Back to Journals » Neuropsychiatric Disease and Treatment » Volume 18
Downregulation of miR-210 Promoted Apoptosis of Hippocampal Neurons by Negatively Regulating the TLR4/NF-кB1 Signaling Pathway in a Rat Model of Status Epilepticus
Authors Zhou Q, Luo H, Wang X, Li P, Kong H, He B
Received 5 May 2022
Accepted for publication 8 August 2022
Published 18 August 2022 Volume 2022:18 Pages 1763—1770
DOI https://doi.org/10.2147/NDT.S371950
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yuping Ning
Qin Zhou,1 Huanjun Luo,2 Xiaowei Wang,2 Peng Li,3 Haibo Kong,1 Baomei He1
1Center for Reproductive Medicine, Department of Pediatrics, Zhejiang Provincial People’s Hospital (Affiliated People’s Hospital, Hangzhou Medical College), Hangzhou, People’s Republic of China; 2Bengbu Medical College, Bengbu, Anhui Province, People’s Republic of China; 3Department of Gastroenterology, Zhejiang Provincial People’s Hospital, Affiliated People’s Hospital, Hangzhou Medical College, Hangzhou, People’s Republic of China
Correspondence: Baomei He, Zhejiang Provincial People’s Hospital (Affiliated People’s Hospital, Hangzhou Medical College), No. 158 Shangtang Road, Hangzhou, Zhejiang Province, 310014, People’s Republic of China, Tel/Fax +86 13588008667, Email [email protected]
Purpose: Status epilepticus (SE) is a life-threatening condition causing brain damage, hippocampal necrosis and apoptosis. This study aimed to determine whether microRNA-210 regulates seizure and apoptosis by targeting the TLR4 /NF-κB1 associated signaling pathway.
Methods: In a pilocarpine-induced epileptic rat model, the expressions of microRNA-210 (miR-210), TLR4, NF-κB1 and caspase-3 were assessed by a quantitative polymerase chain reaction and Western blotting. Tunel detects hippocampal neuron apoptosis.
Results: We found that miR-210, TLR4, NF-κB1 and caspase-3 were upregulated in the hippocampus of the rat model compared with that of control. The knockdown of miR-210 significantly restored the expression levels of TLR4, NF-κB1 and caspase-3 and increased hippocampal apoptosis.
Conclusion: These findings showed that the downregulation of miR-210 promoted apoptosis of hippocampal neurons by negatively regulating the TLR4/NF-кB1 signaling pathway.
Keywords: apoptosis, microRNA, NF-κB1, status epilepticus, TLR4
Introduction
Status epilepticus (SE) is a critical illness in children. The tonic-clonic status epilepticus is the most common form and is associated with high mortality and disability rates.1 Prolonged seizures can cause hypoxia and permanent neurological injury in specific regions of the brain, such as the hippocampus.2,3 The longer duration of attacks is accompanied by serious brain damage, and the fatality rate of SE is close to 20%.4 However, there is currently no disease-specific treatment for this disorder.
Emerging evidence indicates that inflammatory dysregulation at the lesion sites represents a critical factor in the development of epilepsy. However, it remains unclear how unbalanced inflammation contributes to epilepsy.5 The Toll-like receptors (TLRs) are an important family of receptors that mediate systemic inflammatory responses. Among them, TLR4 plays an important role in cell proliferation, apoptosis, differentiation and death.6 After TLR4 activation, the nuclear factor kappa B (NF-κB) participates in the transcriptional regulation of apoptotic genes by regulating downstream proteins, ultimately leading to the activation of caspase-3.7 However, the exact role of TLR4 signaling in the context of epilepsy has not been investigated.
MicroRNAs (or miRNAs) are small non-coding RNA molecules containing about 22 nucleotides that regulate RNA silencing and post-transcriptional gene expression.8 The miR-210 is upregulated during brain hypoxia and protects the brain by inhibiting apoptosis.9,10 However, Chen et al found that miR-210 is abundantly expressed in the hippocampus and the intrahippocampal injection of a miR-210 inhibitor attenuated the apoptosis induced by epileptic activity in rats.11 Therefore, it is meaningful to clarify the real mechanism of miR-210 regulating neuronal apoptosis.
