Back to Journals » International Journal of General Medicine » Volume 17
Drug-Coated Balloon for de-novo Coronary Artery Lesions Exceeding 2.5 mm in Diameter: Optical Coherence Tomography Analysis and Clinical Follow-Up
Authors Liu Y, Zhang B, Lv H, Zhu Y , Zhou X, Zhu H , Guo L
Received 22 November 2023
Accepted for publication 18 January 2024
Published 22 January 2024 Volume 2024:17 Pages 225—236
DOI https://doi.org/10.2147/IJGM.S451329
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Yuriy Sirenko
Yuguo Liu,1 Bo Zhang,1 Haichen Lv,1 Yifan Zhu,2 Xuchen Zhou,1 Hao Zhu,1 Lei Guo1
1Department of Cardiology, The First Affiliated Hospital of Dalian Medical University, Dalian, People’s Republic of China; 2Department of Cardiology, Shengjing Hospital of China Medical University, Shenyang, People’s Republic of China
Correspondence: Lei Guo; Hao Zhu, Department of Cardiology, The First Affiliated Hospital of Dalian Medical University, 222 Zhongshan Road, Dalian City, 116011, People’s Republic of China, Tel +86 411-83635963 ; +86 411-83635963, Email [email protected]; [email protected]
Objective: To investigate the precise changes in the lumen and lesions, and clinical outcomes after DCB treatment for de-novo coronary lesions exceeding 2.5 mm in diameter through a detailed analysis of OCT.
Methods: This is a prospective study including 53 consecutive patients with 55 de-novo coronary lesions, who underwent DCB angioplasty-only between January 2021 and April 2022. Quantitative coronary angiography (QCA) and OCT were performed before percutaneous coronary interventions (PCI), immediately after PCI, and at 6– 9 months follow-up after PCI. Target lesion failure (TLF) was the primary endpoint of the present study. Multivariate logistic regression analysis was performed to identify the predictors or risks for late lumen enlargement (LLE).
Results: A total of 52 patients were successfully treated with DCB. The median follow-up was 7 months, and the incidence of TLF was 7.5%. After the DCB procedure, 43 patients had their scheduled angiographic and OCT examination. QCA demonstrated that the late lumen loss was − 0.79 ± 0.28 mm. OCT demonstrated LLE in 79.1% and dissection healing in 65.1% of lesions. After multivariable logistic analysis, type B dissection (odds ratio [OR] 2.92, 95% confidence interval [CI] 1.34– 7.41, p = 0.037) was found to be a predictor of LLE, but lipid plaque (OR 0.09, 95% CI 0.01– 0.63, p = 0.015) was a risk of LLE.
Conclusion: This is the first and largest prospective study to assess the outcomes of DCB treatment for de-novo coronary lesions exceeding 2.5 mm in diameter and the detection of significant vessel enlargement and dissection healing guide by OCT. DCB could be a novel, safe and effective treatment for de-novo coronary lesions exceeding 2.5 mm in diameter through a detailed analysis of OCT.
Keywords: drug-coated balloon, optical coherence tomography, late lumen enlargement, de novo, coronary artery disease
Introduction
The drug-coated balloon (DCB) is safe and effectively used for in-stent restenosis (ISR), small coronary vessels, patients at high risk of bleeding, coronary bifurcation lesions, and diffuse lesions.1–4 Nonetheless, there are only few prospective data available that report the outcomes of the DCB-only strategy in previously untreated de-novo coronary lesions, and it is associated with low rates of target lesion revascularization (TLR) and major adverse cardiac events (MACE).5,6 Moreover, most studies were conducted in small coronary vessels with less than 2.5 mm diameters.
