Back to Journals » International Journal of General Medicine » Volume 14
Drug–Drug Interactions of Newly Approved Direct-Acting Antiviral Agents in Patients with Hepatitis C
Authors Gao LH, Nie QH, Zhao XT
Received 25 September 2020
Accepted for publication 26 November 2020
Published 28 January 2021 Volume 2021:14 Pages 289—301
DOI https://doi.org/10.2147/IJGM.S283910
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Lu-Hua Gao, Qing-He Nie, Xi-Tai Zhao
Center of Infectious Diseases, Second Affiliated Hospital, Air-Force Military Medical University, Xi’an 710038, People’s Republic of China
Correspondence: Qing-He Nie
Center of Infectious Diseases, Second Affiliated Hospital, Air-Force Military Medical University, 569 Xinsi Road, Baqiao District, Xi’an 710038, People’s Republic of China
Email [email protected]
Abstract: Hepatitis C is a major health problem worldwide, frequently resulting in cirrhosis and increasing the risk of hepatocellular carcinoma significantly. In recent years, the advent of direct-acting antivirals (DAAs) has dramatically improved the therapeutic outcomes in hepatitis C patients. In the last two years, several new DAA combinations have been approved for the treatment of the hepatitis C virus (HCV) infection, including elbasvir/grazoprevir, sofosbuvir/velpatasvir, sofosbuvir/velpatasvir/voxilaprevir, and glecaprevir/pibrentasvir. The newly approved DAA regimens may be prescribed with other drugs simultaneously, increasing the potential of pharmacokinetic interactions. Therefore, the knowledge and management of drug–drug interactions (DDIs) with DAAs should be considered a key issue in HCV therapy. This review summarizes researches of DDIs focusing on newly approved DAAs (elbasvir, grazoprevir, velpatasvir, voxilaprevir, glecaprevir, pibrentasvir) for patients undergoing HCV treatment to provide clinical consideration for comedication. With respect to DDIs, newly approved DAA regimens, including elbasvir/grazoprevir, sofosbuvir/velpatasvir, sofosbuvir/velpatasvir/voxilaprevir, and glecaprevir/pibrentasvir, are safely applicable.
Keywords: drug–drug interaction, direct-acting antiviral, chronic hepatitis C, pharmacokinetic, comedication
Introduction
The hepatitis C virus (HCV) has been a major health problem since its discovery in 1989, affecting over 184 million people globally, and remained mostly undetected.1 About 25% chronic HCV patients develop Cirrhosis and account for a majority of HCV-related morbidity and mortality.1 The conventional therapeutic strategy for an HCV infection is pegylated interferon plus ribavirin with sustained virologic response rates (SVR) of 70–80% for HCV 2 or 3 genotypes and SVR of 45–70% for HCV 1, 4, 5, and 6 genotypes.2 However, the frequent use and severe adverse effects associated with interferon-based regimens give rise to numerous contraindications, resulting in an unsatisfactory overall efficacy of HCV treatment.
Since 2011, improved cure rates have been observed with the first generation of oral direct-acting antivirals (DAAs) combined with interferon and ribavirin.3 Because of fewer side effects, sustained virological response rate over 90%, and shortened treatment durations involved in DAA treatment, the US Food and Drug Administration (FDA) no longer suggests a combination of DAA oral regiments with interferon therapy after 2013.4 Recently, the DAAs have shown dramatic advances in the therapeutic outcomes in hepatitis C patients owing to their high efficacy and favorable safety profile.5 Despite the high cost, all-oral regimens of DAAs are strongly recommended by several hepatitis C treatment guidelines and have been widely accepted by patients.6–8 Advancely, DAA programs have been established to improve liver-related outcomes, such as reducing the risk of cirrhosis, hepatocellular carcinoma, decompensated liver disease, and all-cause mortality.9–12 Since December 2017, more than 10 types of DAAs have been approved for the treatment of hepatitis C. Although highly effective and well tolerable, DAAs can result in drug–drug interactions (DDIs) with multiple drugs as they participate in common metabolic pathways, such as cytochrome P450 (CYP450), organic anion transporting polypeptides (OATP), multidrug resistance protein (MRP), P-glycoprotein (P-gp), and breast cancer resistance protein (BCRP). Functioning as inducers, substrates, and/or inhibitors of metabolizing enzymes and transporters, DAAs can influence the plasma concentrations of coadministered drugs, augmenting toxicity or reducing effectiveness. As the use of comedications is frequent and diversified in chronic hepatitis C patients, a majority of patients are at the risk of DDIs induced lower efficacy or toxicity of DAAs or comedications.13,14 Therefore, none of the DAAs are completely free of DDIs, which can significantly alter drug exposure, and thus their efficacy and toxicity.
Considering a broad range of indications, the newly approved DAA regimens contained sofosbuvir/velpatasvir/voxilaprevir, elbasvir/grazoprevir, and glecaprevir/pibrentasvir, which may incur more interactions with comedications in the last two years. Appropriately, handling the pharmacokinetic interactions is imperative to improve the therapeutic effectiveness and safety in HCV-infected patients. Previous reviews summarized the relationship between DDIs and DAAs in different drug classes or specific drugs.15–19 This review summarizes the researches into newly approved DAAs (elbasvir, grazoprevir, velpatasvir, voxilaprevir, glecaprevir, pibrentasvir) associated DDIs, endeavoring to identify optimal treatment regiment for individual patients.
Elbasvir/Grazoprevir
Elbasvir (ie, MK-8742) and grazoprevir (ie, MK-5172) are inhibitors of the HCV-specific NS5A protein and NS3/4A protease, respectively (Figure 1). Elbasvir (50 mg) and grazoprevir (100 mg) fixed-dose combination have been recommended for the treatment of HCV infection of genotypes 1 and 4. Elbasvir/grazoprevir is administered once a day, with or without the presence of food.20 Given patients who have not been treated or have been treated with pegylated interferon combined with ribavirin, the recommended treatment duration is as follows: (1) In HCV genotype 1a patients without baseline NS5A polymorphisms, the treatment duration is 12 weeks; (2) In HCV genotype 1a and NS5A baseline polymorphism patients treatment is 16 weeks with ribavirin; (3) In HCV genotype 1b, treatment duration is 12 weeks. The approved course of treatment for HCV genotype 1a or 1b patients is 12 weeks prior to treatment with pegylated interferon and ribavirin plus protease inhibitors and ribavirin. The approved treatment period for the treatment-naïve HCV genotype 4 patients is 12 weeks. Finally, in the case of patients with HCV genotype 4, the previously approved treatment period for polyvinyl interferon combined with ribavirin is 16 weeks. These protocols are appropriate in patients with or without compensatory cirrhosis. Dosing of elbasvir/grazoprevir for any level of renal impairment, including hemodialysis, is not recommended based on gender or race/ethnicity. In patients with mild hepatic impairment (Child-Pugh A), elbasvir/grazoprevir can be safely used without dosage adjustment but is contraindicated in patients with moderate or severe hepatic impairment (Child-Pugh B or C) as grazoprevir exposure was significantly increased by 5- to 12-fold. Addition of ribavirin and a longer duration of 16 weeks are recommended in baseline NS5A resistance-associated polymorphism patients. Notably, no dose recommendations have been provided for children under 18 years old and elderly patients (Supplementary Figure 1).
