Back to Journals » Journal of Multidisciplinary Healthcare » Volume 11
Does the medical literature remain inadequately described despite having reporting guidelines for 21 years? – A systematic review of reviews: an update
Authors Jin Y , Sanger N, Shams I, Luo C, Shahid H , Li G, Bhatt M, Zielinski L , Bantoto B, Wang M , Abbade LPF , Nwosu I, Leenus A , Mbuagbaw L, Maaz M, Chang Y, Sun G, Levine MAH , Adachi JD , Thabane L, Samaan Z
Received 25 October 2017
Accepted for publication 10 April 2018
Published 27 September 2018 Volume 2018:11 Pages 495—510
DOI https://doi.org/10.2147/JMDH.S155103
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Yanling Jin,1,* Nitika Sanger,2,* Ieta Shams,3,* Candice Luo,4,* Hamnah Shahid,5,* Guowei Li,1,* Meha Bhatt,1 Laura Zielinski,6 Bianca Bantoto,7 Mei Wang,1 Luciana PF Abbade,8 Ikunna Nwosu,4 Alvin Leenus,1 Lawrence Mbuagbaw,1 Muhammad Maaz,1 Yaping Chang,1 Guangwen Sun,1 Mitchell AH Levine,1,9 Jonathan D Adachi1,9 Lehana Thabane,1,9 Zainab Samaan1,10
1Department of Health Research Methods, Evidence and Impact, McMaster University, Hamilton, ON, Canada; 2Department of Medical Science, Medical Sciences Graduate Program, McMaster University, Hamilton, ON, Canada; 3Department of Psychology, Neuroscience and Behaviour, McMaster University, Hamilton, ON, Canada; 4Faculty of Health Sciences, Bachelors of Health Sciences, McMaster University, Hamilton, ON, Canada; 5Department of Arts and Science, McMaster University, Hamilton, ON, Canada; 6Department of Neuroscience, McMaster Integrative Neuroscience Discovery and Study, McMaster University, Hamilton, ON, Canada; 7Department of Science, Honours Integrated Sciences Program, McMaster University, Hamilton, ON, Canada; 8Department of Dermatology and Radiotherapy, Botucatu Medical School, Universidade Estadual Paulista, UNESP, São Paulo, Brazil; 9St. Joseph’s Healthcare Hamilton, Hamilton, ON, Canada; 10Department of Psychiatry and Behavioural Neurosciences, McMaster University, Hamilton, ON, Canada
*These authors contributed equally to this work
Purpose: Reporting guidelines (eg, Consolidated Standards of Reporting Trials [CONSORT] statement) are intended to improve reporting standards and enhance the transparency and reproducibility of research findings. Despite accessibility of such guidelines, researchers are not required to adhere to them. Our goal was to determine the current status of reporting quality in the medical literature and examine whether adherence of reporting guidelines has improved since the inception of reporting guidelines.
Materials and methods: Eight reporting guidelines, such as CONSORT, Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA), STrengthening the Reporting of OBservational studies in Epidemiology (STROBE), Quality of Reporting of Meta-analysis (QUOROM), STAndards for Reporting of Diagnostic accuracy (STARD), Animal Research: Reporting In Vivo Experiments (ARRIVE), Consolidated Health Economic Evaluation Reporting Standards (CHEERS), and Meta-analysis of Observational Studies in Epidemiology (MOOSE) were examined. Our inclusion criteria included reviews published between January 1996 to September 2016 which investigated the adherence to reporting guidelines in the literature that addressed clinical trials, systematic reviews, observational studies, meta-analysis, diagnostic accuracy, economic evaluations, and preclinical animal studies that were in English. All reviews were found on Web of Science, Excerpta Medical Database (EMBASE), MEDLINE, and Cumulative Index to Nursing and Allied Health Literature (CINAHL).
Results: Among the general searching of 26,819 studies by using the designed searching method, 124 studies were included post screening. We found that 87.9% of the included studies reported suboptimal adherence to reporting guidelines. Factors associated with poor adherence included non-pharmacological interventions, year of publication, and trials concluding with significant results. Improved adherence was associated with better study designs such as allocation concealment, random sequence, large sample sizes, adequately powered studies, multiple authorships, and being published in journals endorsing guidelines.
Conclusion: We conclude that the level of adherence to reporting guidelines remains suboptimal. Endorsement of reporting guidelines by journals is important and recommended.