Coronary artery dissection, a risk factor for acute coronary occlusion, is an inevitable consequence of DCB treatment. Coronary angiography (CAG) is a two-dimensional imaging technique with a low resolution of the dissection and frequently misses its presence, as well as the inability to observe the fine structure of the lumen and describe the composition of plaque.7 With a lateral resolution of up to 20 μm, optical coherence tomography (OCT) is the highest resolution intracavitary imaging technique available, approximately ten times that of intravascular ultrasound (IVUS).8 OCT measures coronary dimensions precisely and detects stent under expansion, incomplete stent apposition, and dissection.9 Compared to coronary angiography-guided interventions, OCT guidance reduces the incidence of cardiac death, myocardial infarction (MI), and revascularization in coronary artery disease (CAD) patients.10 The European Society of Cardiology (ESC) recommends the use of OCT to guide optimal percutaneous coronary interventions (PCI) treatment.11 However, there is no specific report on a DCB-only strategy for large de-novo coronary lesions with a diameter > 2.5 mm, and the precise changes in the lumen and lesions after DCB treatment are remain unknown.
The present study aimed to investigate the precise changes in the lumen and lesions, and clinical outcomes after DCB treatment for de-novo coronary lesions exceeding 2.5 mm in diameter through a detailed analysis of OCT.
Methods
Study Population
This was an observational and prospective study. The registry included all consecutive patients referred to the First Affiliated Hospital of Dalian Medical University for PCI of de-novo lesions between January 2021 and April 2022. Data on patient baseline characteristics, procedural details, and in-hospital outcomes were collected from medical records and a database. Patients aged > 18 years and symptomatic CAD and de-novo lesions in native coronary arteries (vessel diameter > 2.5 mm) were included. Patients with acute MI within 48 h, ISR lesions, lesions in the left main coronary artery, severely calcified lesions, or vessel size ≤ 2.5 mm were excluded. Therefore, 53 patients with 55 de-novo coronary lesions in vessels > 2.5 mm in diameter were included in the present study. This study has been registered on the ClinicalTrials.gov (NCT04984135). Clinical follow-up of the registry after index CAG was performed at 1, 3, 6 and 9 months.
Ethics
This study was approved by the ethics committee of the First Affiliated Hospital of Dalian Medical University (No.: YJ-KY-FB-2021-09) and the study conformed to the ethical guidelines of the Declaration of Helsinki (as revised in 2013) on the principles for medical research involving human subjects. All subjects gave written informed consent to participate in the study.
Procedures
Standard techniques were used to perform coronary interventions. Unless they had previously received these antiplatelet medications, all patients received loading doses of aspirin (300 mg) and clopidogrel (300–600 mg) or ticagrelor (180 mg) before the PCI. Heparin was given as an initial bolus of 70–100 IU/kg body weight, followed by a 1000 IU dose every hour. The use of glycoprotein IIb/IIIa inhibitors was at the discretion of the physician. A semi-compliant balloon is used to pre-dilate the lesion, with a balloon-to-vessel ratio of 0.8–1.0. A DCB-only approach may be considered if an optimal angiographic result is obtained (with ≤ 30% residual stenosis, Thrombolysis in Myocardial Infarction (TIMI) grade 3 flow, and no dissection of the lesion or type A/B dissection based on National Heart, Lung, and Blood Institute (NHLBI) criteria). The present study used SeQuent Please DCB catheters with paclitaxel (B. Braun Melsungen, Vascular Systems, Berlin, Germany). The balloon/vessel diameter ratio for DCB intervention was 0.8–1.0. The balloon length was chosen to cover a minimum of 2 mm across both ends of the lesion. The balloon dilation lasted 30–60s. Bailout drug-eluting stents (DES) implantation was recommended for type D or higher coronary dissections or impaired distal flow after pre-dilation. Angiographic success was defined as final residual stenosis < 30% and a TIMI flow grade ≥ 3.12 After the DCB procedure, dual antiplatelet therapy (DAPT) was recommended for at least three months, followed by aspirin indefinitely. Patients should have at least one year of DAPT after DES implantation.