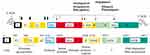 |
Figure 1 Mechanism of DAAs. Elbasvir, an inhibitor of NS5A; Grazoprevir, an inhibitor of the NS3/4A protease; Glecaprevir, an inhibitor of NS3/4A protease; Pibrentasvir, an inhibitor of NS5A; Velpatasvir, an inhibitor of the NS5A protein; Voxilaprevir, an inhibitor of the NS3/4A protease. Solid diamonds denote sites of the hepatitis C virus (HCV) polyprotein precursor cleavaged by the endoplasmic reticulum signal peptidase. Amino acid positions are shown above each protein. The open diamond indicates further processing of the core protein by signal peptide peptidase. Arrows indicate sites cleavaged by HCV NS2–3 and NS3 proteases. Asterisks in the E1 and E2 region indicate the glycosylation of the envelope proteins. Reproduced from Moradpour D, Brass V, Gosert R, Wölk B, Blum HE, Hepatitis C: molecular virology and antiviral targets, Trends in Molecular Medicine, 2002;8:476–482. Copyright (2002), with permission from Elsevier.88 Abbreviation: NCR, noncoding region. |
The absolute bioavailabilities of elbasvir and grazoprevir are 32% and 27%, respectively.21 In the case of grazoprevir, a high-fat diet increases the area under the curve (AUC) by about 1.5-times and Cmax by about 2.8-times. Slight decreases in AUC0-inf and Cmax have been observed with elbasvir and elbasvir/grazoprevir and may be administered without consideration of food (Co.). Elbasvir and grazoprevir are the substrates for CYP3A and P-gp and P-gp seems to have minimal negative effect on the absorption of the two drugs.22 Furthermore, grazoprevir may be transported by BCRP and it is a substrate for OATP1B1/3. Elbasvir is a CYP3A inhibitor in vitro, while grazoprevir is a weak CYP3A inhibitor in humans. Both elbasvir and grazoprevir are inhibitors of BCRP, but elbasvir is a weak inhibitor of P-gp, and grazoprevir is a weak inhibitor of CYP3A4.23 Therefore, strong inducers of CYP3A4, including carbamazepine, phenytoin, rifampicin, and efavirenz are contraindicated and moderate inducers, such as nafcillin, etravirine, and modafinil, are not recommended. Furthermore, CYP3A4 inhibitors are not recommended to applied combined with elbasvir and grazoprevir.24 Additionally, OATP inhibitors including cyclosporine, atazanavir, lopinavir, darunavir, and tipranavir are contraindicated with the elbasvir/grazoprevir regimen. The elimination of elbasvir and grazoprevir mainly occurs in the liver, with an average time of 24 h and 31 h, respectively.25 As Elbasvir and grazoprevir are affected by oxidative metabolism, the combination of mainly CYP3A, whether CYP3A substrate or strong CYP3A inducer, with elbasvir/grazoprevir is forbidden as they can significantly reduce the plasma concentrations of elbasvir and grazoprevir to decrease the therapeutic effect. Whereas with few accurate clinical data, elbasvir/grazoprevir combined with a moderate dose of CYP3A inducer is still not recommended.5 Furthermore, as both drugs are metabolized by the liver, they are prohibited in patients with moderate and severe liver injury (Child-Pugh B or C).26 The peak plasma concentration is attained in 3 h for elbasvir and 2 h for grazoprevir. Elbasvir binds to plasma proteins at a rate of 99.9% and grazoprevir binds to plasma proteins at a rate of 98.8%.27 They are mainly eliminated by feces (>90%) and minimally excreted by urine (<1%), indicating that dosage adjustment is unnecessary in patients with renal impairment (Co.). The C-SURFER study demonstrated that elbasvir/grazoprevir can be safely used in hemodialysis patients with advanced chronic kidney disease.28 Several real-world studies had confirmed the efficacy and safety of elbasvir/grazoprevir in severe chronic kidney disease patients, including those that have renal replacement therapy.29–32
The pharmacokinetics of elbasvir/grazoprevir or comedications may be influenced by potential common metabolic pathways, resulting in reduced efficacy and other adverse events. Studies in healthy subjects evaluated the potential pharmacokinetic interactions of grazoprevir when coadministrated with inducers or inhibitors of CYP3A, BCRP, OATP, and P-gp. The results indicated that grazoprevir (as a CYP3A/BCRP inhibitor) caused 3-fold, 34%, and 43% increase in AUC of atorvastatin, midazolam, and atazanavir, respectively. The AUC of grazoprevir decreased by 84% when coadministrated with efavirenz, and the AUC increased by 10.58, 12.86, 7.5, 12.61, and 8.35 fold, respectively, when coadministrated with efavirenz, atazanavir, ritonavir, or pantoprazole based on the pharmacokinetic parameters of fixed-dose combination of lopinavir/ritonavir, darunavir/ritonavir, and a single intravenous or oral dose of rifampin.22,33,34 Given that the solubility of elbasvir and grazoprevir is pH-dependent, Feng et al35 performed pharmacokinetic studies in 16 healthy subjects to evaluate the efficacy of famotidine, elbasvir, and grazoprevir demonstrated that famotidine and pantoprazole had no clinically relevant effects on the pharmacokinetics of the elbasvir/grazoprevir coadministration. It could be speculated that acid-reducing agents including H2-receptor antagonists, proton-pump inhibitors, and antacids, with pharmacokinetic properties which is similar to famotidine or pantoprazole, may be administrated with elbasvir and grazoprevir without dose adjustment. When elbasvir/grazoprevir regimen was combined with ketoconazole, the AUC and Cmax value for elbasvir were increased by 80% and 29%, respectively, while the AUC and Cmax value for grazoprevir increased by 202% and 13%, respectively.5