Materials and Methods
Rat Model of Status Epilepticus
Juvenile male Sprague Dawley rats at twenty-one days old, weighing from 50 to 80 g, were purchased from the Shanghai SLAC Laboratory Animal, Co., Ltd. (Shanghai, China). They were maintained at a temperature of 23 ± 2°C, with a 12-hour light/dark cycle and food and water ad libitum. All the experiments were performed according to the Guidelines for the Care and Use of Laboratory Animals and approved by the Experimental Animal Ethics Committee of Hangzhou Yingyang Biomedical Center (protocol number SYXK 2020–0024).
The SE model was established as follows: rats were first intraperitoneally injected with lithium chloride (3 mEq/kg), then 18 h later with methylscopolamine bromide (1 mg/kg). After 30 min, rats were intraperitoneally injected with pilocarpine (100 mg/kg). The degree of convulsions was scored as follows: grade 0, without convulsions; grade I, facial twitching; grade II, rhythmic nodding; grade III, local clonic convulsions; grade IV, rigidity with standing; grade V, generalized tonic-clonic convulsions. When the convulsions´ time reached 30 min, rats were intraperitoneally injected with 10% chloral hydrate (400 mg/kg) and atropine (1 mg/kg).12 If convulsions were not alleviated, chloral hydrate was repeated until convulsions stopped. After convulsions, physiological saline and 10% glucose solution (10 mL/kg) were intraperitoneally injected to supplement electrolyte and improve energy supply. The qualified SE model was based on grade IV or above convulsions lasting for 30 min, with rats in good conditions after convulsions.13
Grouping and Interventions
A total of 72 rats were used for two experiments: (1) the expression levels of miR-210, TLR4, NF-κB1 and caspase-3 were evaluated at different time points (day 1, day 2, day 3, and day 7, n = 8 in each group); (2) the effects of miR-210 were evaluated through the implementation of a miR-210 inhibitor. The SE rats were randomly divided into a negative control group (SE + NC) and a miR-210 inhibitor group (SE + miR-210 inhibitor) (n = 8 in each group). The miR-210 inhibitor (sequence: 5’-TCAGCCGCTGTCACACGCACAG-3’) was purchased from Shanghai GeneChem Co., Ltd. (Shanghai, China). The rats were first anesthetized and then placed on a stereotaxic device with the skull flat. After the scalp was incised, a bilateral guide was placed through an indwelling stainless-steel cannula (interior diameter, 0.33 mm; external diameter, 0.63 mm) to reach the hippocampus. The miRNA-210 inhibitor was then injected through an inner cannula (interior diameter, 0.08 mm; external diameter, 0.3 mm) by a microprocessor pump at a rate of 250 nl/min for 2 min.14 The injection needle was left in the site for an additional 2 min until diffusion was complete. The NC rats were injected with a scrambled oligonucleotide as the NC. After modeling, rats were sacrificed at the indicated time points.
Brain Tissues
After sacrifice, the brain was harvested and the hippocampus was isolated. The left hippocampus was removed and placed in liquid nitrogen at −80°C. The right hippocampal tissues were also collected and fixed in 4% paraformaldehyde for 2 h, embedded in paraffin, sectioned and stained.
qPCR
The cDNA was obtained by polymerase chain reaction (PCR) performed on a Mastercycler (Eppendorf, Germany). PCR band densities were analyzed for semiquantification. Real-time quantitative PCR (qPCR) was performed with an iQ5 cycler (Bio-Rad, Hercules, US), while the quantitative analysis was achieved by the delta Ct method. The specific primers for miRNAs are listed in Table 1. All reactions were run in triplicate.
 |
Table 1 Primer Sequences Used in This Study for PCR |
Western Blot
The tissue lysates were loaded and resolved by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). After electrophoresis, the proteins were blotted onto a polyvinylidene difluoride membrane. After blocking with 5% non-fat dry milk, the membrane was incubated with primary antibodies and anti-rabbit or anti-mouse antibodies conjugated with horseradish peroxidase. The bands were visualized with enhanced chemiluminescence reagents and the images were captured by an EMCCD imaging system (Tenon, Shanghai, China). The protein levels were quantified by the Lowry method.
TUNEL Assay
The terminal deoxynucleotidyl transferase (TdT) dUTP Nick-End Labeling (TUNEL) assay was used to detect apoptosis of hippocampal neurons (Servicebio, China). After dewax and rehydration, the paraffin sections were digested with proteinase K (Servicebio, China). The slides were incubated for 20 min. Later, an appropriate amount of TDT enzyme, dUTP and buffer in a ratio of 1:5:50 was added, the slides were then incubated at 37°C for 2 h. The DAPI reagent (Servicebio, China) was used to determine the number of nuclei and to assess gross cell morphology. The images were obtained through a fluorescence microscope (Nikon Eclipse C1, Japan). The apoptotic rate was calculated as follows: TUNEL positive cell numbers/total cell numbers* 100%.