Keywords: guidelines, adherence, review, CONSORT
Introduction
Medical science is an evolving and dynamic field of research that impacts health care, disease outcomes, and health care systems in general. The evidence generated from millions of medical publications is meant to inform these dynamic changes and therefore has to be presented in a clear, consistent, and transparent fashion. There are more than 26 million citations for biomedical literature in the PubMed1 database alone. To understand and evaluate the evidence presented in these citations, a harmonized method of reporting the research findings is needed to ensure clarity, consistency, and the uptake and dissemination of knowledge.2 Tremendous efforts have been made to provide guidelines for different types of research designs to assist in the process of transparent and clear reporting, eg, Enhancing the QUAlity and Transparency Of health Research (EQUATOR) Network website.3 However, despite the wide availability of such guidelines since the inception of the Consolidated Standards of Reporting Trials (CONSORT4) statement in 1996, the uptake remains suboptimal in the face of the exponential volume of medical literature leaving the readers confused. For example, some studies show positive harmful results from eating red meat on the risk of having colorectal cancer,5 while others are showing inconsistent effect marked by substantial methodological differences, type of red meat investigated, and the population selection limitations.6 Therefore, the reader is unable to decide whether red meat has an effect on bowel cancer risk. Poor reporting without using well-designed guidelines in primary studies may lead to a bias in the treatment effects found in systematic reviews. In addition, poorly conducted systematic reviews may not be able to detect the bias effect that the studies included. In a previous study, we conducted a scoping review and examined the level of adherence to six reporting guidelines and found the level of adherence to be suboptimal in 86% of the included studies.7
The aim of this review was to conduct a systematic review of reviews to update the state of adherence to guidelines since 2012 and to identify factors associated with improved adherence. Our hypothesis was that the reporting standards have improved since our last examination in 2012 given that a longer period has passed after guideline statements were first introduced for researchers and more journals started to endorse the guidelines. Our search was looking at reviews published between January 1, 1996, and September 30, 2016.
Materials and methods
This systematic review was performed and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.8 A protocol for a series of three reviews including the current systematic review has been peer reviewed and published elsewhere.9
Study inclusion and exclusion criteria
Systematic reviews which investigated the adherence to commonly used reporting guidelines in medical literature that addressed clinical trials, systematic reviews, observational studies, meta-analysis, diagnostic accuracy, economic evaluations, and preclinical animal studies that have been reported in English were selected. Eight guidelines included in this review were as follows: CONSORT,4 PRISMA,8 STrengthening the Reporting of OBservational studies in Epidemiology (STROBE),10 Quality of Reporting of Meta-analysis (QUOROM),11 STAndards for Reporting of Diagnostic accuracy (STARD),12 Animal Research: Reporting In Vivo Experiments (ARRIVE),13 Consolidated Health Economic Evaluation Reporting Standards (CHEERS),14 and Meta-analysis of Observational Studies in Epidemiology (MOOSE).15
The exclusion criteria included studies that 1) were not systematic reviews; 2) did not explore adherence to the aforementioned reporting guidelines; 3) did not provide data on guideline adherence; 4) were subsets of the included studies; 5) published abstracts, letters, editorials, or commentaries; and 6) reviews in languages other than English for feasibility and resource purposes.
Search strategy
The search strategy was based on the previously published review7 and was updated for this systematic review. We searched four databases (Excerpta Medical Database [EMBASE], MEDLINE, Cumulative Index to Nursing, and Allied Health Literature [CINAHL], and Web of Science) from 1996 (CONSORT inception – first created guideline among all eight included guidelines) to September 30, 2016.
We used the following search terms for each of the four databases: (Systematic reviews OR reviews OR quality of reporting OR completeness of reporting) AND (CONSORT OR STROBE OR QUOROM OR PRISMA OR MOOSE OR STARD OR ARRIVE OR CHEERS) OR adherence. Detailed search terms have been reported in the published protocol.9 All stages of search, inclusion, exclusion, and data abstraction were performed independently in duplicate, and agreement was reached through team discussion and consensus.
Outcome measures
The primary outcome was the level of adherence to reporting guidelines and their checklists as reported in the systematic reviews. The secondary outcome included the factors that were associated with improved adherence to guidelines.
Data extraction
A specific data abstraction form was designed to include the following data: 1) general characteristics of the included studies (first author, publication year, country, journal, study field, search time frame, data sources, numbers of included primary studies, and study design), 2) main findings from the included studies, 3) authors’ summaries and conclusions, and 4) factors reported to be related to improved guideline reporting adherence. Each assessment of the systematic reviews was conducted in duplicate. Calibration was performed on the data extraction form. If the pair of evaluators was unable to come to a conclusion, a third-party reviewer would have settled the dispute.
Quality evaluation
We used the modified Assessing the Methodological Quality of Systematic Reviews/Overview of Quality Assessment Questionnaire (Assessment of Multiple Systematic Reviews [AMSTAR]/Overview Quality Assessment Questionnaire [OQAQ]), a 10-item scale,7 to assess the quality of the systematic reviews included in this review. We assigned a number out of a maximum of 20 points for each included study. The higher the number assigned, the better the quality of the systematic review.
Data synthesis
We provided a qualitative summary and characteristics of the included studies. We summarized the factors associated with adherence based on the included study results; no quantitative analysis was possible in this review. We also reported the percentage of studies in which the level of adherence to reporting each guideline was suboptimal. This was calculated by dividing the number of studies with this finding by the total number of studies evaluating the guideline.
Results
Our search resulted in a total of 9,123 publications, of which 124 systematic reviews that included 26,819 primary studies were included in this systematic review of reviews. Figure 1 shows the PRISMA flowchart for the included studies.
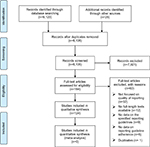  | Figure 1 PRISMA flow diagram. Abbreviation: PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses. |
The characteristics of the included studies are described in Table 1. The majority of the studies (65% of the total 124 included studies) investigated the adherence to the CONSORT statement as expected since it is the first and oldest guideline. The second most commonly investigated guideline is the PRISMA with 19 studies (15%; Table 1).