Coinfection with HCV is extremely common in patients with HIV infection (10%–30%). In HIV-infected patients who received injectable treatment (90%), HCV treatment faced toa therapeutic challenge due to the complex interactions between antiretroviral agents and DAAs.36 A pharmacokinetic study had assessed the potential interactions between grazoprevir and ritonavir-boosted HIV protease inhibitors, indicating that the atazanavir AUC0-24 was modestly increased by 43% with co-administrated grazoprevir, while atazanavir AUC0-24 was not impacted by lopinavir and darunavir. Additionally, the grazoprevir AUC0-24 was significantly increased by atazanavir/ritonavir, lopinavir/lopinavir, and darunavir/ritonavir by 10.58-fold, 12.86-fold, and 7.5-fold, respectively.34 It is clear that ritonavir-boosted HIV protease inhibitors are not eligible for coadministration combined with fixed-dose elbasvir/grazoprevir. However, other antiretroviral agents such as tenofovir, abacavir, emtricitabine, lamivudine, raltegravir, dolutegravir, and rilpivirine can be safely administered with elbasvir/grazoprevir without adverse effects during the HCV or HIV therapy confirmed in the C-EDGE CO-INFECTION and C-WORTHY studies.37,38
Frequent HIV/HCV coinfection and opioid agonist therapy lead to poor patients compliance and complicated DDIs, chronic hepatitis C therapy remains a huge challenge in subjects who used injectable drugs. In addition, no clinically meaningful pharmacokinetic interactions have been observed among elbasvir, grazoprevir, buprenorphine, and naloxone.39,40 Results from a placebo-controlled, randomized, and double-blind trial documented that the fixed-dose combination of elbasvir (50 mg) and grazoprevir (100 mg) was effective in the treatment of HCV infection in patients receiving opioid agonist therapy and with ongoing drug use, including amphetamines, barbiturates, benzodiazepines, cannabinoids, cocaine, opioids, phencyclidine, and propoxyphene.41 Moreover, DDIs between DAAs and immunosuppressant agents remain a major concern for HCV infection therapy in solid organ transplantation recipients. Previously, a study conducted in healthy subjects indicated that elbasvir/grazoprevir resulted in a 43% increase in the tacrolimus AUC, while cyclosporine was not recommended for concomitant use with elbasvir/grazoprevir owing to the 15.2-fold increase of grazoprevir AUC. Notably, mycophenolate mofetil and prednisone did not incur any DDIs with elbasvir/grazoprevir.42 Clinical trials have demonstrated that coadministration of elbasvir/grazoprevir and recommended immunosuppressants is a safe and effective treatment for HCV infection in post-liver or renal transplantation patients with careful monitoring and appropriate dose adjustment.43,44 Elbasvir/grazoprevir and oral contraceptives ethinyl estradiol and levonorgestrel were found to lack pharmacokinetic interactions indicate that elbasvir/grazoprevir could be concomitantly administrated to female HCV patients with childbearing potential to prevent pregnancy.45
A large number of studies have demonstrated that the dosage of atorvastatin should not exceed 20 mg/day and the dose of rosuvastatin should not exceed 10 mg/day when used in combination with elbasvir/grazoprevir. The maximum recommended dosage of fluvastatin, lovastatin, and simvastatin should not exceed 80 mg/day.46 In order to prevent elbasvir/grazoprevir from increasing the concentration of statins, close monitoring the reduction of the amiodarone dose is necessary to assist the treatment to proceed safely.47 According to relevant research, when the elbasvir/grazoprevir regimen is coadministered with phenytoin, carbamazepine, rifampin, cyclosporine, and other drugs, significant interactions occur.46 In summary, cyclosporine, rosuvastatin, atorvastatin are not recommended for combination with elbasvir/grazoprevir.47 Table 1 summarizes the available evidence and clinical recommendations for the concomitant administration of elbasvir/grazoprevir and other drugs.
 |
Table 1 Drug–Drug Interactions Associated with Elbasvir and Grazoprevir# |
Sofosbuvir/Velpatasvir/Voxilaprevir
Sofosbuvir is a nucleotide-like inhibitor of HCV NS5B polymerase and a prodrug. The active metabolite of sofosbuvir is metabolized to GS-461203 and the inactive metabolite of sofosbuvir is dephosphorylated to GS-331007. Velpatasvir (ie, GS-5816) and voxilaprevir (ie, GS-9857) are pangenotypic HCV NS5A and NS3/4A protease inhibitors, respectively (Figure 1). The sofosbuvir and velpatasvir fixed-dose combination was approved for the treatment of HCV infection of all six genotypes consisting of 400 mg sofosbuvir and 100 mg velpatasvir. Sofosbuvir and velpatasvir are administered once a day, irrespective of food.33 As for sofosbuvir/velpatasvir regimens, a fixed-dose combination of sofosbuvir, velpatasvir, and voxilaprevir (400/100/100 mg, once daily) is available for the treatment of HCV-infected patients who are untreated or previously treated with a regimen containing sofosbuvir and/or an NS5A inhibitor.34,48 Sofosbuvir/velpatasvir/voxilaprevir is suitable for chronic hepatitis C adult patients, with or without compensatory cirrhosis. These patients were (1) genotype 1 to 6, who had received an NS5A inhibitor treatment or (2) genotype 1a or 3 who had been administered sofosbuvir without an NS5A inhibitor. For patients with mild or moderate renal impairment or mild liver impairment (Child-Pugh A), it is unnecessary to adjust the dose of sofosbuvir/velpatasvir/voxilaprevir. The sofosbuvir/velpatasvir/voxilaprevir regimen is not recommended in patients with severe renal injury, severe liver injury, or end-stage renal disease (Child-Pugh B or C). No dosage adjustment for the sofosbuvir/velpatasvir/voxilaprevir regimen is warranted in geriatric patients, while its use in pediatric patients remains unclear (Supplementary Figure 2). Sofosbuvir has been well studied and is prescribed for chronic HCV infection patients for over 4 years, so it will not be discussed further in this review.