Quantitative Coronary Angiography (QCA) Analysis
QCA analysis was carried out using commercially available software (QCA-CMS, Medis, Leiden, the Netherlands). QCA was performed before and after PCI and at the follow-up angioplasty. Angiographic follow-up was routinely scheduled 6–9 months after the procedure. If clinically indicated, follow-up angiograms were obtained earlier. The reference vessel diameter (RVD), minimal lumen diameter (MLD), lesion length, percent diameter stenosis, and percent area stenosis were all measured and recorded by two blinded personnel. The SYNTAX score was not recorded. The difference between post- and pre-procedure MLD was used to calculate acute gain. In contrast, the MLD difference between post-procedure and follow-up measurements was used to calculate the late lumen loss (LLL). Late lumen enlargement (LLE) was defined as an increase in MLD at follow-up compared to MLD immediately after PCI.
OCT Analysis
In the present study, frequency-domain (FD) OCT was performed before PCI, immediately after PCI, and at 6–9 months follow-up after PCI using the ILUMIEN™ OPTIS™ PCI optimization System (Abbott Vascular, USA) with a pullback speed of 20 mm/s. An experienced investigator blinded to the clinical and lesional information analyzed all OCT images. All cross-sectional images were measured at 1-mm intervals for quantitative measurement. Type of plaques (fibrous, lipid, mixed, or calcified plaque), dissection, MLD, minimal lumen area (MLA), percent diameter stenosis, percent area stenosis, and lesion length were all measured.9 Neointimal dissections were analyzed after procedure. All FD-OCT images were analyzed at 0.2-mm intervals. Dissections were defined as disruption of the vessel luminal surface, including flaps and cavities, in at least two consecutive cross-sectional images. Dissection flap reattachment and healing was defined as the dissection flap protruding into the vessel lumen being reattached to the tunica media and healing.
Clinical Follow-Up
Clinical outcomes such as cardiac death, MI, TLR, and target lesion failure (TLF) were recorded during the follow-up. TLF, defined as the composite of cardiac death, target vessel MI, and clinically driven TLR at follow-up, was the primary endpoint of the present study. MI was defined as typical clinical symptoms, relevant electrocardiogram changes, and elevated troponin T or troponin I levels (3 × the upper limit of normal). TLR was defined as any repeated PCI or bypass surgery of the target vessel due to target lesion restenosis.
Statistical Analysis
Continuous variables were presented as means ± standard deviation (SD) and compared using the t-test or Wilcoxon rank sum test, as appropriate. Categorical variables were expressed as percentages and compared using the chi-square or Fisher exact tests, as appropriate. Multivariate logistic regression analysis was performed to identify the predictors or risks for LLE. Variables were included in the model if their P-value in the bivariate analysis was < 0.10 or if they were clinically relevant. A forward procedure was used to build the multivariable model. The risk was represented by odds ratios (ORs) with 95% confidence intervals (CIs). SPSS Statistics version 24 (IBM SPSS Inc.) and Stata Version 15.1 (StataCorp LLC) were used for statistical analyses.
Results
Baseline Characteristics
The mean patient age in the overall study population was 58.3 ± 12.1 years, with 42 male patients (79.2) (Table 1). Approximately 54.7% of the patients had hypertension, 39.6% had diabetes mellitus, 39.6% smoked, and 41.5% had acute coronary syndrome (ACS). The mean left ventricular ejection fraction (LVEF) was 55.2 ± 5.7%. 19 (34.5%) lesions were in the left anterior descending artery (LAD), 26 (47.3%) in the left circumflex artery (LCX) and 10 (18.2%) in the right coronary artery (RCA). Complex (American College of Cardiology/American Heart Association type B2/C) accounted for 47.2% of the total. Table 2 presents the angiographic and procedural characteristics. In 98.1% of cases, pre-dilation with a scoring balloon was performed. DCB had an average diameter and length of 2.8 ± 0.5 and 27.9 ± 7.2 mm, respectively. RVD and lesion length for the total population were 2.89 ± 0.51 and 19.3 ± 10.8 mm, respectively. Due to type D dissection, one patient (1.8%) required bailout stenting. Table 3 details the QCA analysis at baseline, post-intervention, and follow-up. MLD and residual stenosis were 1.81 ± 0.54 mm and 29.9 ± 13.4%, respectively, immediately after DCB treatment. The acute luminal gain was 0.97 ± 0.51 mm.
