Back to Journals » International Journal of General Medicine » Volume 17
Downregulation of INPP4B is Associated with Poor Prognosis in Epithelial Ovarian Carcinoma
Received 23 October 2023
Accepted for publication 11 March 2024
Published 20 March 2024 Volume 2024:17 Pages 1059—1072
DOI https://doi.org/10.2147/IJGM.S445491
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Scott Fraser
Liangliang Jiang, Jing Wang
Department of Gynecological Tumor, Harbin Medical University Cancer Hospital, Harbin, People’s Republic of China
Correspondence: Jing Wang, Department of Gynecological Tumor, Harbin Medical University Cancer Hospital, Harbin, Heilongjiang Province, People’s Republic of China, Tel +8646022627, Email [email protected]
Background: INPP4B is a tyrosine-specific phosphatase in the human body, which plays an important role in the developing process of carcinogenesis. However, The correlation between INPP4B and epithelial ovarian cancer is rarely explored. In this study, the expression of INPP4B in human epithelial ovarian carcinoma and normal ovaries was detected, to explore the correlation between INPP4B expression and clinicopathological risk factors of epithelial ovarian carcinoma and to clarify its significance in the developing process of and prognosis of epithelial ovarian carcinoma.
Methods: The expression of INPP4B in various tumors was detected by bioinformatics method, and the expression in epithelial ovarian cancer and normal control group was detected by Elisa. The immunohistochemical method was used in this experiment to analyze the expression of INPP4B in specimens of 100 cases of epithelial ovarian carcinoma and 20 cases of normal ovaries. Analysis of clinicopathological risk factors and related survival analysis was carried out on the expression of INPP4B in 100 cases of epithelial ovarian carcinoma.
Results: The results showed that the positive expressed INPP4B protein in epithelial ovarian carcinoma was significantly less, compared with that in normal ovaries (P < 0.05). The expression of INPP4B was significantly associated with many clinicopathologic factors, such as tumor differentiation (P < 0.001), FIGO stage (P < 0.001), lymph node metastasis (P < 0.001) and distant metastasis at recurrence (P=0. 009), but not with age, pathologic type of tumor, serum CA125 at recurrence and chemotherapy sensitivity.
Conclusion: In epithelial ovarian carcinoma, there is a downregulation of INPP4B expression, which may be related to poor tumor differentiation, late FIGO stage, lymph node metastasis, distant metastasis at recurrence and insensitivity to chemotherapy. Under-expression of INPP4B, lymph node metastasis, FIGO stage, and distant metastasis at recurrence are factors of poor prognostic. The under-expression level of INPP4B may be involved in the progression of epithelial ovarian carcinoma.
Keywords: epithelial ovarian carcinoma, INPP4B, immunohistochemistry: clinical significance, survival
Introduction
Epithelial ovarian carcinoma is one of the gynecological malignant tumors, with the third highest mortality rate after cervical cancer in the world.1 Epithelial ovarian cancer has a genetic predisposition, and germline BRCA1/2 mutations are the strongest known genetic risk factor for epithelial ovarian cancer. The lifetime risk of ovarian cancer for carriers of germline mutations in the BRCA1 and BRCA2 genes is 35–60% and 12–25%, respectively. BRCA1/2 status can be used to predict a patient’s expected survival.2
Although the surgical procedure and chemotherapeutics have been improved, and the overall survival rate of patients with epithelial ovarian carcinoma has improved over the past 50 years, the five-year survival rate is still below 50%.3 The cost of treatment per patient with ovarian cancer remains the highest among all types of cancer. For instance, the average initial cost in the first year can amount to approximately USD 80,000, while the final year cost may escalate to USD 100,000.4 In order to mitigate mortality associated with ovarian cancer, public health experts are also seeking an economical and convenient screening strategy. Women with BRCA mutations have a significantly elevated lifetime risk of developing ovarian cancer, typically presenting as high-grade serous carcinoma which is highly aggressive and has a substantial mortality rate. Consequently, current treatment guidelines for epithelial ovarian cancer also encompass various preventive strategies for patients with hereditary ovarian cancer including genetic counseling for women at high risk and risk-reducing interventions (RRI) such as surgical procedures or chemoprevention through contraceptive medications. However, there still exist several unresolved challenges.