As the perpetrators of DDIs, velpatasvir and voxilaprevir inhibit drug transporters, including OATP1B1, P-gp, BCRP, and OATP1B3. Specifically, velpatasvir is an inhibitor of OATP2B1 and a substrate of P-gp and BCRP in vivo, and in vitro, which is slowly metabolized by CYP2B6, CYP2C8, and CYP3A4. Velpatasvir administration resulted in increased pravastatin, rosuvastatin, and digoxin exposure by 35%, 160–170%, and 34%, respectively.48,49 As the substrate of OATP, P-gp, and CYP, the AUC of velpatasvir increased by 47%, 103%, and 70% when coadministered a single dose of rifampin, cyclosporine, or ketoconazole, respectively, but decreased by 82% following multiple doses of rifampin.50 Food slightly altered the velpatasvir AUC and significantly increased the voxilaprevir AUC.34,51 Velpatasvir was demonstrated to have higher aqueous solubility in acidic conditions, indicating that the coadministration of acid-reducing agents should be handled cautiously.51 Famotidine (40 mg twice daily) does not impact the velpatasvir AUC, while omeprazole (20 mg or 40 mg) can reduce the velpatasvir AUC by 37–56%.49,50 Voxilaprevir is a substrate of P-gp, BCRP, OATP1B1, and OATP1B3 in vivo, and in vitro, and slowly metabolized in the liver by CYP1A2, CYP2C8, and CYP3A4.34,52 Meanwhile, voxilaprevir is an inhibitor of drug transporters P-gp, BCRP, OATP1B1, and OATP1B3, demonstrating a biliary elimination with a t1/2 of approximately 33 h.6 Voxilaprevir helps to increase the AUC of pravastatin, rosuvastatin, and dabigatran by 116%, 639%, and 161%, respectively, while the pharmacokinetics of bictegravir, cobicistat, darunavir, elvitegravir, emtricitabine, rilpivirine, tenofovir alafenamide, tenofovir, ethinyl estradiol, and norgestrel remain unaltered.53 The voxilaprevir AUC was increased by 84%, 691%, 839%, 331% and 143–171%, or decreased by 73%, when coadministered with voriconazole, single-dose rifampin, cyclosporine A, a single dose of atazanavir/r, boosted antiretroviral regimens, or multiple-dose rifampin, respectively, but remained unaltered by unboosted antiretroviral regimens.53
Velpatasvir and voxilaprevir are primarily eliminated through biliary excretion and merely excreted in the urine. Thus, it was unnecessary to adjust the dose when velpatasvir and voxilaprevir are administrated to patients with mild to severe renal impairment.54,55 .56 It was demonstrated that liver injury has no significant effect on the clinical exposure of velpatasvir. Contrarily, voxilaprevir is not approved in patients with moderate or severe liver function injury (Child-Pugh B or C) since the exposure level in patients was significantly increased compared to healthy subjects.54,57,58 Healthy or HCV/HIV co-infected subjects participated in Phase 1 studies, evaluating DDIs between sofosbuvir/velpatasvir and HIV antiretroviral agents, demonstrated the absence of clinically relevant changes in darunavir/ritonavir, emtricitabine, lopinavir/ritonavir, atazanavir/ritonavir (r), raltegravir, efavirenz, elvitegravir, dolutegravir, cobicistat, or tenofovir alafenamide pharmacokinetics. Rilpivirineand tenofovir (as tenofovir disoproxil fumarate, but not as tenofovir alafenamide) exposure was increased to 40–81% when coadministered with sofosbuvir/velpatasvir. Furthermore, velpatasvir exposure was decreased to 53% and increased to 142% by efavirenz and atazanavir/r, respectively.49 Velpatasvir does not interfere with the efficacy of norgestimate/ethinyl estradiol.58, A post-analysis of the three ASTRAL studies indicated that the efficacy and safety of sofosbuvir/velpatasvir was not affected by methadone or buprenorphine in HCV-infected patients receiving 12-week-opioid replacement treatment.59 No clinically relevant interactions were observed with combination of velpatasvir and cyclosporine.49 Rindone et al60 reported an apparent interaction between sofosbuvir/velpatasvir and warfarin that resulted in a subtarget International Normalized Ratio (INR) and subsequent thrombosis. It is unsuggestive that drugs including St. John’s wort (Hypericum perforatum), carbamazepine, oxcarbazepine, phenobarbitone, phenytoin, rifabutin, rifapentine, atazanavir, lopinavir, efavirenz, tipranavir/ritonavir, amiodarone, cyclosporin, rosuvastatin, ethinyl oestradiol, pitavastatin to be combined with sofosbuvir/velpatasvir/voxilaprevir.61 In addition, the coadministration of rifampicin/darunavir with sofosbuvir/velpatasvir/voxilaprevir is contraindicated.61 In a French study, mainly including the failure of sofosbuvir + NS5 inhibitors, 43 patients were treated with sofosbuvir/velpatasvir/voxilaprevir + ribavirin for 8 (n=34) or 12 weeks (n = 9), and the initial detection rate of SVR12 was about 95%.62 Statins, including atorvastatin, lovastatin, simvastatin, and fluvastatin, are recommended to be combined with sofosbuvir/velpatasvir/voxilaprevir with the lowest dose.61 Based on the reported side-effects, such as symptomatic bradycardia and heart block, amiodarone is not recommended in combination with sofosbuvir/velpatasvir/voxilaprevir. Velpatasvir and voxilaprevir related DDIs have been confirmed by clinical studies summarized in Table 2.
 |
Table 2 Drug–Drug Interactions Associated with Velpatasvir and Voxilaprevir# |
Glecaprevir/Pibrentasvir
Glecaprevir (ie, ABT-493) is an HCV NS3/4A protease inhibitor and pibrentasvir (ie, ABT-530) is an NS5A inhibitor (Figure 1). A fixed-dose combination of glecaprevir and pibrentasvir (300/120 mg, once daily) is available for the treatment of patients with all 6 genotypes of chronic HCV infection, without cirrhosis or with compensated cirrhosis (Child-Pugh A).63 The approved dose is 3 tablets once daily. For the HCV genotype 2, 4, 5, or 6 infection patients, no ribavirin treatment was recommended with shorter than 12 weeks of treatment duration. Only patients with genotype 1 infection can be treated for 8 weeks, but this limitation highlighted the need for an 8-week pan-genotype treatment for other HCV genotype-infected patients is requisite.64 Most patients with genotype 2, 4, or 6 have baseline polymorphisms in NS5A, but with none in NS3.65 No dosage adjustment is recommended for compensated cirrhosis (Child-Pugh A), any degree of renal impairment including patients receiving dialysis, or geriatric patients. Glecaprevir/pibrentasvir has not been approved for moderate liver damage patients (Child-Pugh B) and is forbidden for patients with severe liver damage (Child-Pugh C). The safety and effectiveness of glecaprevir/pibrentasvir in children under the age of 18 have not been illuminated (Supplementary Figure 3).