Statistical Analysis
All experiments were conducted independently at least three times. Data are expressed as means and standard errors. Groups were compared by independent t-test followed by post hoc analysis. The statistical analysis was performed with SPSS 17.0 (SPSS Inc., Chicago, IL, US). Alpha was set at 0.05, and all tests were two-tailed.
Results
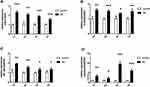
MiR-210, TLR4, NF-κB1 and Caspase-3 in the Hippocampus
The expression levels in the hippocampus of miR-210, TLR4, NF-κB1 and caspase-3 were evaluated by qPCR. Compared with control rats, they were significantly upregulated in SE rats (Figure 1).
Hippocampal Injection of a miR-210 Inhibitor
When a miR-210 inhibitor was microinjected in the hippocampus of SE rats, the expression level of miR-210 significantly decreased (Figure 2).
 |
Figure 2 The expression of miR-210 in the hippocampus after microinjection of a miR-210 inhibitor. ***p < 0.001. |
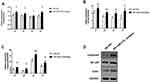
Expression of TLR4, NF-κB1 and Caspase-3 After Knockdown of miR-210
We measured the expression levels of TLR4, NF-κB1 and caspase3 in the hippocampus with qPCR. Compared with the SE + NC group, the hippocampal tissues of SE rats showed higher expression levels of TLR4, NF-κB1 and caspase-3 after microinjection of a miR-210 inhibitor (Figure 3A–C). The Western blot of brain homogenates showed higher expression levels of TLR4, NF-κB1 and caspase-3 after microinjection of a miR-210 inhibitor (Figure 3D).
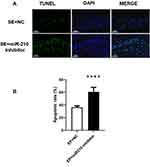
Knockdown of miR-210 Promoted Apoptosis in the Hippocampus
Compared with the EP + NC group, the number of apoptotic cells in the hippocampal CA1 region was significantly increased in the EP + miR-210 inhibitor group (Figure 4).
Discussion
Epilepsy is a chronic disorder of the brain characterized by recurrent epileptic seizures.15 The hippocampus is often involved in seizures, even if they are not generated there.16 However, the epileptic mechanisms causing inflammation and apoptosis in the hippocampus remain to be explored.
TLR4 is an important mediator in the innate and cytokine-activated immune systems.17 It is abundantly expressed in hippocampal neurons and glial cells,18 while its inhibition prevented pilocarpine-induced seizures in rats.19 In this study, we showed that the expression of TLR4 increased in the hippocampus of SE rats. The expression levels of NF-κB1 and caspase-3 were also significantly increased, suggesting that NF-κB1 promoted the synthesis and release of caspase-3. The TLR4/NF-κB1 signaling pathway might be involved in the immune-inflammatory response in the hippocampus of SE rats. Consistent with our results, miR-210 was described as abundantly expressed in the hippocampus of SE rats (10, 24). In SE, hypoxia is one of the main drivers of brain injury. The miR-210 becomes highly upregulated in response to cerebral hypoxia, protecting the brain and inhibiting neuronal apoptosis.20 In the nervous system, miR-210 is not only involved in the occurrence of gliomas, peripheral nerve sheath tumors and neuroblastomas, but also in inflammation and neuronal apoptosis.21,22 The miR-210 has a proapoptotic activity at physiological oxygen levels, but an anti-apoptotic activity under hypoxic conditions.9 A previous study found that the downregulation of miR-210 reduced the anti-apoptotic and antioxidant effects induced by vagus nerve stimulation in rats.23 In another study, miR-210 suppressed neuronal apoptosis by inhibiting caspase activity and regulating the balance between Bcl-2 and Bax levels in a murine model of hypoxic-ischemic encephalopathy.24 After intracerebral microinjection of a miR-210 inhibitor, we found an increased expression of caspase-3. The degree of hippocampal apoptosis was also augmented, suggesting that the downregulation of miR-210 can promote apoptosis of hippocampal neurons. Our findings are consistent with the anti-apoptotic effect of miR-210 under hypoxic conditions described by several authors, but contradict the results of other studies11,25 which requires further research.