Inositol polyphosphate-4-phosphatase type II (INPP4B) is a phosphoinositol phosphatase, which is a negative regulator of PI3K/AKT signaling pathway.5 It is noteworthy to mention that the PI3K pathway is frequently upregulated in epithelial ovarian cancer and plays a pivotal role in chemoresistance and preservation of genomic stability, as it is implicated in numerous processes of DNA replication and cell cycle regulation. Inhibiting the PI3K may result in genomic instability and mitotic catastrophe by reducing the activity of Aurora kinase B, a spindle assembly checkpoint protein, thereby increasing the occurrence of lagging chromosomes during prometaphase.6 INPP4B plays different roles and functions in different tumors, and the anti-cancer effect of INPP4B was first reported in breast cancer.7 INPP4B is low-expressed in basal cell-like breast cancer, which is related to tumor grade and survival.8 In addition, INPP4B also plays the role of tumor suppressor gene in prostate cancer, and the under-expression of INPP4B is closely related to poor prognosis.9
Accumulating evidence supports that INPP4B is closely linked to the pathogenesis of cancer, and it is promising to be a new tumor therapeutic target. In this paper, we first applied bioinformatics methods to detect the expression of INPP4B in pan-cancer. Immunohistochemical and Elisa method was used to detect the expression of INPP4B in epithelial ovarian carcinoma and normal ovarian control group, to explore the relationship between INPP4B and the pathogenesis of epithelial ovarian carcinoma. INPP4B has the opportunity to be a new and efficient oncological marker for epithelial ovarian carcinoma. It can be used in early diagnosis and targeted therapy of epithelial ovarian carcinoma, inhibiting the proliferation and metastasis of tumor cells as a tumor suppressor gene, to achieve more efficient treatment. In addition, it can provide evidence for further exploring its mechanism in epithelial ovarian carcinoma, and provide theoretical support and research direction guidance for the pathogenesis of epithelial ovarian carcinoma.
Methods
Gene Expression Analysis
TIMER2 (tumor immune estimation resource, version 2, http://timer.cistrome.org/), GEPIA2 (Gene Expression Profiling Interactive Analysis, version 2) tool (http://gepia2.cancer-pku.cn/#analysis) and UALCAN (The University of ALabama at Birmingham CANcer data analysis Portal) (https://ualcan.path.uab.edu/) was exploited to investigate INPP4B expression profiling spectrum.10–12
Human Tissues
Patients’ tissue samples and normal clinical tissue samples were taken from 100 patients with epithelial ovarian carcinoma and 20 patients with normal ovaries. The patients received surgeries at Harbin Medical University Cancer Hospital from January 2011 to January 2013. The paraffin-embedded samples were selected for immunohistochemistry. Inclusion criteria of ovarian carcinoma: 1. Epithelial ovarian carcinoma diagnosed by pathology; 2. No other malignant tumors; 3. The patient had not undergone preoperative neoadjuvant chemotherapy or radiotherapy; 4. The patient’s case data was complete. Exclusion criteria: 1) Patients with severe medical complications; 2) Patients with irregular follow-up. A total of 100 patients who met the inclusion criteria were enrolled, aged from 22 to 73 years (median of 53 years). The patients suffered cytoreductive surgery for ovarian carcinoma, followed by six courses of platinum-based chemotherapy, repeated every three weeks. Ovarian specimens from 20 patients in the control group were benign and resected preventively. This study was approved by the Ethics Committee of Harbin Medical University Cancer Hospital (No.: KY2022-68).