In healthy subjects, glecaprevir and pibrentasvir exposures are minimally affected by food.66,67 Glecaprevir and pibrentasvir are weakly inhibited by CYP3A, CYP1A2, and uridine glucuronosyltransferase 1A1.68 Both drugs are substrates of P-gp and BRCP transporters, and glecaprevir is additionally a substrate of OATP1B1/3. Furthermore, these drugs are inhibitors of P-gp, OATP1B1/3, BCRP, and weak inhibitors of CYP3A4 and UGT1A1.32,69 The biliary-fecal pathway is the major excretion route of glecaprevir/pibrentasvir, and the mean t1/2 of glecaprevir/pibrentasvir are 7.5 h and 26 h, respectively.69,70 No dosage adjustment is required in patients with renal function impairment. The AUC in patients with moderate liver dysfunction was 100% higher than those observed in patients with severe liver dysfunction. Similarly, the pibrentasvir AUC in patients with moderate and severe liver dysfunction increased by 26% and 115%, respectively. Therefore, glecaprevir/pibrentasvir is not recommended in patients with moderate or severe liver function impairment.71 No dosage adjustment for glecaprevir and pibrentasvir is necessary in subjects with mild to severe renal impairment or end-stage renal disease, with or without hemodialysis.72
The safety and efficacy of fixed-dose glecaprevir/pibrentasvir were confirmed in Phase 3 trials, EXPEDITION-4 and CERTAIN-1, which enrolled HCV-infected patients with stage 4/5 chronic kidney disease, or dialysis-dependent end-stage renal disease.73,74 The phase 1 studies demonstrated a weak effect of ritonavir on glecaprevir and pibrentasvir pharmacokinetics. Furthermore, raltegravir, dolutegravir, or rilpivirine anchor antiretroviral regimens were safe when co-administrated with glecaprevir/pibrentasvir in an ongoing phase 3 trial EXPEDITION-2.63,75–77 Hence, substrates of CYP including caffeine, dextromethorphan hydrobromide, midazolam, omeprazole, and tolbutamide were not affected by coadministered glecaprevir/pibrentasvir.78,79 Continued monitoring and reduction of digoxin dose or dosing frequency are needed when used concomitantly with glecaprevir/pibrentasvir.80 Glecaprevir and pibrentasvir exposures are not affected by losartan or valsartan, while losartan and valsartan AUCs increased by 151% and 36%, respectively. Nevertheless, according to losartan and valsartan label recommendations, no dosage adjustments are required.80 Cyclosporine does not induce significant DDIs when coadministered with glecaprevir/pibrentasvir, while tacrolimus dosage adjustment may be necessary due to increased exposure by 45% when coadministered with glecaprevir/pibrentasvir.81 A phase 1 study conducted in healthy subjects revealed that no clinically significant pharmacokinetic interactions were observed between glecaprevir/pibrentasvir and felodipine or amlodipine.82 In the EU, the combination of glecaprevir/pibrentasvir with formulations containing atazanavir, atorvastatin, simvastatin, dabigatran ester, estradiol alkyne, and strong P-gp and CYP3A inducers (such as rifampin, carbamazepine, St. John’s wort, phenobarbital, phenytoin sodium, and primidone) is prohibited.68 In the according study, the SVR12 was 100% (n/N = 9/9) after simeprevir, daclatasvir, and sofosbuvir were administered for 8 weeks in a small number of genotype 1 HCV-infected patients with compensated cirrhosis.83 Glecaprevir/pibrentasvir with ombitasvir/paritaprevir/ritonavir and dasabuvir were coadministered to three patients who had failed to respond in their last treatment regimen within 9 months.84 The combination of dabigatran, atazanavir, simvastatin, atorvastatin, ethinyl estradiol containing contraceptives, and rifampicin could induce multiple drug interactions.85 Furthermore, methadone or buprenorphine/naloxone combined with glecaprevir/pibrentasvir demonstrated good safety. In addition, in a comprehensive analysis of a glecaprevir/pibrentasvir phase 3 trial, subjects receiving opioid substitution therapy achieved the same high virological cure rates as those not receiving opioid substitution treatment.86 Additionally, drugs including St. John’s wort (Hypericum perforatum), carbamazepine, darunavir, lopinavir, efavirenz, ritonavir, ethinyl estradiol, atorvastatin, lovastatin, simvastatin should not be combined with glecaprevir/pibrentasvir.61 Coadministration of rifampicin and atazanavir with glecaprevir/pibrentasvir is contraindicated. Pitavastatin and fluvastatin are recommended in the lowest dose when combined with glecaprevir/pibrentasvir.61 Drugs, including oxcarbazepine, phenobarbitone, phenytoin, rifabutin, and rifapentine, should be avoided in combination with glecaprevir/pibrentasvir.61 Detailed DDIs with glecaprevir and pibrentasvir are presented in Table 3.
 |
Table 3 Drug–Drug Interactions Between Glecaprevir/Pibrentasvir and Co-Administered Drugs# |
Discussion
Given the advent of new drugs and targeted screening campaigns, hepatitis C could be eliminated in the near future. Multiple interferon-free and oral DAA regimens are available for the treatment of all patients with cirrhosis, HIV-coinfection, and other disease populations historically considered difficult to cure.87 Owing to the favorable efficacy, safety profile, and relatively short duration (typically 12 weeks), DAA regimens can be used to treat more patients than interferon-based regimens. However, frequent and multiple comorbidities and complications associated with chronic hepatitis C can significantly affect the safety and effectiveness of therapeutics. Therefore, the simultaneous use of several drugs in HCV-infected patients, with concomitant chronic diseases, is markedly prevalent. Moreover, this favorable efficacy and safety profile of DAA regimens has been accelerating the widespread use of DAAs in HCV-infected patients. The DDIs between DAAs and other drugs should be particularly concerning as they may directly influence the therapeutic effects and increase the frequency and severity of adverse events, potentially leading to treatment failure of the HCV infection or disturbances in comorbidity therapy. The present review summarized the research related to DDIs in newly approved DAAs.
Classical DAAs (including simeprevir, daclatasvir, ledipasvir, sofosbuvir, paritaprevir, ombitasvir, and dasabuvir) have been extensively used in clinical applications and their DDIs with commonly prescribed medications have been evaluated by physicians.19 The evaluation of DDIs is crucial in the research and development of drugs from preclinical studies to post-market clinical observations. However, unknown DDIs with DAAs are almost inevitable due to the complexity of clinical medications, with continuous monitoring and evaluation required for further evidence. The possibility of DDIs must be considered in determining the best treatment for individual patients. Furthermore, access to a drug interaction database is recommended during the treatment process. In addition, whether DDIs should be used for HCV treatment as indicated following the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America (AASLD-IDSA) guideline was carefully checked by consulting the Liverpool HEP drug interactions checker. Importantly, the patient’s medication chart needs to be carefully examined, including self-medication, which is time-consuming and requires sufficient interaction with pharmaceutical companies.
Conclusions
In conclusion, newly approved DAA regimens including elbasvir/grazoprevir, sofosbuvir/velpatasvir, sofosbuvir/velpatasvir/voxilaprevir, and glecaprevir/pibrentasvir are safe to take with other drugs, in the respect of DDIs. This review will provide useful information for the treatment of HCV infection.
Funding
There is no funding to report.
Disclosure
The authors declare that they have no potential conflicts of interest.
References
1. Thrift AP, El-Serag HB, Kanwal F. Global epidemiology and burden of HCV infection and HCV-related disease. Nat Rev Gastroenterol Hepatol. 2017;14:122–132.
2. Ghany MG, Liang TJ. Current and future therapies for hepatitis C virus infection. N Engl J Med. 2013;369:679–680.
3. Strader DB, Seeff LB. A brief history of the treatment of viral hepatitis C. Clin Liver Dis. 2012;1:6–11. doi:10.1002/cld.1
4. Smith BD, Jorgensen C, Zibbell JE, Beckett GA. Centers for disease control and prevention initiatives to prevent hepatitis C virus infection: a selective update. Clin Infect Dis. 2012;55:S49–S53. doi:10.1093/cid/cis363
5. Garrison KL, German P, Mogalian E, Mathias A. The drug-drug interaction potential of antiviral agents for the treatment of chronic hepatitis C infection. Drug Metab Dispos. 2018;117:079038.
6. Chahine EB, Kelley D, Childs-Kean LM. Sofosbuvir/velpatasvir/voxilaprevir: a pan-genotypic direct-acting antiviral combination for hepatitis C. Ann Pharmacother. 2017;52:1060028017741508.
7. Karaoui LR, Mansour H, Chahine EB. Elbasvir-grazoprevir: a new direct-acting antiviral combination for hepatitis C. Am J Health Syst Pharm. 2017;74:1533–1540. doi:10.2146/ajhp160558
8. Yinan Y, Ming Y, Jie W, et al. Grazoprevir and elbasvir in patients with genotype 1 hepatitis C virus infection: a comprehensive efficacy and safety analysis. Can J Gastroenterol Hepatol. 2017;2017:1–7.