The majority of studies used the guideline checklist to evaluate the level of adherence and generated a mean score as summarized in Table S1. Table S1 summarizes the studies’ findings by guideline with authors’ conclusions for each study. Most studies described the adherence to the different guidelines using the following qualitative descriptors:
deficient, not adequately reported, generally poor, suboptimal, poor, medium, low, poor to moderate, lack of CONSORT adherence, bad, far from satisfactory, lack of standard reporting, improvement over the years has been minor, weak, quality of the articles varied substantially, insufficient, missed reporting some important factors, deficiencies in reporting, inconsistent, needs to be improved, inadequate, there is a need for improvement in quality of reporting, overall adherence is low.
A summary of the quantitative assessment of adherence to guidelines is presented in Table 2.
The level of adherence to all included reporting guidelines was 87.9% of all guidelines combined showing a need for improvement in reporting. Factors associated with poor adherence to CONSORT guideline included trials with significantly positive results, trials with the categorical outcome, trials conducted in North America compared to Europe, and trials funded by nonindustry source. A summary of factors associated with adherence standards is summarized in Table 3. Several factors were associated with better reporting standards relating to authors, study design, outcome specifications, year of publication (recent years of publications are associated with better reporting standards), journal, funding source, and study/author country.
Factors associated with improved adherence to reporting guidelines
Author factors
The included studies reported that the expertise of the author team, for example, an epidemiologist, improved the quality of reporting the study. In addition, having multiple authors also improved reporting quality.
Study factors
Study design with detailed methods including allocation concealment, randomization, specific outcome measures, sample size and power calculations, acknowledgment of limitations and sources of bias, larger sample size, registration of clinical trials, pharmacological interventions, and detailed statistical analysis plan were associated with better reporting and adherence to reporting guidelines. Year of publication was also associated with adherence in which the more recently published articles had increased adherence.
Journal factor
Publications in journals endorsing reporting guidelines have better adherence to these guidelines than articles published in journals that do not endorse such guidelines. In addition, journals’ impact factor, medical journals, and journals with restriction on the number of words per article also had articles with better reporting standards. Publication in a general medical journal was associated with better reporting quality than a specialty journal.
Ethics and funding factors
Articles that reported ethical approval, participants’ consent, and the source of funding were associated with improved adherence to reporting guidelines.
Country of study factors
Geographic location of the study has an impact on the quality of reporting and adherence to reporting guidelines, for example, studies reported from Europe had better reporting standards compared to studies from North America. Studies reported from China had lower adherence to guidelines than elsewhere indicating geographical variations may directly or indirectly impact the level of adherence to reporting guidelines in the medical literature.
Quality assessment of included studies
For each included systematic review, we performed a quality assessment using the modified AMSTAR/OQAQ score. Table 4 provides the total score out of 20 for each study. The scores varied from 9 to 20. The average score for all the included studies is 16.14. The lowest scores were related to items 5 and 6 of the quality assessment related to the availability of the primary studies’ characteristics similar to a previously reported study.7 Items 5 and 6 were evaluated if there was information on included and excluded studies provided and if the characteristics of included studies provided, respectively.
Discussion
The medical literature is paramount to the progression of the understanding of health and disease and the establishment of priorities and recommendations for prevention, diagnosis, treatment, and measurement of outcomes. To implement research findings, transparent and consistent reporting standards are needed to help make informed decisions. Such standards have been set by the CONSORT working group and others for the past 2 decades with the aim of improving the reporting standards in biomedical research. It is expected that the introduction of new change to the current practice will take time to adopt and disseminate. However, the uptake of the widely available guidelines has been less than ideal. We define suboptimal and less than ideal as <100%. The whole idea of a systematic review is to have completely transparent methods reported, so everyone can follow and reproduce the results. Inherently, systematic reviews are meant to be a more rigorous study design. This allows them to produce meaningful results than individual studies. Thus, when reviews fail to adhere to reporting guidelines, it calls into question the consistency of their results. Given the weight that systematic reviews have in the scientific community, it is imperative that we hold reviews to a high standard.
Five years ago, we investigated the level of adherence to reporting standards in the medical literature, and we identified 86% of the systematic reviews conducted on the level of adherence to reporting guidelines of the medical literature to be less than ideal.7 Since our previous scoping review, many new revisions and updates to reporting guidelines have been introduced. Currently, there are 358 reporting guidelines on the EQUATOR Network website16 for many study types that are freely available. However, endorsement of reporting guidelines by journals still remains low.
Among all the factors that can improve the reporting quality, such as author factors, study factors, journal factors, ethics and funding factors, and country of study factors, author factors as well as their limitations have been studied in other researches. The author factors were the number of the authors of the publication and the level of expertise in the different research methods. Multiple authorships were shown to be an important determinant of the impact of the research being produced and its likelihood of being cited.17 The complexity and cost of medical research today requires multiple levels of expertise in various disciplines as well as accountability and oversight by study team members, institutions, and funding bodies. It is known that the number of authors per article has increased over the past few decades18,19 with a concern posed to question the roles of multiple authors and the most senior academics holding senior authorship at the expense of others in the team.20 Other studies have reported that the research produced by teams rather than single authors was impactful and more frequently cited, at least in certain fields.21 It is likely that multiple authorships arising from collaborative efforts have advantages of producing good quality impactful research; however, multiple authorships also have limitations and may not be feasible at every setting due to geographical limitations or strict timeline to follow as bringing more authors is time-consuming.22 In this review, we found that having multiple authorships is important to have publications with better adherence to reporting guidelines. However, the role of each author and the hierarchy of authorship should be clarified for successful collaborations and research impact as discussed earlier.