FIGO staging of International Federation of Gynecology and Obstetrics was adopted for surgical staging. There were four stages: Stage I, II, III and IV, the former two were defined as early stages, while the latter two were designated as late stages. The classification standards of the World Health Organization were adopted for pathological types of tumors. Tumors were differentiated into well-differentiated (G1) and poorly differentiated (G2/G3). A complete set of data was obtained in patients, including age, pathological type, differentiation degree, FIGO stage, CA125 value at recurrence, lymph node metastasis, distant metastasis at recurrence, chemotherapy sensitivity, overall survival time (OS) and disease-free survival time (DFS).
All of the patients recruited in the study were followed up regularly until death or five years after the operation. The follow-up interval ranged from 3 to 60 months, 48 months on average. DFS was defined as the interval from the date of surgery to the date of diagnosis of recurrence or distant metastasis, and OS was defined as the interval from the date of surgery to the death of the patient or to the time of data collection.
Elisa
We used an Elisa kit (Abbexa UK) to detect serum from 10 patients with epithelial ovarian cancer and 10 normal controls in Harbin Medical University Cancer Hospital from January to February 2024. Blood samples were placed at room temperature for 2 hours, centrifuged at 1000×g for 20 minutes, and supernatant was collected for later use.
Set standard, test samples and control wells. Aliquot 100 µL of diluted standard into the standard wells. Aliquot 100 µL of Standard Diluent buffer into control (zero) well. Aliquot 100 µL of diluted samples into the sample wells. Incubate for 1 hr at 37 °C. Aliquot 100 µL of Detection Reagent A to each well. Incubate for 1 hr at 37 °C. Wash 3 times. Aliquot 100 µL of Detection Reagent B to each well. Incubate for 30 mins at 37 °C. Wash 5 times. Aliquot 90 µL of TMB Substrate to each well. Incubate for 15 mins at 37 °C. Aliquot 50 µL of Stop Solution. Measure the OD at 450 nm.
Immunohistochemistry
After paraffin embedding, the tissue was sectioned. Then the tissue sections were dewaxed, rinsed with distilled water and soaked in PBS solution for 6 minutes. The sections were rinsed and placed in a pressure cooker containing EDTA repair solution. The sections were boiled for 3 min, and then were taken out and cooled to room temperature to retrieval antigen. The sections were rinsed with distilled water again, and placed in PBS solution for 3 times (4 min each time). The tissue section was incubated with 3% hydrogen peroxide (room temperature, 8 min). Thus, the endogenous peroxidase activity was removed, and the PBS solution was washed 3 times (5 min each time). It was added with a blocking reagent. After incubation (room temperature, 8 min). The ratio of Anti-INPP4B antibody (Abcam USA) to PBS buffer is 1:50, and each slide is slowly dripped with an antibody of 50 μ Cover all tissues to prevent the formation of bubbles. Add an appropriate amount of diluted primary antibody and incubate at 37 ° C for about 2 hours. The section was rinsed with PBS (3 times, 5 min each time). Add biotin labeled secondary antibody working solution (Quick enzyme-linked sheep anti mouse/rabbit IgG polymer 50 μL) and incubate at 37 °C for 20 minutes. It was rinsed with PBS (3 times, 4 min each time). It was added with HRP-SPTM and incubated (37.5 °C, 15 min). It was rinsed with PBS (3 times, 3 min each time). DAB color development: A drop of DAB color reagents A, B and C was added into 1 mL of distilled water respectively. The tissue section was rested at room temperature for 3 to 15 minutes and then observed under a microscope. After satisfaction, the section was fully rinsed with water. The section was re-dyed with hematoxylin, dehydrated, hyalinized and sealed, and then observed under a microscope.