9. Backus LI, Belperio PS, Shahoumian TA, Mole LA. Direct-acting antiviral sustained virologic response: impact on mortality in patients without advanced liver disease. Hepatology. 2018;68.
10. Butt AA, Yan P, Simon TG, Abou-Samra AB. Effect of paritaprevir/ritonavir/ombitasvir/dasabuvir and ledipasvir/sofosbuvir regimens on survival compared with untreated hepatitis C virus-infected persons: results from ERCHIVES. Clin Infect Dis. 2017;65:1006. doi:10.1093/cid/cix364
11. Foster GR, Irving WL, Cheung MC, et al. Impact of direct acting antiviral therapy in patients with chronic hepatitis C and decompensated cirrhosis. J Hepatol. 2016;64:1224–1231. doi:10.1016/j.jhep.2016.01.029
12. Lin CW, Dutta S, Asatryan A, et al. Pharmacokinetics, safety, and tolerability of single and multiple doses of ABT-493: a first-in-human study. J Pharm Sci. 2017;106:645–651. doi:10.1016/j.xphs.2016.10.007
13. Lauffenburger JC, Mayer CL, Hawke RL, Brouwer KL, Fried MW, Farley JF. Medication use and medical comorbidity in patients with chronic hepatitis C from a US commercial claims database: high utilization of drugs with interaction potential. Eur J Gastroenterol Hepatol. 2014;26:1073. doi:10.1097/MEG.0000000000000152
14. Smolders EJ, Berden FA, de Kanter CT, Kievit W, Drenth JP, Burger DM. The majority of hepatitis C patients treated with direct acting antivirals are at risk for relevant drug-drug interactions. United European Gastroenterol J. 2016;5:648–657. doi:10.1177/2050640616678151
15. Burger D, Back D, Buggisch P, et al. Clinical management of drug–drug interactions in HCV therapy: challenges and solutions. J Hepatol. 2013;58:792–800. doi:10.1016/j.jhep.2012.10.027
16. Elsherif O, Khoo S, Solas C. Key drug-drug interactions with direct-acting antiviral in HIV-HCV coinfection. Curr Opin HIV AIDS. 2015;10:348–354. doi:10.1097/COH.0000000000000185
17. Garrison KL, German P, Mogalian E, Mathias A. The drug-drug interaction potential of antiviral agents for the treatment of chronic hepatitis C infection. Drug Metab Dispos. 2018;46:1212–1225. doi:10.1124/dmd.117.079038
18. Soriano V, Labarga P, Fernandez-Montero JV, et al. Drug interactions in HIV-infected patients treated for hepatitis C. Expert Opin Drug Metab Toxicol. 2017;13(8):807–816. doi:10.1080/17425255.2017.1351942
19. Talavera PS, Boyer A, Lamblin G, et al. Managing drug-drug interactions with new direct-acting antiviral agents in chronic hepatitis C. Br J Clin Pharmacol. 2016;83:269. doi:10.1111/bcp.13095
20. Keating GM. Elbasvir/grazoprevir: first global approval. Drugs. 2016;76:617–624. doi:10.1007/s40265-016-0558-3
21. Co., M. Zepatier (elbasvir/grazoprevir): US prescribing information. 2017. Available from: https://wwwaccessdatafdagov/drugsatfda_docs/label/2017/208261Orig1s003lbledtpdf.
22. Caro L, Talaty JE, Guo Z, et al. Pharmacokinetic interaction between the HCV protease inhibitor MK-5172 and midazolam, pitavastatin, and atorvastatin in healthy volunteers [abstract]. Hepatology. 2013;58:437A–438A.
23. Caro L, Talaty JE, Guo Z, Butterfield K, Prueksaritanont T. Pharmacokinetic interaction between the HCV protease inhibitor MK-5172 and IV and oral rifampin in healthy volunteers. Hepatology. 2013;58:446A–447A.
24. Keating GM. Elbasvir/grazoprevir: first global approval. Drugs. 2016;76:1–8.
25. Agency., E. M. Summary product of characteristics ZEPATIER. 2016. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_Product_Information/human/004126/WC500211235.pdf.
26. Caro L, Wenning L, Guo Z, et al. Effect of hepatic impairment on the pharmacokinetics of grazoprevir, a hepatitis C virus protease inhibitor. Antimicrob Agents Chemother. 2017;61:00813–00817. doi:10.1128/AAC.00813-17
27. Kassas ME, Elbaz T, Latif YAE, Esmat G. Elbasvir and grazoprevir for chronic hepatitis C genotypes 1 and 4. Expert Rev Clin Pharmacol. 2016;9:1413. doi:10.1080/17512433.2016.1233813
28. Roth D, Nelson DR, Bruchfeld A, et al. Grazoprevir plus elbasvir in treatment-naive and treatment-experienced patients with hepatitis C virus genotype 1 infection and stage 4–5 chronic kidney disease (the C-SURFER study): a combination phase 3 study. Lancet. 2015;386:1537–1545. doi:10.1016/S0140-6736(15)00349-9
29. Alric L, Ollivier-Hourmand I, Hillaire S, Guillaume M. Real-world efficacy and safety of Elbasvir and Grazoprevir in HCV genotype 1 or 4 infected patients with severe chronic kidney disease stage 4/5 or hemodialyzed. Hepatology. 2017;66:582A–583A.
30. Yanny B, Sahota A. Grazoprevir-elbasvir based therapy is safe and effective in patients with chronic kidney disease GFR < 30 and patients with end stage renal disease who are on renal replacement therapy in a community based setting. J Hepatol. 2017;66:S292–S293.
31. Younossi Z, Bacon B, Curry M, Dieterich D. Elbasvir/grazoprevir effectiveness in patients with chronic hepatitis C and chronic kidney disease: real-world experience from the TRIO network [abstract]. J Hepatol. 2017;66:S748–S749. doi:10.1016/S0168-8278(17)31991-8
32. Zuckerman E, Ashkenasi E, Kovalev Y, et al. The real-world Israeli experience of treating chronic hepatitis C (CHC), genotype 1 (GT1) and genotype 4 (GT4) patients with advanced fibrosis with elbasvir/grazoprevir: a large multi-center cohort. Hepatology. 2017;66:822A–823A.
33. Caro L, Talaty JE, Guo Z, et al. Pharmacokinetic interactions between the HCV protease inhibitor MK-5172 and ritonavir-boosted HIV protease inhibitors. Hepatology. 2013;58:442A–443A.
34. Talaty JE, Caro L, Yeh WW, Fraser IP. Pharmacokinetic interaction between the HCV protease inhibitor MK-5172 and efavirenz in normal healthy volunteers. Hepatology. 2013;58:445A.