There are few reports describing a correlation between TLR and miR-210. The miR-210 modulates TLR gene expression, diminishing the downstream production of reactive oxygen species.26 The long non-coding RNA (lncRNA) maternally expressed gene 3 (MEG3) positively regulates the expression of TLR4 through the miR-210, modulating the inflammatory response and apoptosis of porcine alveolar macrophages.27 Other studies found that miR-210 can be regulated by downstream molecules of the TLR signaling pathway. After lipopolysaccharides (LPS) stimulation, the expression of miR-210 was upregulated in mouse macrophages, and miR-210 targeted NF-κB1 in the downstream of the LPS/TLR4 signaling pathway to inhibit the expression of p50 and subsequent NF-κB activation.28 In this study, we found that the expression of TLR4 and NF-κB1 significantly increased after microinjection of a miR-210 inhibitor in the hippocampus of SE rats. It is possible that miR-210 negatively regulated the TLR4/NF-κB1 signaling pathway in the hippocampus of SE rats. However, further studies are needed to verify this hypothesis.
Conclusion
The present study demonstrated that miR-210 might be involved in neuroinflammation and apoptosis induced by pilocarpine-induced epilepsy. The downregulation of miR-210 increased inflammation and neuronal apoptosis in the hippocampus of SE rats through the TLR4/NF-κB1 signaling pathway. Our findings provide valuable insights to better understand the role of miR-210 in epileptic seizures, but further research is warranted to clarify the issue.
Acknowledgments
This work was supported by the Science and Technology Program of Zhejiang province (No. 2018C37086), the Traditional Chinese Medicine Science and Technology Program of Zhejiang province (No.2020ZB022), Zhejiang medical and health science and technology plan project (No.2022KY073).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ascoli M, Ferlazzo E, Gasparini S, et al. Epidemiology and outcomes of status epilepticus. Int J Gen Med. 2021;14:2965–2973. doi:10.2147/IJGM.S295855
2. Dingledine R, Varvel NH, Dudek FE. When and how do seizures kill neurons, and is cell death relevant to epileptogenesis? Adv Exp Med Biol. 2014;813:109–122.
3. Gualtieri F, Marinelli C, Longo D, et al. Hypoxia markers are expressed in interneurons exposed to recurrent seizures. Neuromolecular Med. 2013;15(1):133–146. doi:10.1007/s12017-012-8203-0
4. Betjemann JP, Josephson SA, Lowenstein DH, Burke JF. Trends in status epilepticus-related hospitalizations and mortality: redefined in us practice over time. JAMA Neurol. 2015;72(6):650–655. doi:10.1001/jamaneurol.2015.0188
5. Rana A, Musto AE. The role of inflammation in the development of epilepsy. J Neuroinflammation. 2018;15(1):144. doi:10.1186/s12974-018-1192-7
6. Adhikarla SV, Jha NK, Goswami VK, et al. TLR-mediated signal transduction and neurodegenerative disorders. Brain Sci. 2021;11(11):1373. doi:10.3390/brainsci11111373
7. Rahimifard M, Maqbool F, Moeini-Nodeh S, et al. Targeting the TLR4 signaling pathway by polyphenols: a novel therapeutic strategy for neuroinflammation. Ageing Res Rev. 2017;36:11–19. doi:10.1016/j.arr.2017.02.004
8. Adams BD, Kasinski AL, Slack FJ. Aberrant regulation and function of microRNAs in cancer. Curr Biol. 2014;24(16):R762–776. doi:10.1016/j.cub.2014.06.043
9. Favaro E, Ramachandran A, McCormick R, et al. MicroRNA-210 regulates mitochondrial free radical response to hypoxia and krebs cycle in cancer cells by targeting iron sulfur cluster protein ISCU. PLoS One. 2010;5(4):e10345. doi:10.1371/journal.pone.0010345
10. Bertero T, Grosso S, Robbe-Sermesant K, et al. “Seed-Milarity” confers to hsa-miR-210 and hsa-miR-147b similar functional activity. PLoS One. 2012;7(9):e44919. doi:10.1371/journal.pone.0044919