Result Assessment
Two experienced pathologists observed the staining degree of the specimen under a microscope and determined that cells with brown granules in the nucleus or cytoplasm as positive cells. Five visual fields (x 400) were taken from each positive section, and the percentage of 100 positive cells counted in each group was recorded separately. In terms of the proportion of positive cells: < 5%, 5–25%, 26–50%, 51–75% and ≥ 76% were counted as 0–4 points respectively; In terms of the proportion of staining intensity, strong, medium, weak and none of dyeing were recorded as 3–0 points respectively. Finally, the two indexes were scored synthetically, and the scores were divided into four grades: 6–7 for strongly positive, 4–5 for positive, 1–3 for weakly positive, and 0 for negative. The score of each slice was divided into two grades: with 4 points as the boundary. Those with ≥ 4 points were overexpressed, and those with < 4 points were under-expression.
Statistical Analysis
The statistical software used in this paper was spss22.0 (IBM, Armonk, New York, USA). The enumeration data were expressed by composition ratio or rate, and the comparison between groups was carried out by chi-square test. Kaplan-Meier analysis was adopted to draw the survival curves while the Log-rank method to compare the survival rates. Independent prognostic factors including OS and DFS were evaluated by Cox proportional hazard model. Test level p < 0.05 suggested statistically significant differences.
Results
Differential Expression of INPP4B Between Tumour and Normal Tissue Samples
Firstly, we compared INPP4B expression levels among tumors and matched normal tissues from 33 cancers using the TIMER database (Figure 1A). Elevated INPP4B expression was observed in BLCA (bladder urothelial carcinoma), CHOL((Cholangio carcinoma), ESCA (Esophageal carcinoma), KICH (Kidney Chromophobe) et al. INPP4B was low expression in GBM (Glioblastoma multiforme) and KIRP (Kidney renal papillary cell carcinoma). Subsequently, we combined TCGA and GTEx datasets to further validate the INPP4B expression differences in multiple normal tissues and tumor tissues (Figure 1B). INPP4B was significantly low expression in tumor tissues when compared with normal tissues (Figure 2).
 |
Figure 2 Expression level of INPP4B in Ovarian cancer. (*P<0.05). |
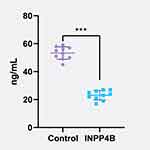
The Expression of INPP4B in Epithelial Ovarian Cancer Was Detected by ELISA
The results showed that the expression level of INPP4B in epithelial ovarian cancer was 23.54±3.37, and that in normal ovarian tissue of the control group was 54.05±4.68. INPP4B was significantly lower in epithelial ovarian cancer, and the difference was statistically significant (p < 0.05) (Figure 3).
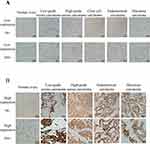
Immunohistochemistry Detection of the Expression of INPP4B Protein
INPP4B was expressed in the cytoplasm of both the lesion tissues and the normal ovarian control group. The overexpression rate of INPP4B in epithelial ovarian carcinoma was 30% (30/100). The overexpression rate of INPP4B in normal ovaries was 80.0% (16/20). Immunohistochemical results showed that INPP4B protein was low expressed in epithelial ovarian cancer than in normal ovarian tissue. (P<0.05) (Figure 4).
Relationship Between INPP4B Expression and Clinicopathological Factors
The expression of INPP4B was significantly associated with many clinicopathological factors. There were significant differences in tumor differentiation degree (P < 0.001), FIGO stage (P < 0.001), lymph node metastasis (P < 0.001) and distant metastasis at recurrence (P=0. 009), but no significant differences were found among other indexes (Table 1).
 |
Table 1 Relationship Between INPP4B Expression and Clinicopathological Factors |
Survival Analysis of Patients with Epithelial Ovarian Carcinoma
Comparison of Kaplan-Meier Survival Rate and Comparison of Survival Rate with Log-Rank Method
To explore the potential application value of INPP4B expression, a retrospective analysis was carried out on 100 epithelial ovarian carcinoma specimens. Kaplan-Meier was used to draw survival curves, and the Log-rank method was used to compare survival rates among groups. The results showed that the low expression group of INPP4B had shorter OS and DFS than the overexpression group (Figure 5).