Study factors that improved adherence to reporting guidelines included well-designed, detailed study methods and adequately powered studies. Study results could be altered regarding trial designs, qualities, and methods.23 Therefore, guidelines such as CONSORT statement that is designed for randomized control trials (RCTs), STROBE guideline for observational studies, and PRISMA guideline for systematic reviews were invented accordingly based on different study designs. RCTs are also considered as the highest level of primary evidence in the clinical practice, and therefore it is vital that these trials are reported according to the expected standards.24
Other factors reported that might improve the level of adherence to reporting guidelines included journals endorsing these guidelines. The Internal Committee of Medical Journal Editors (ICMJEs) recognized the importance of reporting guidelines in ensuring study details that are described adequately to be evaluated appropriately and encouraged journals to request these reporting standards from authors.25 The EQUATOR Network has valuable resources and tool kits to assist authors and journal editors to adopt the reporting guidelines and provide case studies of journals endorsing the guidelines. Since journals that endorsed reporting guidelines often ask authors to submit a completed checklist regarding the guidelines, it improves the quality of reporting for those journals endorsing these guidelines. Yet, not all journals currently endorse the guidelines. According to the CONSORT website, there are 585 journals that endorse CONSORT,26 while there are about 30,000 journals indexed in PubMed.27 While not all of these indexed journals publish RCTs, many of them do publish them, but do not adhere to CONSORT guidelines.27
The EQUATOR Network also has tool kits for ethics boards and study sponsors to ensure that the reporting guidelines are considered when these agencies review research submissions for ethical approval or funding requests. It is therefore important that all stakeholders take part in the use and dissemination of the reporting guidelines to enhance the quality of medical research and biomedical literature.
Limitations
The included studies are limited to only eight of the reporting guidelines, and therefore the current study lacks the generalizability to other guidelines that may have a better adherence standard. In addition, there was no comparison between studies to ensure that they are using qualitative descriptors such as “inadequate” or “suboptimal” with the same operational definition. The studies do not provide sufficient information regarding the operationalization of qualitative descriptors to allow us to adequately compare descriptors across studies.
In addition, the study was limited to systematic reviews that present with its own set of limitations. The most notable limitation is the low mean score on the quality assessment since each systematic review follows different reporting guidelines or does not follow guidelines at all and the lack of detailed data on the included studies’ characteristics. Furthermore, a quantitative analysis was not conducted, as not all included studies provided relevant data. Strict inclusion criteria may have allowed a quantitative analysis. However, for the sake of a more representative sample, such criteria were not implemented.
The inclusion of studies in English only is also a limitation to a selected section of the medical literature and did not include other reporting guidelines that may be in use in other languages.
Despite the limited scope of inclusion criteria and quality limitation of the included studies, this review provides an insight into the limited uptake of reporting guidelines and calls for exploring barriers to such uptake. Future studies may include broad surveys of authors, journal editors, funding agencies, ethics boards, and readers to solicit opinions and understanding of the role of reporting guidelines in the medical research and literature.
Conclusion
Current adherence to reporting guidelines in the medical literature is suboptimal. However, there are factors associated with better reporting upon which we can develop strategies for better reporting. Reporting guidelines are an imperative tool in the endeavor to improve the consistency of reporting in the medical literature. However, the suboptimal uptake and correct usage of reporting guidelines demonstrate the need for further emphasis in the scientific community to encourage the use of reporting guidelines. The responsibility for improving the transparency, quality, and reproducibility of medical literature lies with all stakeholders from the research participants to regulatory authorities and everyone in between including authors, readers, educators, funders, academic and health care institutions, editors, peer reviewers, and guideline developers. Future studies may include broad surveys of authors, journal editors, funding agencies, ethics boards, and readers to solicit opinions and understanding of the role of reporting guidelines in the medical research and literature.
Data sharing statement
Unpublished study data are available upon request.