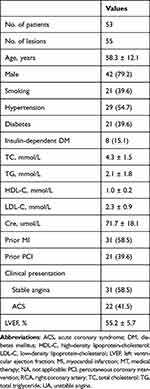 |
Table 1 Baseline Clinical Characteristics |
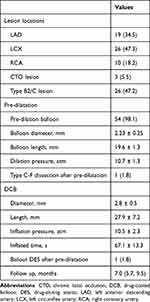 |
Table 2 Lesional and Procedural Characteristics |
 |
Table 3 Quantitative Coronary Angiography Analysis |
After the DCB procedure, 43 patients had their scheduled angiographic and OCT examination. MLD and residual stenosis in these patients was 1.87 ± 0.54 mm and 26.8 ± 15.5%, respectively. The LLL was −0.79 ± 0.28 mm (Table 3). Figures 1 and 2 are representative CAG and OCT images of late lumen enlargement after DCB treatment for de-novo coronary lesions exceeding 2.5 mm in diameter, respectively. Figure 3 shows the OCT analysis of the DCB for de-novo coronary lesions exceeding 2.5 mm in diameter.
 |
Figure 3 Optical coherence tomography analysis of the drug-coated balloon for de-novo coronary lesions exceeding 2.5 mm in diameter. |
Clinical Outcomes at Seven‑month Follow‑up
The median follow-up was seven months. Clinical follow-up data were collected from all patients. Table 4 displays the clinical event data. During the follow-up period, the incidence of TLF was 7.5%. MI and TLR occurred in one (1.8%) and three patients (5.4%), respectively. There was no cardiac death among the patients.
 |
Table 4 Clinical Outcomes of All 53 Patients During Follow-Up |
The Differences Between LLE and Non-LLE Groups Depending on OCT
Table 5 indicates the differences in lesional and procedural characteristics based on the OCT-detected LLE. However, the mixed fibrous-lipid plaque was more common, while lipid plaque was relatively less common in the LLE group than in the non-LLE group. The LLE group had more type B dissection (52.9 vs 11.1%, p < 0.025). Dissection flap healing was more common in the LLE group (73.5 vs 33.3%, p < 0.024). The LLE group had significantly higher late lumen gain (0.80 ± 0.45 vs 0.36 ± 0.44 mm, p < 0.012). There were no differences in pre and immediately post-DCB procedural characteristics. MLD and MLA were significantly higher, while the percentage of lumen stenosis was lower in the LLE group than in the non-LLE group.
 |
Table 5 OCT Analysis According to Late Lumen Enlargement |
Predictors Associated with LLE
After multivariable logistic analysis, type B dissection (odds ratio [OR] 2.92, 95% confidence interval [CI] 1.34–7.41, p = 0.037) was found to be a predictor of LLE, but lipid plaque (OR 0.09, 95% CI 0.01–0.63, p = 0.015) was a risk of LLE (Table 6).
 |
Table 6 Univariate and Multivariable Predictors of Late Lumen Enlargement |
Discussion
To the best of our knowledge, this is the first and largest study to assess the outcomes of DCB treatment for de-novo coronary lesions exceeding 2.5 mm in diameter and detect significant vessel enlargement and dissection healing using OCT.