Univariate Analysis of OS
Univariate analysis found that differentiation degree, FIGO stage, lymph node metastasis, distant metastasis at recurrence, chemotherapy sensitivity and INPP4B were the influencing factors of OS, but the interference of confounding factors was not controlled. Thus, multivariate regression analysis was needed to control the interference of confounding factors and find independent influencing factors of OS (Table 2).
 |
Table 2 Single Factor Analysis of OS |
Multivariate Regression Analysis of OS
Multivariate regression analysis showed that FIGO stage, lymph node metastasis, distant metastasis at recurrence and INPP4B were independent influencing factors of OS; FIGO stage, lymph node metastasis and distant metastasis at recurrence were independent risk factors, while INPP4B was independent protective factor. This indicates that patients with higher expression survived longer, and other indicators were not independent influencing factors of OS (Table 3).
 |
Table 3 Multivariate Regression Analysis of OS |
Univariate Analysis of DFS
Univariate analysis found that differentiation degree, FIGO stage, lymph node metastasis, chemotherapy sensitivity and INPP4B were the influencing factors of DFS, but the interference of confounding factors was not controlled. Thus, multivariate regression analysis was needed to control the interference of confounding factors and find independent influencing factors of DFS (Table 4).
 |
Table 4 Single Factor Analysis of DFS |
Multivariate Regression Analysis of DFS
Multivariate regression analysis showed that FIGO stage, lymph node metastasis and INPP4B were independent influencing factors of DFS; FIGO stage and lymph node metastasis were independent risk factors, while INPP4B was an independent protective factor. This indicates that patients with higher expression survived longer, and other indicators were not independent influencing factors of DFS (Table 5).
 |
Table 5 Multivariate Regression Analysis of DFS |
Discussion
This study aims to explore whether the expression of INPP4B in epithelial ovarian cancer is related to clinicopathological factors and prognosis. We first analyzed the expression of INPP4B in different tumors by bioinformatics methods and found that its expression was increased in BLCA, CHOL and other tumors, but decreased in GBM and KIRP. Further studies showed that the expression of INPP4B was decreased in epithelial ovarian cancer. This study also detected low expression of INPP4B in epithelial ovarian cancer by Elisa. It continued to expand the sample size and immunohistochemical examination was applied to explore the expression of INPP4B in 100 cases of epithelial ovarian cancer tissues and 20 cases of tissues from the control group. The pathological types of epithelial ovarian carcinoma in this study included high-grade serous carcinoma, clear cell carcinoma, low-grade serous carcinoma, endometrioid carcinoma and mucinous carcinoma. Among them, there were serous carcinoma (n=60), mucinous carcinoma (n=19), endometrioid carcinoma (n=17) and clear cell carcinoma (n=4). Other types of epithelial ovarian carcinoma were not included in this study. There are two different carcinogenic pathways for epithelial ovarian cancer. Type I epithelial cell carcinoma is taken as a relatively indolent and genetically stable tumor, while type II epithelial cell carcinoma is a biologically invasive tumor, which tends to metastasize from the primary site in a small size.13 High-grade serous ovarian cancer is the most common epithelial ovarian cancer type, which is about 75% of epithelial ovarian cancer. It develops according to the type II pathway, with p53 and BRCA mutations. Ovarian cancer is one of the gynecological tumors, which has the highest mortality and morbidity.14 At present, there is a lack of specific and effective methods to diagnose ovarian cancer. Most patients are diagnosed as clinically advanced (stage III or IV), and some even have distant metastasis.15 Therefore, searching for tumor markers and new drug targets for early diagnosis of diseases is the key to improving the survival and prognosis of patients, with broad application prospects.16 Proteomics can identify protein targets and pathways related to cancer cell growth and metastasis. Proteomic