Author contributions
Contributed to the conception and design of the study, development of data extraction forms, search strategy, analysis of results, manuscript writing, and final review of the manuscript: YJ, NS, IS, CL, HS, and GL. Contributed to the methodological design, critical revision, and final review of the manuscript: MB, LZ, BB, MW, LPFA, IN, AL, LM, MM, YC, GS, MAHL, JDA, and LT. Substantially contributed to the conception and design of the study, critical revision, and final approval of the manuscript: ZS. All the authors read and approved the final manuscript. All the authors consented and approved the manuscript for publication. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
NCBI [database on the Internet]. PUBMED. 2017. Available from: https://www.ncbi.nlm.nih.gov/pubmed. Accessed June 16, 2018. | ||
Simera I, Altman DG, Moher D, Schulz KF, Hoey J. Guidelines for reporting health research: the EQUATOR network’s survey of guideline authors. PLoS Med. 2008;5(6):e139. | ||
Altman DG, Simera I, Hoey J, Moher D, Schulz K. EQUATOR: reporting guidelines for health research. Lancet. 2008;371(9619):1149–1150. | ||
Schulz KF, Altman DG, Moher D; CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332. | ||
Bernstein AM, Song M, Zhang X, et al. Processed and unprocessed red meat and risk of colorectal cancer: analysis by tumor location and modification by time. PLoS One. 2015;10(8):e0135959. | ||
Alexander DD, Weed DL, Miller PE, Mohamed MA. Red meat and colorectal cancer: a quantitative update on the state of the epidemiologic science. J Am Coll Nutr. 2015;34(6):521–543. | ||
Samaan Z, Mbuagbaw L, Kosa D, et al. A systematic scoping review of adherence to reporting guidelines in health care literature. J Multidiscip Healthc. 2013;6:169–188. | ||
Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. | ||
Li G, Mbuagbaw L, Samaan Z, et al. State of reporting of primary biomedical research: a scoping review protocol. BMJ Open. 2017;7:e014749. | ||
von Elm E, Altman DG, Egger M, et al; STROBE Initiative. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457. | ||
Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of reporting of meta-analyses. Lancet. 1999;354(9193):1896–1900. | ||
Bossuyt PM, Reitsma JB, Bruns DE, et al; Standards for Reporting of Diagnostic Accuracy. The STARD statement for reporting studies of diagnostic accuracy: explanation and elaboration. Clin Chem. 2003;49(1):7–18. | ||
Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8(6):e1000412. | ||
Husereau D, Drummond M, Petrou S, et al. Consolidated health economic evaluation reporting standards (CHEERS) statement. Cost Eff Res Allocation. 2013;11:6. | ||
Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283(15):2008–2012. | ||
EQUATOR [home page on the Internet]. Available from: http://www.equator-network.org. Accessed June 16, 2018. | ||
Thelwall M, Sud P. National, disciplinary and temporal variations in the extent to which articles with more authors have more impact: Evidence from a geometric field normalised citation indicator. J Inf. 2016;10(1):48–61. | ||
Schrock JB, Kraeutler MJ, McCarty EC. Trends in authorship characteristics in the American journal of sports medicine, 1994 to 2014. Am J Sports Med. 2016;44(7):1857–1860. | ||
Geminiani A, Ercoli C, Feng C, Caton JG. Bibliometrics study on authorship trends in periodontal literature from 1995 to 2010. J Periodontol. 2013;85(5):e136–e143. | ||
Drenth JH. Multiple authorship: the contribution of senior authors. JAMA. 1998;280(3):219–221. | ||
Wuchty S, Jones BF, Uzzi B. The increasing dominance of teams in production of knowledge. Science. 2007;316(5827):1036–1039. | ||
Bozeman B, Fay D, Slade CP. Research collaboration in universities and academic entrepreneurship: the-state-of-the-art. J Technol Transf. 2013;38(1):1–67. | ||
Moher D, Pham B, Jones A, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet. 1998;352(9128):609–613. | ||
Atkins D, Best D, Briss PA, et al; GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):1490. | ||
ICMJE. Recommendations for the Conduct, Reporting, Editing, and Publication of Scholarly Work in Medical Journals. Citeseer. 2016; (December):1–17. doi: 10.1080/08941920.2016.1150542 | ||
CONSORT [webpage on the Internet]. Consort Endorsers. 2018. Available from: http://www.consort-statement.org/about-consort/endorsers. Accessed June 16, 2018. | ||
NIH [webpage on the Internet]. U.S National Library of Medicine. 2018. Available from: https://www.nlm.nih.gov/bsd/serfile_addedinfo.html. Accessed June 16, 2018. | ||
Adie S. CONSORT compliance in surgical randomized trials: possible solutions. Ann Surg. 2013;261(5):4932–4932. | ||
Adie S, Ma D, Harris IA, Naylor JM, Craig JC. Quality of conduct and reporting of meta-analyses of surgical interventions. Ann Surg. 2015;261(4):685–694. | ||
Agha RA, Lee S-Y, Jeong KJL, Fowler AJ, Orgill DP. Reporting quality of observational studies in plastic surgery needs improvement: a systematic review. Ann Plast Surg. 2015;76(5):585–589. | ||