PCI with DES implantation is still the mainstay of intervention therapy for symptomatic CAD. Unfortunately, late stent thrombosis and restenosis, with a hazard of nearly 2% per year after implantation,13 and the need for prolonged DAPT, which increased the risk of bleeding, remained a concern and contributed to the development of DCB.14
DCBs were developed to deliver antiproliferative drugs such as paclitaxel to the coronary vessel wall to inhibit neointimal proliferation after angioplasty.14,15 In the treatment of ISR, DCB is safe and effective.16 DCB has a class IA ESC recommendation for this indication.17 In addition, DCB has revealed potential in small coronary vessels, diffuse CAD, and bifurcations, where stenting may be technically challenging and yield suboptimal results.12 However, only a few studies report the outcomes of the DCB-only strategy in previously untreated large de-novo coronary lesions.18
CAG has only a rudimentary capability for detecting true-diameter stenosis due to limitations inherent in bi-dimensional imaging, foreshortening effects, and overlapping vessel segments.19 It frequently misses eccentric plaques, resulting in the DCB not completely covering the lesion. Nevertheless, OCT can identify the type of plaque, whether fibrous, lipid, calcified, or mixed, and provide a comprehensive assessment of pre-expansion before DCB treatment.8 OCT can precisely define the lesion location and provide precise information to the operator to develop a DCB treatment plan. Coronary dissection after balloon angioplasty may progress to an intramural coronary hematoma, resulting in vessel lumen size restriction and acute occlusion. However, CAG has a low resolution of the dissection, and about 30% of intramural hematomas cannot be detected by angiography. However, they are observed by OCT,20 providing a precise and detailed assessment of the size and depth of the dissection and the information on whether DES implantation should be performed.
Several studies demonstrated that DCB is safe and feasible in non-small de-novo coronary lesions.18 However, these studies did not include intravascular imaging, which was a limitation. OCT detects dissections more clearly than IVUS. Therefore, authors recommend and demand that OCT be used in pre-, post-procedure, and at follow-ups of DCB treatment for de-novo lesions to obtain more confirmatory information.21–23
In the present study, all of the target lesion diameters were > 2.5 mm, and OCT was performed before PCI, immediately after PCI, and as a follow-up after PCI. Our study exhibited chronic LLE, and most of these dissections healed completely within seven months of DCB treatment, consistent with previous research.24 These lumen increases are most likely due to the effects of paclitaxel on the vascular wall. One possible explanation is vascular remodeling. Paclitaxel inhibits smooth muscle cell (SMC) proliferation by modulating microtubule formation and upregulating pro-apoptotic factors.25 Initially, it is found in much higher concentrations in the vascular wall after DCB use than after DES implantation,26 resulting in both cryostasis and mitotic and post-mitotic arrest.15 Pires et al indicated higher SMC content reductions in the intima and media after DES implantations while using comparable paclitaxel and sirolimus doses. Furthermore, deep dissection after pre-dilation can reach the tunica media, causing vascular enlargement if high doses of paclitaxel are administered directly into the tunica media.24
Multivariable analysis revealed that the lipid plaque was a negative predictor of future LLE after DCB angioplasty for de-novo CAD. Because lipid plaques have more lipid nuclei and are more prone to non-reflow and slow flow, as well as a thinner fibrous cap, the probability of dissection and vessel occlusion after DCB is higher than in other types of plaque. Therefore, avoid applying higher pressures during DCB pre-dilation.27
Furthermore, we found that type B dissection positively predicted future LLE after DCB angioplasty for de-novo CAD. Coronary artery dissection is an inevitable side effect of DCB treatment. However, previous studies indicated that non-occlusive residual coronary dissection was associated with a better long-term outcome due to the positive effect of chronic vessel enlargement.22,28,29 Funatsu et al demonstrated that acute and midterm outcomes were favorable despite dissections complicating 80% of lesions.30 He also revealed that lesions with type B dissection had a higher net gain than lesions with type A or no dissection. In contrast, type C-E dissections independently predicted TLR after DCB treatment.30 Another study revealed that leaving a non-flow-limiting dissection untreated after DCB angioplasty is safe and does not increase TLR.29 Moreover, at the six-month follow-up, repeat angiography revealed that 93.8% of patients with type A-C dissection had complete vessel healing.29 Therefore, dissection following treatment may not be unfavorable, and non-flow-limiting larger dissection may be associated with low late lumen loss and favorable clinical outcomes after DCB treatment.