techniques like protein array analysis and mass spectrometry can promote the anatomy of potential molecular signal events and proteomic characteristics in ovarian cancer. Proteomic analysis of ovarian cancer and its adaptive response may reveal new treatment options, and this may reduce the occurrence of drug resistance and improve the prognosis of patients. This study analyzed the expression of INPP4B in 100 epithelial ovarian cancer tissues and 20 controls. The expression of INPP4B was low in epithelial ovarian cancer. Further statistical analysis showed that INPP4B expression was significantly correlated with tumor differentiation, FIGO stage, lymph node metastasis at recurrence and distant metastasis. These results also suggested that INPP4B was associated with epithelial ovarian cancer, suggesting that INPP4B may act as a tumor suppressor gene in the pathogenesis of epithelial ovarian cancer. The advantage of this study is that the OS and DFS of INPP4B low expression group were shorter than those of INPP4B high expression group through univariate and multivariate analysis. This study shows that NPP4B is an independent prognostic factor of epithelial ovarian cancer, and is related to the progression and metastasis of epithelial ovarian cancer. In previous studies, Gewinner et al17 found that the low expression of INPP4B in epithelial ovarian cancer was significantly related to the decrease of overall survival and lymph node metastasis, which was consistent with this study. Salmena et al18 found that abnormal expression of P53 was very common in serous ovarian cancer and was significantly related to the mortality of ovarian cancer. In univariate analysis, low expression of INPP4B in subtypes of endometrial cancer was related to poor survival rate, which was consistent with this study. It is worth noting that they found that patients with BRCA1 or BRCA2 mutation were more likely to have low expression of INPP4B and abnormal expression of p53. Low expression of INPP4B was more likely to occur in high-grade serous carcinoma and endometrial carcinoma. The main advantage of this research is the large sample size and stratified analysis based on histological subtypes. INPP4B belongs to the phosphatase family, and its main function is to maintain the homeostasis of intracellular phosphoinositol.19 The role of INPP4B in malignant tumors has been studied.20 INPP4B plays the role of a tumor suppressor gene in some malignant tumors including gastric cancer, breast cancer and colon cancer. Its low expression is associated with poor clinical prognosis.21–24 At present, studies on the pathogenesis of epithelial ovarian cancer generally believe that abnormal PI3K/ATK signal is closely related to the pathogenesis of epithelial ovarian cancer and this signal pathway is one of the important signal pathways of cell metabolism and anti-apoptosis. Studies have shown that INPP4B plays a physiological role by negatively regulating PI3K/Akt signaling pathway.25,26 In breast cancer, INPP4B inhibits the PI3K/Akt pathway, thus resulting in the effect of a tumor suppressor gene.8 INPP4B inhibits PI3K/AKT signaling by converting PI(3,4)P into PI(3)P. ER breast cancer with PIK3CA mutation showed increased expression of INPP4B mRNA and protein, while inhibition of AKT signal transduction. Therefore, INPP4B, as a regulatory factor of PI3K/Akt signal, may act as a suppressor gene in the pathogenesis of ovarian cancer and become a new target for clinical treatment. In conclusion, compared with the normal control group, the expression level of INPP4B in epithelial ovarian cancer tissues decreased significantly. This also indicated that INPP4B was related to epithelial ovarian cancer, and this result also suggested that INPP4B might act as a tumor suppressor gene in the pathogenesis of epithelial ovarian cancer. There are also some limitations and deficiencies in this study. In this study, the number of patients with rare pathological types of epithelial ovarian cancer is small, which may lead to errors in experimental results. In addition, animal and cell experiments have not been carried out in this study for pathogenesis. Therefore, the mechanism of INPP4B in epithelial ovarian cancer remains unclear due to the limitations of the present method.