11. Chen L, Zheng H, Zhang S. Involvement of upregulation of miR-210 in a rat epilepsy model. Neuropsychiatr Dis Treat. 2016;12:1731–1737. doi:10.2147/NDT.S108190
12. Gao F, Gao Y, Liu YF, Wang L, Li YJ. Berberine exerts an anticonvulsant effect and ameliorates memory impairment and oxidative stress in a pilocarpine-induced epilepsy model in the rat. Neuropsychiatr Dis Treat. 2014;10:2139–2145. doi:10.2147/NDT.S73210
13. Reddy DS, Kuruba R. Experimental models of status epilepticus and neuronal injury for evaluation of therapeutic interventions. Int J Mol Sci. 2013;14(9):18284–18318. doi:10.3390/ijms140918284
14. Wang D, Li Z, Zhang Y, et al. Targeting of microRNA-199a-5p protects against pilocarpine-induced status epilepticus and seizure damage via SIRT1-p53 cascade. Epilepsia. 2016;57(5):706–716. doi:10.1111/epi.13348
15. Zavala-Tecuapetla C, Cuellar-Herrera M, Luna-Munguia H. Insights into potential targets for therapeutic intervention in epilepsy. Int J Mol Sci. 2020;21(22). doi:10.3390/ijms21228573
16. Seinfeld S, Goodkin HP, Shinnar S. Status Epilepticus. Cold Spring Harb Perspect Med. 2016;6(3):a022830. doi:10.1101/cshperspect.a022830
17. Zusso M, Lunardi V, Franceschini D, et al. Ciprofloxacin and levofloxacin attenuate microglia inflammatory response via TLR4/NF-kB pathway. J Neuroinflammation. 2019;16(1):148. doi:10.1186/s12974-019-1538-9
18. Wang FX, Xiong XY, Zhong Q, Meng ZY, Yang H, Yang QW. Foxp3 exhibits antiepileptic effects in ictogenesis involved in TLR4 signaling. FASEB J. 2017;31(7):2948–2962. doi:10.1096/fj.201600989R
19. Qu Z, Jia L, Xie T, et al. (-)-Epigallocatechin-3-gallate protects against lithium-pilocarpine-induced epilepsy by inhibiting the Toll-Like Receptor 4 (TLR4)/nuclear factor-kappaB (NF-kappaB) signaling pathway. Med Sci Monit. 2019;25:1749–1758. doi:10.12659/MSM.915025
20. Chan YC, Banerjee J, Choi SY, Sen CK. miR-210: the master hypoxamir. Microcirculation. 2012;19(3):215–223. doi:10.1111/j.1549-8719.2011.00154.x
21. Yan R, Xu H, Fu X. Salidroside protects hypoxia-induced injury by up-regulation of miR-210 in rat neural stem cells. Biomed Pharmacother. 2018;103:1490–1497. doi:10.1016/j.biopha.2018.04.184
22. Lai NS, Wu DG, Fang XG, et al. Serum microRNA-210 as a potential noninvasive biomarker for the diagnosis and prognosis of glioma. Br J Cancer. 2015;112(7):1241–1246. doi:10.1038/bjc.2015.91
23. Jiang Y, Li L, Tan X, Liu B, Zhang Y, Li C. miR-210 mediates vagus nerve stimulation-induced antioxidant stress and anti-apoptosis reactions following cerebral ischemia/reperfusion injury in rats. J Neurochem. 2015;134(1):173–181. doi:10.1111/jnc.13097
24. Qiu J, Zhou XY, Zhou XG, Cheng R, Liu HY, Li Y. Neuroprotective effects of microRNA-210 on hypoxic-ischemic encephalopathy. Biomed Res Int. 2013;2013:350419. doi:10.1155/2013/350419
25. Bie B, Wang Z, Chen Y, et al. Vagus nerve stimulation affects inflammatory response and anti-apoptosis reactions via regulating miR-210 in epilepsy rat model. Neuroreport. 2021;32(9):783–791. doi:10.1097/WNR.0000000000001655
26. Li C, Zhao M, Zhang C, et al. miR210 modulates respiratory burst in Apostichopus japonicus coelomocytes via targeting Toll-like receptor. Dev Comp Immunol. 2016;65:377–381. doi:10.1016/j.dci.2016.08.008
27. Yin RH, Guo ZB, Zhou YY, Wang C, Yin RL, Bai WL. LncRNA-MEG3 regulates the inflammatory responses and apoptosis in porcine alveolar macrophages infected with haemophilus parasuis through modulating the miR-210/TLR4 axis. Curr Microbiol. 2021;78(8):3152–3164. doi:10.1007/s00284-021-02590-x
28. Qi J, Qiao Y, Wang P, Li S, Zhao W, Gao C. microRNA-210 negatively regulates LPS-induced production of proinflammatory cytokines by targeting NF-kappaB1 in murine macrophages. FEBS Lett. 2012;586(8):1201–1207. doi:10.1016/j.febslet.2012.03.011
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.