35. Feng HP, Vaddady P, Guo Z, et al. No pharmacokinetic interaction between the hepatitis C virus inhibitors elbasvir/grazoprevir and famotidine or pantoprazole. Clin Transl Sci. 2017;10(5):360–365. doi:10.1111/cts.12465
36. Wyles DL, Sulkowski MS, Dieterich D. Management of hepatitis C/HIV coinfection in the era of highly effective hepatitis C virus direct-acting antiviral therapy. Clin Infect Dis. 2016;63(Suppl 1):S3. doi:10.1093/cid/ciw219
37. Rockstroh JK, Nelson M, Katlama C, Lalezari J. Efficacy and safety of grazoprevir (MK-5172) and elbasvir (MK-8742) in patients with hepatitis C virus and HIV co-infection (C-EDGE CO-INFECTION): a non-randomised. Open-Label Trial Lancet HIV. 2015;2:e319–327. doi:10.1016/S2352-3018(15)00114-9
38. Sulkowski M, Hezode C, Gerstoft J, et al. Efficacy and safety of 8 weeks versus 12 weeks of treatment with grazoprevir (MK-5172) and elbasvir (MK-8742) with or without ribavirin in patients with hepatitis C virus genotype 1 mono-infection and HIV/hepatitis C virus co-infection (C-WORTHY): a randomised, open-label phase 2 trial. Lancet. 2015;385:1087–1097.
39. Marshall WL, Marenco T, Feng HP, Barbour AM. No pharmacokinetic interaction between HCV NS5A inhibitor elbasvir and buprenorphine/naloxone in healthy volunteers. Hepatology. 2015;62:573A.
40. Meemken L, Hanhoff N, Tseng A, Christensen S, Gillessen A. Drug-drug interactions with antiviral agents in people who inject drugs requiring substitution therapy. Ann Pharmacother. 2015;49:796–807. doi:10.1177/1060028015581848
41. Dore GJ, Altice F, Litwin AH, et al. Elbasvir-grazoprevir to treat hepatitis C virus infection in persons receiving opioid agonist therapy: a randomized trial. Ann Intern Med. 2016;165:625. doi:10.7326/M16-0816
42. Yeh WW, Feng HP, Dunnington KM, et al. No clinically meaningful pharmacokinetic interactions between HCV inhibitors grazoprevir/elbasvir with tacrolimus, mycophenolate mofetil, and prednisone, but cyclosporine increases grazoprevir/elbasvir exposures in healthy subjects [abstract]. Hepatology. 2015;62:570A–571A.
43. Eisenberger U, Friebus-Kardash J, Guberina H. Feasibility of elbasvir/grazoprevir for treatment of chronic hepatitis C virus infection in renal transplant recipients with impaired allograft function. Transpl Int. 2017;30:28.
44. Martin MT, Koppe S. Elbasvir/grazoprevir use in postliver transplantation patients on hemodialysis. Transplantation. 2017;101:1. doi:10.1097/TP.0000000000001758
45. Marshall WL, Feng HP, Caro L, et al. No clinically meaningful pharmacokinetic interaction between the hepatitis C virus inhibitors elbasvir and grazoprevir and the oral contraceptives ethinyl estradiol and levonorgestrel. Eur J Clin Pharmacol. 2017;73:1–8. doi:10.1007/s00228-017-2216-4
46. Tsai TC, Deng ST, Hsu CW. The efficacy and safety of elbasvir/grazoprevir treatment in HCV genotype 1 patients in Taiwan. J Med Virol. 2020;92(2):219–226. doi:10.1002/jmv.25605
47. Liu CJ, Tseng KC, Lo CC, Tseng IH, Cheng PN. Limited drug-drug interaction of elbasvir/grazoprevir for chronic hepatitis C. J Formos Med Assoc. 2019.
48. Sciences, G. Epclusa (sofosbuvir/velpatasvir): US prescribing information. Available from: https://wwwaccessdatafdagov/drugsatfda_docs/label/2017/208341s009lblpdf.
49. Mogalian E, McNally J, Shen G, et al. Drug-drug interaction profile of sofosbuvir/velpatasvir fixed-dose combination [abstract]. J Hepatol. 2016;64:S613–S614. doi:10.1016/S0168-8278(16)01136-3
50. Mogalian E, Osinusi A, Shen G, Sajwani K, McNally J, Ling J. Effect of food and acid reducing agents on the relative bioavailability and pharmacokinetics of sofosbuvir/velpatasvir fixed-dose combination tablet. Clin Pharmacol Ther. 2016;99:S43–S44.
51. Mogalian E, German P, Kearney BP, et al. Preclinical pharmacokinetics and first-in-human pharmacokinetics, safety, and tolerability of velpatasvir, a pangenotypic hepatitis C virus NS5A inhibitor, in healthy subjects. Antimicrob Agents Chemother. 2017;61:e02084–02016. doi:10.1128/AAC.02084-16
52. Mogalian E, German P, Kearney BP, et al. Use of multiple probes to assess transporter- and cytochrome P450-mediated drug–drug interaction potential of the pangenotypic HCV NS5A inhibitor velpatasvir. Clin Pharmacokinet. 2016;55:605–613. doi:10.1007/s40262-015-0334-7
53. Garrison KL, Kirby B, Stamm LM, et al. Drug-drug interaction profile of sofosbuvir/velpatasvir/voxilaprevir fixed-dose combination. J Hepatol. 2017;66:S492–S493. doi:10.1016/S0168-8278(17)31381-8
54. Lawitz E, Marbury T, Kirby BJ, et al. The effect of renal or hepatic impairment on the pharmacokinetics of GS-9857, a pan-genotypic HCV NS3/4A protease inhibitor. Antivir Ther. 2016;64:S613.
55. Mogalian E, Mathias A, Brainard D, et al. The pharmacokinetics of GS-5816, a pangenotypic HCV-specific NS5A inhibitor, in HCV-uninfected subjects with severe renal impairment. J Hepatol. 2015;62:S590–S591. doi:10.1016/S0168-8278(15)30915-6
56. Heo Y-A, Deeks ED. Sofosbuvir/velpatasvir/voxilaprevir: a review in chronic hepatitis C. Drugs. 2018;78(5):577–587. doi:10.1007/s40265-018-0895-5
57. Curry MP, O’Leary JG, Bzowej N, Muir AJ. Sofosbuvir and velpatasvir for HCV in patients with decompensated cirrhosis. N Engl J Med. 2015;373:2618–2628. doi:10.1056/NEJMoa1512614
58. Mogalian E, Brainard DM, McNally J, Shen G, Cuvin J. Lack of clinically relevant pharmacokinetic drug-drug interaction between norgestimate/ethinyl estradiol and pangenotypic HCV NS5A inhibitor GS-5816 in HCV-uninfected female subjects. Hepatology. 2014;60:1173A.