Conclusion
INPP4B has an under-expression in epithelial ovarian carcinoma, which may be related to poor tumor differentiation, late FIGO stage, lymph node metastasis, distant metastasis at recurrence and insensitivity to chemotherapy. Under-expression of INPP4B, lymph node metastasis and FIGO stage are factors of poor prognosis. The under-expression of INPP4B in patients with epithelial ovarian carcinoma may be related to the progression and poor prognosis of tumor. INPP4B can be used as a new biomarker to analyze the prognosis of epithelial ovarian carcinoma.
Abbreviations
TIMER2, tumor immune estimation resource version 2; OS, overall survival time; DFS, disease-free survival time; IHC, immunohistochemistry; GEPIA2, Gene Expression Profiling Interactive Analysis version 2; UALCAN, The University of ALabama at Birmingham CANcer data analysis Portal.
Data Sharing Statement
The data were all included in this paper. The original data are available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
The studies involving human participants were reviewed and approved by the Institutional Ethical Committee of Harbin Medical University Cancer Hospital (Ethical Review Number: No.: KY2022-68). The patients/participants provided their written informed consent to participate in this study. The present study fulfils the requirements of the Declaration of Helsinki.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; JL took part in drafting, WJ revising or critically reviewing the article; All authors gave final approval of the version to be published; All authors have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Haiyan Foundation Youth Project JJQN2022-12.
Disclosure
All authors disclosed no relevant relationships.
References
1. Feng J, Wang Q. Correlation of systemic immune-inflammatory response index with clinical data in patients with malignant ovarian tumor. Am J Transl Res. 2023;15(5):3309–3317.
2. Shah S, Cheung A, Kutka M, Sheriff M, Boussios S. Epithelial ovarian cancer: providing evidence of predisposition genes. Int J Environ Res Public Health. 2022;19(13):8113. doi:10.3390/ijerph19138113
3. Zhu J, Wong F, Szymiczek A, et al. Evaluating the utility of ctDNA in detecting residual cancer and predicting recurrence in patients with serous ovarian cancer. Int J Mol Sci. 2023;24(18). doi:10.3390/ijms241814388
4. Ghose A, Bolina A, Mahajan I, et al. Hereditary ovarian cancer: towards a cost-effective prevention strategy. Int J Environ Res Public Health. 2022;19(19):12057. doi:10.3390/ijerph191912057
5. Xu M, Ren J, Jia W, et al. Regulation of B-1 cell numbers and B cell-mediated antibody production by Inpp4b. Scand J Immunol. 2023;98(4):e13309. doi:10.1111/sji.13309
6. Aliyuda F, Moschetta M, Ghose A, et al. Advances in ovarian cancer treatment beyond PARP inhibitors. Curr Cancer Drug Targets. 2023;23(6):433–446. doi:10.2174/1568009623666230209121732
7. Liu H, Paddock M, Wang H, et al. The INPP4B tumor suppressor modulates EGFR trafficking and promotes triple-negative breast cancer. Cancer Discovery. 2020;10(8):1226–1239. doi:10.1158/2159-8290.Cd-19-1262
8. Rodgers S, Ooms L, Oorschot V, et al. INPP4B promotes PI3Kα-dependent late endosome formation and Wnt/β-catenin signaling in breast cancer. Nat Commun. 2021;12(1):3140. doi:10.1038/s41467-021-23241-6
9. Chen H, Li H, Chen Q. INPP4B overexpression suppresses migration, invasion and angiogenesis of human prostate cancer cells. Clin Exp Pharmacol Physiol. 2017;44(6):700–708. doi:10.1111/1440-1681.12745
10. Li T, Fu J, Zeng Z, et al. TIMER2.0 for analysis of tumor-infiltrating immune cells. Nucleic Acids Res. 2020;48(W1):W509–W514. doi:10.1093/nar/gkaa407
11. Tang Z, Kang B, Li C, Chen T, Zhang Z. GEPIA2: an enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 2019;47(W1):W556–W560. doi:10.1093/nar/gkz430
12. Song J, Wu Y, Chen Z, Zhai D, Zhang C, Chen S. Clinical significance of KRT7 in bladder cancer prognosis. Int J Biol Markers. 2024;3936155231224798. doi:10.1177/03936155231224798
13. Pavlidis N, Rassy E, Vermorken J, et al. The outcome of patients with serous papillary peritoneal cancer, fallopian tube cancer, and epithelial ovarian cancer by treatment eras: 27 years data from the SEER registry. Cancer Epidemiol. 2021;75:102045. doi:10.1016/j.canep.2021.102045
14. Zachou G, El-Khouly F, Dilley J. Evaluation of follow-up strategies for women with epithelial ovarian cancer following completion of primary treatment. Cochrane Database Syst Rev. 2023;8(8):CD006119. doi:10.1002/14651858.CD006119.pub4
15. Yang H, Gao D, Wang C, Jiao J, Yu X, Li X. Effect of different metastasis patterns on the prognosis of patients with stage III high-grade serous ovarian cancer. Am J Cancer Res. 2023;13(8):3599–3606.