Agha RA, Fowler AJ, Limb C, et al. Impact of the mandatory implementation of reporting guidelines on reporting quality in a surgical journal: a before and after study. Int J Surg. 2016;30:169–172. | ||
Aguiar PM, Brito GD, Correr CJ, Lyra DP, Storpirtis S. Exploring the quality of systematic reviews on pharmacist interventions in patients with diabetes: an overview. Ann Pharmacother. 2014;48(7):887–896. | ||
Aguiar PM, Lima TM, Storpirtis S. Systematic review of the economic evaluations of novel therapeutic agents in multiple myeloma: what is the reporting quality? J Clin Pharm Ther. 2016;41(2):189–197. | ||
Al Faleh K, Al-Omran M. Reporting and methodologic quality of Cochrane Neonatal review group systematic reviews. BMC Pediatr. 2009;9:38. | ||
Al-Namankany AA, Ashley P, Moles DR, Parekh S. Assessment of the quality of reporting of randomized clinical trials in paediatric dentistry journals. Int J Paediatr Dent. 2009;19(5):318–324. | ||
Alvarez F, Meyer N, Gourraud PA, Paul C. CONSORT adoption and quality of reporting of randomized controlled trials: a systematic analysis in two dermatology journals. Br J Dermatol. 2009;161(5):1159–1165. | ||
Anttila H, Malmivaara A, Kunz R, Autti-Rämö I, Mäkelä M. Quality of reporting of randomized, controlled trials in cerebral palsy. Pediatrics. 2006;117(6):2222–2230. | ||
Areia M, Soares M, Dinis-Ribeiro M. Quality reporting of endoscopic diagnostic studies in gastrointestinal journals: where do we stand on the use of the STARD and CONSORT statements? Endoscopy. 2010;42(2):138–147. | ||
Augestad KM, Berntsen G, Lassen K, et al; Study Group of Research Quality in Medical Informatics and Decision Support (SQUID). Standards for reporting randomized controlled trials in medical informatics: a systematic review of CONSORT adherence in RCTs on clinical decision support. J Am Med Inf Assoc. 2012;19(1):13–21. | ||
Balasubramanian SP, Wiener M, Alshameeri Z, Tiruvoipati R, Elbourne D, Reed MW. Standards of reporting of randomized controlled trials in general surgery: can we do better? Ann Surg. 2006;244(5):663–667. | ||
Bath FJOVE, Bath PM. Quality of full and final publications reporting acute stroke trials. Stroke. 2000;29(10):2203–2210. | ||
Bereza BG, Machado M, Einarson TR. Assessing the reporting and scientific quality of meta-analyses of randomized controlled trials of treatments for anxiety disorders. Ann Pharmacother. 2008;42:1402–1409. | ||
Bian ZX, Moher D, Dagenais S, et al. Improving the quality of randomized controlled trials in Chinese herbal medicine, part IV: applying a revised CONSORT checklist to measure reporting quality. Zhong Xi Yi Jie He Xue Bao. 2006;4(3):233–242. | ||
Biondi-Zoccai G, Lotrionto M, Abbate A, Testa L. Compliance with QUOROM and quality of reporting of over-lapping meta-analyses on the role of acetylcysteine in the prevention of contrast associated nephropathy. BMJ. 2006;332(7535):199–204. | ||
Borg Debono V, Zhang S, Ye C, et al. The quality of reporting of RCTs used within a postoperative pain management meta-analysis, using the CONSORT statement. BMC Anesthesiol. 2012;12:13. | ||
Bousquet PJ, Calderón MA, Demoly P, et al. The consolidated standards of reporting trials (CONSORT) statement applied to allergen-specific immunotherapy with inhalant allergens: A global allergy and asthma European network (GA2LEN) article. J Allergy ClinImmunol. 2011;127(1):49–56, 56.e1–e11. | ||
Bramhall M, Florez-Vargas O, Stevens R, Brass A, Cruickshank S. Quality of methods reporting in animal models of colitis. Inflamm Bowel Dis. 2015;21(6):1248–1259. | ||
Cairo F, Sanz I, Matesan P, Nieri M, Pagliaro U. Quality of reporting of randomized control trials to implant in dentistry. J Clin Periodontol. 2012;39:202–206. | ||
Capili B, Anastasi JK, Geiger JN. Adverse event reporting in acupuncture clinical trials focusing on pain. Clin J Pain. 2010;26(1):43–48. | ||
Cavadas V, Branco F, Carvalho FL, Osório L, Gomes MJ, Silva-Ramos M. The quality of reporting of randomized controlled trials in pelvic organ prolapse. Int Urogynecol J. 2011;22(9):1117–1125. | ||
Choi J, Jun JH, Kang BK, Kim KH, Lee MS. Endorsement for improving the quality of reports on randomized controlled trials of traditional medicine journals in Korea: a systematic review. Trials. 2014;15:429. | ||
Chowers MY, Gottesman BS, Leibovici L, Pielmeier U, Andreassen S, Paul M. Reporting of adverse events in randomized controlled trials of highly active antiretroviral therapy: systematic review. J Antimicrob Chemother. 2009;64(2):239–250. | ||
Cook DA, Levinson AJ, Garside S. Method and reporting quality in health professions education research: a systematic review. Med Educ. 2011;45(3):227–238. | ||
Daitch V, Babich T, Singer P, Leibovici L. Quality of reporting nutritional randomized controlled trials in patients with cystic fibrosis. J Pediatr Gastroenterol Nutr. 2016;63(2):265–269. | ||
Dasi F, Navarro-García MM, Jiménez-Heredia M, et al. Evaluation of the quality of publications on randomized clinical trials using the consolidated standards of reporting trials (CONSORT) statement guidelines in a Spanish tertiary hospital. J Clin Pharmacol. 2012;52(7):1106–1114. | ||