59. Grebely J, Dore GJ, Zeuzem S, et al. Efficacy and safety of sofosbuvir/velpatasvir in patients with chronic hepatitis C virus infection receiving opioid substitution therapy: analysis of phase 3 ASTRAL trials. Clin Infect Dis. 2016;63:1479. doi:10.1093/cid/ciw579
60. Rindone JP, Mellen CK. Reduction in warfarin effect associated with sofosbuvir-velpatasvir. Am J Health Syst Pharm. 2017;74:1308. doi:10.2146/ajhp170324
61. Pearlman BL, Hinds AE. Review article: novel antivirals for hepatitis C—sofosbuvir/velpatasvir/voxilaprevir, glecaprevir/pibrentasvir. Aliment Pharmacol Ther. 2018;48:914–923. doi:10.1111/apt.14977
62. Llaneras J, Riveiro-Barciela M, Lens S, et al. Effectiveness and safety of sofosbuvir/velpatasvir/voxilaprevir in patients with chronic hepatitis C previously treated with DAAs. J Hepatol. 2019;71:666–672. doi:10.1016/j.jhep.2019.06.002
63. Rockstroh JK, Lacombe K, Viani RM, et al. Efficacy and safety of glecaprevir/pibrentasvir in patients co-infected with hepatitis C virus and human immunodeficiency virus-1: the EXPEDITION-2 study. Clin Infect Dis. 2017;67.
64. Pawlotsky JM, Negro F, Aghemo A, Berenguer M, Wedemeyer H. EASL recommendations on treatment of hepatitis C 2018. J Hepatol. 2018;69:373–395. doi:10.1016/j.jhep.2018.03.026
65. Asselah T, Kowdley KV, Zadeikis N, et al. Efficacy of glecaprevir/pibrentasvir for 8 or 12 weeks in patients with HCV genotype 2, 4, 5, or 6 infection without cirrhosis. Clin Gastroenterol Hepatol. 2018;16:417–426.
66. Lin CW, Dutta S, Asatryan A, et al. Pharmacokinetics, safety, and tolerability following single and multiple doses of pibrentasvir in a first-in-human study. Clin Pharmacol Drug Dev. 2018;7:44–52. doi:10.1002/cpdd.350
67. Lin CW, Dutta S, Asatryan A, et al. Pharmacokinetics, safety, and tolerability of single and multiple doses of ABT-493: a first-in-human study. J Pharm Sci. 2017;106:645–651.
68. Lamb YN. Glecaprevir/pibrentasvir: first global approval. Drugs. 2017;77:1797–1804. doi:10.1007/s40265-017-0817-y
69. Néant N, Solas C. Drug-drug interactions potential of direct acting antivirals for the treatment of chronic hepatitis C infection. Int J Antimicrob Agents. 2018.
70. Hubbard H, Lawitz E. Glecaprevir + pibrentasvir (ABT493 + ABT-530) for the treatment of hepatitis C. Expert Rev Gastroenterol Hepatol. 2017.
71. Agency, E. M. Summary product of characteristics MARIVET. 2017. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/004430/WC500233677.pdf.
72. Kosloski MP, Zhao W, Marbury TC, et al. Effects of renal impairment and hemodialysis on the pharmacokinetics and safety of the glecaprevir and pibrentasvir combination in HCV-negative subjects. Antimicrob Agents Chemother. 2017;62. doi:10.1128/AAC.01990-17
73. Gane E, Lawitz E, Pugatch D, et al. Glecaprevir and pibrentasvir in patients with HCV and severe renal impairment. N Engl J Med. 2017;377:1448–1455. doi:10.1056/NEJMoa1704053
74. Kumada H, Watanabe T, Suzuki F, et al. Efficacy and safety of glecaprevir/pibrentasvir in HCV-infected Japanese patients with prior DAA experience, severe renal impairment, or genotype 3 infection. J Gastroenterol. 2018;53:566–575. doi:10.1007/s00535-017-1396-0
75. AASLD-IDSA. Patients with HIV/HCV coinfection. Recommendations for testing, managing, and treating hepatitis C. 2018. Available from: https://www.hcvguidelines.org/unique-populations/hiv-hcv.
76. AbbVie Inc. Mavyret (glecaprevir/pibrentasvir): US prescribing information. 2017. Available from: https://wwwaccessdatafdagov/drugsatfda_docs/label/2017/209394s003lblpdf.
77. Lin CW, Dutta S, Asatryan A, et al. Pharmacokinetics, safety, and tolerability following single and multiple doses of pibrentasvir in a first-in-human study: clinical pharmacology in drug development. Clin Pharmacol Drug Dev. 2017.
78. Kosloski MP, Dutta S, Ding B, et al. Drug-drug interactions between next generation direct acting antivirals ABT-493 and ABT-530 with digoxin. Clin Pharmacokinet. 2016;99:S65.
79. Kosloski MP, Dutta S, Pugatch D, et al. ABT-493 and ABT-530 combination demonstrated minimal potential for CYP-mediated drug-drug interactions. J Hepatol. 2016;64:S405.
80. Kosloski MP, Dutta S, Jiang Q, et al. Drug-drug interactions between direct acting antivirals ABT-493 and ABT-530 with angiotensin II receptor blockers (losartan or valsartan). Hepatology. 2016;64:420A–421A.
81. Kosloski MP, Dutta S, Zhao WH, Wu JT. Drug-drug interactions between next generation direct acting antivirals ABT-493 and ABT-530 with cyclosporine or tacrolimus in healthy subjects [abstract]. Antimicrob Agents Chemother. 2015;62:561A.
82. Kosloski MP, Dutta S, Zhao W, et al. Lack of significant drug-drug interactions between direct acting antivirals glecaprevir and pibrentasvir with calcium channel blockers (felodipine or amlodipine). Clin Pharmacol Ther. 2017;101:S70.
83. Brown RS, Buti M, Rodrigues L, et al. Glecaprevir/pibrentasvir for 8weeks in treatment-naive patients with chronic HCV genotypes 1-6 and compensated cirrhosis: the EXPEDITION-8 trial. J Hepatol. 2019.
84. Poordad F, Pol S, Asatryan A, et al. Glecaprevir/pibrentasvir in patients with HCV genotype 1 or 4 and prior direct-acting antiviral treatment failure. Hepatology. 2017.
85. Anonymous. Glecaprevir/pibrentasvir for hepatitis C. Aust Prescr. 2018;41:169–170.
86. Kosloski MP, Zhao W, Asatryan A, Kort J, Geoffroy P, Liu W. No clinically relevant drug-drug interactions between methadone or buprenorphine-naloxone and antiviral combination glecaprevir and pibrentasvir. Antimicrob Agents Chemother. 2017;61.
87. Falade-Nwulia O, Suarez-Cuervo C, Nelson DR, et al. Oral direct-acting agent therapy for hepatitis C virus infection: a systematic review. Ann Intern Med. 2017;166:637–648.
88. Moradpour D, Brass V, Gosert R, Wölk B, Blum HE, Hepatitis C. molecular virology and antiviral targets. Trends in Molecular Medicine 2002;8.476–482. doi:10.1016/S1471-4914(02)02395-X
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