16. Veneziani A, Gonzalez-Ochoa E, Alqaisi H, et al. Heterogeneity and treatment landscape of ovarian carcinoma. Nat Rev Clin Oncol. 2023;20(12):820–842. doi:10.1038/s41571-023-00819-1
17. Gewinner C, Wang Z, Richardson A, et al. Evidence that inositol polyphosphate 4-phosphatase type II is a tumor suppressor that inhibits PI3K signaling. Cancer Cell. 2009;16(2):115–125. doi:10.1016/j.ccr.2009.06.006
18. Salmena L, Shaw P, Fans I, et al. Prognostic value of INPP4B protein immunohistochemistry in ovarian cancer. Eu J Gynaecol Oncol. 2015;36(3):260–267.
19. Wu Y, Meng D, Xu X, et al. Expression and functional characterization of INPP4B in gallbladder cancer patients and gallbladder cancer cells. BMC Cancer. 2021;21(1):433. doi:10.1186/s12885-021-08143-6
20. Wang Y, Chen L, Li Q, et al. Inositol Polyphosphate 4-Phosphatase Type II Is a tumor suppressor in multiple myeloma. Front Oncol. 2021;11:785297. doi:10.3389/fonc.2021.785297
21. Chaurasiya S, Wu W, Strom A, Warner M, Gustafsson J. Estrogen receptor β regulates AKT activity through up-regulation of INPP4B and inhibits migration of prostate cancer cell line PC-3. Proc Natl Acad Sci USA. 2020;117(42):26347–26355. doi:10.1073/pnas.2007160117
22. Wu Y, Wang X, Lu Y, et al. INPP4B exerts a dual role in gastric cancer progression and prognosis. J Cancer. 2021;12(23):7201–7213. doi:10.7150/jca.58397
23. Chen X, Theobard R, Zhang J, Dai X. Genetic interactions between INPP4B and RAD50 is prognostic of breast cancer survival. Biosci Rep. 2020;40(1). doi:10.1042/bsr20192546
24. Yang L, Ding C, Tang W, et al. INPP4B exerts a dual function in the stemness of colorectal cancer stem-like cells through regulating Sox2 and nanog expression. Carcinogenesis. 2020;41(1):78–90. doi:10.1093/carcin/bgz110
25. Sun X, Chen Y, Tao X, et al. viaINPP4B inhibits glioma cell proliferation and immune escape inhibition of the PI3K/AKT signaling pathway. Front Oncol. 2022;12:983537. doi:10.3389/fonc.2022.983537
26. Hu T, Zhang H, Du Y, et al. ELOVL2 restrains cell proliferation, migration, and invasion of prostate cancer via regulation of the tumor suppressor INPP4B. Cell Signalling. 2022;96:110373. doi:10.1016/j.cellsig.2022.110373
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.