Delaney M, Meyer E, Cserti-Gazdewich C, et al. A systematic assessment of the quality of reporting for platelet transfusion studies. Transfusion. 2010;50(10):2135–2144. | ||
DeMauro SB, Giaccone A, Kirpalani H, Schmidt B. Quality of reporting of neonatal and infant trials in high-impact journals. Pediatrics. 2011;128(3):e639–e644. | ||
de Vries TW, van Roon EN. Low quality of reporting adverse drug reactions in paediatric randomised controlled trials. Arch Dis Child. 2010;95(12):1023–1026. | ||
Dias S, McNamee R, Vail A. Evidence of improving quality of reporting of randomized controlled trials in subfertility. Hum Reprod. 2006;21(10):2617–2627. | ||
Ethgen M, Boutron L, Steg PG, Roy C, Ravaud P. Quality of reporting internal and external validity data from randomized controlled trials evaluating stents for percutaneous coronary intervention. BMC Med Res Methodol. 2009;9:24–24. | ||
Eyawo O, Lee C-W, Rachlis B, Mills EJ. Reporting of noninferiority and equivalence randomized trials for major prostaglandins: a systematic survey of the ophthalmology literature. Trials. 2008;9:69–69. | ||
Fan F-F, Xu Q, Sun Q, Zhao SJ, Wang P, Guo XR. Assessment of the reporting quality of randomized controlled trials on treatment of coronary heart disease with traditional Chinese medicine from the Chinese journal of integrated traditional and Western medicine: a systematic review. PLoS One. 2014;9(1):e86360. | ||
Farrokhyar F, Chu R, Whitlock R, Thabane L. A systematic review of the quality of publications reporting coronary artery bypass grafting trials. Can J Surg. 2007;50(4):266–277. | ||
Fidalgo BMR, Crabb DP, Lawrenson JG. Methodology and reporting of diagnostic accuracy studies of automated perimetry in glaucoma: evaluation using a standardised approach. Ophthalmic Physiol Opt. 2015;35(3):315–323. | ||
Fleming PS, Seehra J, Polychronopoulou A, Fedorowicz Z, Pandis N. A PRISMA assessment of the reporting quality of systematic reviews in orthodontics. Angle Orthod. 2013;83(1):158–163. | ||
Fontela PS, Pant Pai N, Schiller I, Dendukuri N, Ramsay A, Pai M. Quality and reporting of diagnostic accuracy studies in TB, HIV and malaria: evaluation using QUADAS and STARD standards. PLoS One. 2009;4(11):e7753. | ||
Freeman K, Szczepura A, Osipenko L. Non-invasive fetal RHD genotyping tests: a systematic review of the quality of reporting of diagnostic accuracy in published studies. Eur J Obstet Gynecol Reprod Biol. 2009;142(2):91–98. | ||
Froud R, Eldridge S, Diaz Ordaz K, Marinho VCC, Donner A. Quality of cluster randomized controlled trials in oral health: a systematic review of reports published between 2005 and 2009. Community Dent Oral Epidemiol. 2012;40(suppl 1):3–14. | ||
Fung AE, Palanki R, Bakri SJ, Depperschmidt E, Gibson A. Applying the CONSORT and STROBE statements to evaluate the reporting quality of neovascular age-related macular degeneration studies. Ophthalmology. 2009;116(2):286–296. | ||
Gagnier JJ, DeMelo J, Boon H, Rochon P, Bombardier C. Quality of reporting of randomized controlled trials of herbal medicine interventions. Am J Med. 2006;119(9):800.e1–e11. | ||
Gao J, Deng G, Hu Y, et al. Quality of reporting on randomized controlled trials on recurrent spontaneous abortion in China. Trials. 2015;16:172–172. | ||
Gianola S, Gasparini M, Agostini M, et al. Survey of the reporting characteristics of systematic reviews in rehabilitation. Phys Ther. 2013;93(11):1456–1466. | ||
Gohari F, Baradaran HR, Tabatabaee M, et al. Quality of reporting randomized controlled trials (RCTs) in diabetes in Iran; a systematic review. J Diabetes Metab Disord. 2015;15(1):36. | ||
Gulin JEN, Rocco DM, García-Bournissen F. Quality of reporting and adherence to ARRIVE guidelines in animal studies for Chagas disease preclinical drug research: a systematic review. PLoS Negl Trop Dis. 2015;9(11):e0004194. | ||
Halpern SH, Darani R, Douglas MJ, Wight W, Yee J. Compliance with the CONSORT checklist in obstetric anaesthesia randomised controlled trials. Int J Obstet Anesth. 2004;13(4):207–214. | ||
Hemels MEH, Vicente C, Sadri H, Masson MJ, Einarson TR. Quality assessment of metaanalyses of RCTs of pharmacotherapy in major depressive disorder. Curr Med Res Opin. 2004;20(4):477–484. | ||
Herdan A, Roth R, Grass D, et al. Improvement of quality of reporting in randomised controlled trials to prevent hypotension after spinal anaesthesia for caesarean section. Gynecol Surg. 2011;8:121–127. | ||
Huang D, Jin X, Gao J, et al. Quality evaluation of randomized controlled trials reports of laparoscopy compared with open colorectal resection for colorectal cancer. Expert Rev Anticancer Ther. 2015;15(6):727–732. | ||
Hui D, Arthur J, Dalal S, Bruera E. Quality of the supportive and palliative oncology literature: A focused analysis on randomized controlled trials. Support Care Cancer. 2012;20(8):1779–1785. | ||