Several previous related studies on DCB combined with OCT included only 31, 36, and 15 patients, with small sample sizes,7,31,32 and the proportion of completed CAG+OCT follow-up was low in all of them (approximately 40%), whereas the present study included a total of 53 patients with 55 lesions, which is the prospective study on DCB and OCT with the largest sample size included so far. Notably, all 53 patients in this study completed clinical follow-up, and 42 patients completed follow-up with CAG+OCT, with a high completion rate of 79.3% (42/53), which is much higher than that of previous OCT studies (approximately 40%).33 The reason for the small enrolled sample size of such studies is mainly the high cost of DCB+OCT, which is unaffordable for most patients; on the other hand, the application of DCB+OCT requires high requirements for the type of lesion and the condition of the dilated lesion, and the proportion of lesions and vascular conditions that can be combined with the use of DCB and OCT at the same time is low. In fact, most of the clinical studies containing CAG follow-up had a low percentage of imaging follow-up, mainly because of poor patient compliance and fear of imaging, arterial puncture pain and higher cost concerns.
Limitations
This was a single-center study, and the number of patients included was relatively small mainly due to the very high cost of DCB combined with OCT for patients. However, the present study had the largest sample compared to previous study. During the seven-month follow-up after DCB, there were very few adverse cardiovascular outcomes, and significant vessel enlargement and dissection healing were detected by OCT. Long-term follow-up of more than five years is necessary to get a full picture and what events these patients have suffered. Because CAD is becoming one of the leading causes of cardiovascular mortality worldwide, we believe our findings will provide a new safe and effective treatment for patients with CAD.
Conclusions
This is the first and largest prospective study to assess the outcomes of DCB treatment for de-novo coronary lesions exceeding 2.5 mm in diameter and the detection of significant vessel enlargement and dissection healing guide by OCT. Therefore, DCB could be a novel, safe and effective treatment for de-novo CAD exceeding 2.5 mm in diameter. A more extensive study is required to validate the findings.
Abbreviations
DCB, drug coated balloon; OCT, optical coherence tomography PCI, percutaneous coronary intervention; LLE, late lumen enlargement; CAD, coronary artery disease.
Data Sharing Statement
The data used and/or analyzed during the current study are available from the corresponding author (Dr. Lei Guo) on reasonable request.
Acknowledgment
The authors acknowledge all participants of the study.
Funding
This study was supported by the National Natural Science Foundation of China (82170252).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Lee JM, Park J, Kang J, et al. Comparison among drug-eluting balloon, drug-eluting stent, and plain balloon angioplasty for the treatment of in-stent restenosis: a network meta-analysis of 11 randomized, controlled trials. JACC Cardiovasc Interv. 2015;8(3):382–394. doi:10.1016/j.jcin.2014.09.023
2. Latib A, Colombo A, Castriota F, et al. A randomized multicenter study comparing a paclitaxel drug-eluting balloon with a paclitaxel-eluting stent in small coronary vessels: the BELLO (Balloon Elution and Late Loss Optimization) study. J Am Coll Cardiol. 2012;60(24):2473–2480. doi:10.1016/j.jacc.2012.09.020
3. Rissanen TT, Uskela S, Eränen J, et al. Drug-coated balloon for treatment of de-novo coronary artery lesions in patients with high bleeding risk (DEBUT): a single-blind, randomised, non-inferiority trial. Lancet. 2019;394(10194):230–239. doi:10.1016/s0140-6736(19)31126-2
