Treatment of Stable COPD. Chest. 2016;149(5):1181–1196. doi:10.1016/j.chest.2016.02.646
10. Nici L, Mammen MJ, Charbek E, et al. Pharmacologic management of chronic obstructive pulmonary disease. an official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med. 2020;201(9):e56–e69. doi:10.1164/rccm.202003-0625ST
11. National Institute for Health and Care Excellence (NICE). Chronic obstructive pulmonary disease in over 16s: diagnosis and management; 2019. Available from: https://goldcopd.org/wp-content/uploads/2019/12/GOLD-2020-FINAL-ver1.2-03Dec19_WMV.pdf.
12. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease; 2021. Available from: https://goldcopd.org/wp-content/uploads/2019/12/GOLD-2020-FINAL-ver1.2-03Dec19_WMV.pdf.
13. Maleki-Yazdi MR, Singh D, Anzueto A, Tombs L, Fahy WA, Naya I. Assessing Short-term Deterioration in Maintenance-naive Patients with COPD Receiving Umeclidinium/Vilanterol and Tiotropium: a Pooled Analysis of Three Randomized Trials. Adv Ther. 2016;33(12):2188–2199. doi:10.1007/s12325-016-0430-6
14. Takahashi T, Ichinose M, Inoue H, Shirato K, Hattori T, Takishima T. Underdiagnosis and undertreatment of COPD in primary care settings. Respirology. 2003;8(4):504–508. doi:10.1046/j.1440-1843.2003.00501.x
15. Singh D, D’Urzo AD, Donohue JF, et al. An Evaluation Of Single And Dual Long-Acting Bronchodilator Therapy As Effective Interventions In Maintenance Therapy-Naive Patients With COPD. Int J Chron Obstruct Pulmon Dis. 2019;14:2835–2848. doi:10.2147/COPD.S217710
16. Singh D, Maleki-Yazdi MR, Tombs L, Iqbal A, Fahy WA, Naya I. Prevention of clinically important deteriorations in COPD with umeclidinium/vilanterol. Int J Chron Obstruct Pulmon Dis. 2016;11:1413–1424. doi:10.2147/COPD.S101612
17. Zheng J, Xu JF, Jenkins M, Assam PN, Wang L, Lipworth BJ. Glycopyrrolate/formoterol fumarate metered dose inhaler for maintenance-naïve patients with chronic obstructive pulmonary disease: a post-hoc analysis of the randomized PINNACLE trials. Respir Res. 2020;21(1):69. doi:10.1186/s12931-020-1332-3
18. Maltais F, Bjermer L, Kerwin EM, et al. Efficacy of umeclidinium/vilanterol versus umeclidinium and salmeterol monotherapies in symptomatic patients with COPD not receiving inhaled corticosteroids: the EMAX randomised trial. Respir Res. 2019;20(1):238. doi:10.1186/s12931-019-1193-9
19. Maltais F, Naya I, Vogelmeier CF, et al. Salbutamol use in relation to maintenance bronchodilator efficacy in COPD: a prospective subgroup analysis of the EMAX trial. Respir Res. 2020;21(1):280. doi:10.1186/s12931-020-01451-8
20. Lange P, Halpin DM, O’Donnell DE, MacNee W. Diagnosis, assessment, and phenotyping of COPD: beyond FEV(1). Int J Chron Obstruct Pulmon Dis. 2016;11:3–12. doi:10.2147/COPD.S85976
21. Kaplan A, Thomas M. Screening for COPD: the gap between logic and evidence. Eur Respir Rev. 2017;26(143):143. doi:10.1183/16000617.0113-2016
22. Naya I, Tombs L, Lipson DA, Boucot I, Compton C. Impact of prior and concurrent medication on exacerbation risk with long-acting bronchodilators in chronic obstructive pulmonary disease: a post hoc analysis. Respir Res. 2019;20(1):60. doi:10.1186/s12931-019-1027-9
23. Punekar YS, Sharma S, Pahwa A, Takyar J, Naya I, Jones PW. Rescue medication use as a patient-reported outcome in COPD: a systematic review and regression analysis. Respir Res. 2017;18(1):86. doi:10.1186/s12931-017-0566-1
24. Sion KYJ, Huisman EL, Punekar YS, Naya I, Ismaila AS. A Network Meta-Analysis of Long-Acting Muscarinic Antagonist (LAMA) and Long-Acting β2-Agonist (LABA) Combinations in COPD. Pulm Ther. 2017;3(2):297–316. doi:10.1007/s41030-017-0048-0
25. Vestbo J, Anderson JA, Calverley PMA, et al. Bias due to withdrawal in long-term randomised trials in COPD: evidence from the TORCH study. Clin Respir J. 2010;5:44–49. doi:10.1111/j.1752-699X.2010.00198.x
26. Donohue JF, Betts KA, Du EX, et al. Comparative efficacy of long-acting beta2-agonists as monotherapy for chronic obstructive pulmonary disease: a network meta-analysis. Int J Chron Obstruct Pulmon Dis. 2017;12:367–381. doi:10.2147/COPD.S119908
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.