4. López Mínguez JR, Nogales Asensio JM, Doncel Vecino LJ, et al. A prospective randomised study of the paclitaxel-coated balloon catheter in bifurcated coronary lesions (BABILON trial): 24-month clinical and angiographic results. EuroIntervention. 2014;10(1):50–57. doi:10.4244/eijv10i1a10
5. Venetsanos D, Lawesson SS, Panayi G, et al. Long-term efficacy of drug coated balloons compared with new generation drug-eluting stents for the treatment of de novo coronary artery lesions. Catheter Cardiovasc Interv. 2018;92(5):E317–E326. doi:10.1002/ccd.27548
6. Waksman R, Serra A, Loh JP, et al. Drug-coated balloons for de novo coronary lesions: results from the Valentines II trial. EuroIntervention. 2013;9(5):613–619. doi:10.4244/eijv9i5a98
7. de la Torre Hernandez JM, Garcia Camarero T, Lozano Ruiz-Poveda F, et al. Angiography and optical coherence tomography assessment of the drug-coated balloon ESSENTIAL for the treatment of in-stent restenosis. Cardiovasc Revasc Med. 2020;21(4):508–513. doi:10.1016/j.carrev.2019.07.021
8. Truesdell AG, Alasnag MA, Kaul P, et al. Intravascular imaging during percutaneous coronary intervention: JACC state-of-the-art review. J Am Coll Cardiol. 2023;81(6):590–605. doi:10.1016/j.jacc.2022.11.045
9. Tearney GJ, Regar E, Akasaka T, et al. Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: a report from the international working group for intravascular optical coherence tomography standardization and validation. J Am Coll Cardiol. 2012;59(12):1058–1072. doi:10.1016/j.jacc.2011.09.079
10. Meneveau N, Souteyrand G, Motreff P, et al. Optical coherence tomography to optimize results of percutaneous coronary intervention in patients with non-ST-elevation acute coronary syndrome: results of the multicenter, randomized DOCTORS study (does optical coherence tomography optimize results of stenting). Circulation. 2016;134(13):906–917. doi:10.1161/circulationaha.116.024393
11. Räber L, Mintz GS, Koskinas KC, et al. Clinical use of intracoronary imaging. Part 1: guidance and optimization of coronary interventions. An expert consensus document of the European association of percutaneous cardiovascular interventions. Eur Heart J. 2018;39(35):3281–3300. doi:10.1093/eurheartj/ehy285
12. Kleber FX, Rittger H, Bonaventura K, et al. Drug-coated balloons for treatment of coronary artery disease: updated recommendations from a consensus group. Clin Res Cardiol. 2013;102(11):785–797. doi:10.1007/s00392-013-0609-7
13. Kirtane AJ, Gupta A, Iyengar S, et al. Safety and efficacy of drug-eluting and bare metal stents: comprehensive meta-analysis of randomized trials and observational studies. Circulation. 2009;119(25):3198–3206. doi:10.1161/circulationaha.108.826479
14. Jeger RV, Eccleshall S, Wan Ahmad WA, et al. Drug-coated balloons for coronary artery disease: third report of the international DCB consensus group. JACC Cardiovasc Interv. 2020;13(12):1391–1402. doi:10.1016/j.jcin.2020.02.043
15. Axel DI, Kunert W, Göggelmann C, et al. Paclitaxel inhibits arterial smooth muscle cell proliferation and migration in vitro and in vivo using local drug delivery. Circulation. 1997;96(2):636–645. doi:10.1161/01.cir.96.2.636
16. Scheller B, Hehrlein C, Bocksch W, et al. Treatment of coronary in-stent restenosis with a paclitaxel-coated balloon catheter. N Engl J Med. 2006;355(20):2113–2124. doi:10.1056/NEJMoa061254
17. Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2019;40(2):87–165. doi:10.1093/eurheartj/ehy394
18. Yerasi C, Case BC, Forrestal BJ, et al. Drug-coated balloon for de novo coronary artery disease: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75(9):1061–1073. doi:10.1016/j.jacc.2019.12.046
19. Garrone P, Biondi-Zoccai G, Salvetti I, et al. Quantitative coronary angiography in the current era: principles and applications. J Interv Cardiol. 2009;22(6):527–536. doi:10.1111/j.1540-8183.2009.00491.x
20. Tweet MS, Gulati R, Williamson EE, Vrtiska TJ, Hayes SN. Multimodality imaging for spontaneous coronary artery dissection in women. JACC Cardiovasc Imaging. 2016;9(4):436–450. doi:10.1016/j.jcmg.2016.01.009
21. Kleber FX, Schulz A, Waliszewski M, et al. Local paclitaxel induces late lumen enlargement in coronary arteries after balloon angioplasty. Clin Res Cardiol. 2015;104(3):217–225. doi:10.1007/s00392-014-0775-2
22. Liu Y, Zhang YJ, Deng LX, et al. 12-month clinical results of drug-coated balloons for de novo coronary lesion in vessels exceeding 3.0 mm. Int J Cardiovasc Imaging. 2019;35(4):579–586. doi:10.1007/s10554-018-1505-z
23. Yamamoto T, Sawada T, Uzu K, Takaya T, Kawai H, Yasaka Y. Possible mechanism of late lumen enlargement after treatment for de novo coronary lesions with drug-coated balloon. Int J Cardiol. 2020;321:30–37. doi:10.1016/j.ijcard.2020.07.028
24. Sogabe K, Koide M, Fukui K, et al. Optical coherence tomography analysis of late lumen enlargement after paclitaxel-coated balloon angioplasty for de-novo coronary artery disease. Catheter Cardiovasc Interv. 2021;98(1):E35–E42. doi:10.1002/ccd.29435
25. Pires NM, Eefting D, de Vries MR, Quax PH, Jukema JW. Sirolimus and paclitaxel provoke different vascular pathological responses after local delivery in a murine model for restenosis on underlying atherosclerotic arteries. Heart. 2007;93(8):922–927. doi:10.1136/hrt.2006.102244
26. Speck U, Cremers B, Kelsch B, et al. Do pharmacokinetics explain persistent restenosis inhibition by a single dose of paclitaxel? Circ Cardiovasc Interv. 2012;5(3):392–400. doi:10.1161/circinterventions.111.967794
27. Kotani J, Nanto S, Mintz GS, et al. Plaque gruel of atheromatous coronary lesion may contribute to the no-reflow phenomenon in patients with acute coronary syndrome. Circulation. 2002;106(13):1672–1677. doi:10.1161/01.cir.0000030189.27175.4e
28. Cappelletti A, Margonato A, Rosano G, et al. Short- and long-term evolution of unstented nonocclusive coronary dissection after coronary angioplasty. J Am Coll Cardiol. 1999;34(5):1484–1488. doi:10.1016/s0735-1097(99)00395-2
29. Cortese B, Silva Orrego P, Agostoni P, et al. Effect of drug-coated balloons in native coronary artery disease left with a dissection. JACC Cardiovasc Interv. 2015;8(15):2003–2009. doi:10.1016/j.jcin.2015.08.029
30. Funatsu A, Kobayashi T, Mizobuchi M, Nakamura S. Clinical and angiographic outcomes of coronary dissection after paclitaxel-coated balloon angioplasty for small vessel coronary artery disease. Cardiovasc Interv Ther. 2019;34(4):317–324. doi:10.1007/s12928-019-00571-3
31. Fukushima T, Ashikaga T, Yoshikawa S, et al. Effect of drug-coated balloon on stent restenosis, neointimal proliferation, and coronary dissection: an optical coherence tomography analysis. Coron Artery Dis. 2018;29(1):39–45. doi:10.1097/MCA.0000000000000552
32. Akutsu N, Ogaku A, Koyama Y, et al. Effect of drug-coated balloon angioplasty on in-stent restenotic coronary lesions analyzed with optical coherence tomography and serial coronary artery angioscopy. Heart Vessels. 2019;34(12):1925–1935. doi:10.1007/s00380-019-01447-5
33. Li L, Zhao L, Wang J, et al. Optical coherence tomography-guided drug coated balloon in non-small de novo coronary artery lesions: a prospective clinical research. Am J Transl Res. 2021;13(10):11617–11624.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