Junhua Z, Hongcai S, Xiumei G, et al. Methodology and reporting quality of systematic review/meta-analysis of traditional Chinese medicine. J Altern Complement Med. 2007;13(8):797–805. | ||
Karpouzis F, Bonello R. Quality of reporting of randomised controlled trials in chiropractic using the CONSORT checklist. Man Ther. 2016;24:19. | ||
Kiehna EN, Starke RM, Pouratian N, Dumont AS. Standards for reporting randomized controlled trials in neurosurgery. J Neurosurg. 2010;114(2):280–285. | ||
Kim KH, Kang JW, Lee MS, Lee J-D. Assessment of the quality of reporting in randomised controlled trials of acupuncture in the Korean literature using the CONSORT statement and STRICTA guidelines. BMJ Open. 2014;4(7):e005068. | ||
Kober T, Trelle S, Engert A. Reporting of randomized controlled trials in Hodgkin lymphoma in biomedical journals. J Natl Cancer Inst. 2006;98(9):620–625. | ||
Ladd BO, McCrady BS, Manuel JK, Campbell W. Improving the quality of reporting alcohol outcome studies: effects of the CONSORT statement. Addict Behav. 2010;35:660–666. | ||
Lee S-Y, Teoh PJ, Camm CF, Agha RA. Compliance of randomized controlled trials in trauma surgery with the CONSORT statement. J Trauma Acute Care Surg. 2013;75(4):562–572. | ||
Lee SY, Sagoo H, Whitehurst K, et al. Compliance of systematic reviews in plastic surgery with the PRISMA statement. JAMA Facial Plast Surg. 2016;18(2):101–105. | ||
Li JY, Zhang YF, Smith GS, et al. Quality of reporting of randomized clinical trials in tai chi interventions-a systematic review. Evid Based Complement Alternat Med. 2011;2011:383245. | ||
Li J-L, Ge L, Ma JC, et al. Quality of reporting of systematic reviews published in “evidence-based” Chinese journals. Syst Rev. 2014;3:58–58. | ||
Li J, Liu Z, Chen R, et al. The quality of reports of randomized clinical trials on traditional Chinese medicine treatments: a systematic review of articles indexed in the China National Knowledge Infrastructure database from 2005 to 2012. BMC Complement Altern Med. 2014;14:362–362. | ||
Liu D, Jin J, Tian J, Yang K. Quality assessment and factor analysis of systematic reviews and meta-analyses of endoscopic ultrasound diagnosis. PLoS One. 2015;10:1–13. | ||
Liu LQ, Morris PJ, Pengel LHM. Compliance to the CONSORT statement of randomized controlled trials in solid organ transplantation: a 3-year overview. Transpl Int. 2013;26:300–306. | ||
Liu XT, Zhang X, Wen S, Peng L, Hong Q, Kang D. Impact of the Consolidated Standards of Reporting Trials (CONSORT) checklist on reporting of randomized clinical trials in traditional Chinese medicine. J Evid Based Med. 2015;8:192–208. | ||
Liu Y, Zhang R, Huang J, et al. Reporting quality of systematic reviews/meta-analyses of acupuncture. PLoS One. 2014;9(11):e113172. | ||
Liu Y, Zhao X, Mai Y, et al. Adherence to ARRIVE guidelines in Chinese journal reports on neoplasms in animals. PLoS One. 2016;11:e0154657. | ||
Lu J, Gary KW, Copolillo A, Ward J, Niemeier JP, Lapane KL. Randomized controlled trials in adult traumatic brain injury: a review of compliance to CONSORT statement. Arch Phys Med Rehabil. 2015;96:702–714. | ||
Lu L, Zeng J, Chen Y. Quality of reporting in randomized controlled trials conducted in China on the treatment of cancer pain. Expert Rev Anticancer Ther. 2011;11:871–877. | ||
Ma B, Guo J, Qi G, et al. Epidemiology, quality and reporting characteristics of systematic reviews of traditional Chinese medicine interventions published in Chinese journals. PLoS One. 2011;6:e20185. | ||
Ma B, Qi GQ, Lin XT, Wang T, Chen ZM, Yang KH. Epidemiology, quality, and reporting characteristics of systematic reviews of acupuncture interventions published in Chinese journals. J Altern Complement Med. 2012;18:813–817. | ||
Marshman Z, Farid F. The quality of reporting of randomised controlled trials in dental public health. Community Dent Health. 2010;27:253–256. | ||
McCormick F, Cvetanovich GL, Kim JM, et al. An assessment of the quality of rotator cuff randomized controlled trials: utilizing the Jadad score and CONSORT criteria. J Shoulder Elbow Surg. 2013;22:1180–1185. | ||
Miller E, Roposch A, Uleryk E, Doria AS. Juvenile idiopathic arthritis of peripheral joints. Quality of reporting of diagnostic accuracy of conventional MRI1. Acad Radiol. 2009;16:739–757. | ||
Moberg-Mogren E, Nelson DL. Evaluating the quality of reporting occupational therapy randomized controlled trials by expanding the CONSORT criteria. Am J Occup Ther. 2006;60:226–235. | ||
Moher D, Sampson M, Campbell K, et al. Assessing the quality of reports of randomized trials in pediatric complementary and alternative medicine. BMC Pediatr. 2002;12:20–21. | ||
Montané E, Vallano A, Vidal X, Aguilera C, Laporte J-R. Reporting randomised clinical trials of analgesics after traumatic or orthopaedic surgery is inadequate: a systematic review. BMC Clin Pharmacol. 2010;10:2–2. | ||
Montgomery AA, Astin MP, Peters TJ. Reporting of factorial trials of complex interventions in community settings: a systematic review. Trials. 2011;12:179. | ||
Nicolau I, Ling D, Tian L, Lienhardt C, Pai M. Methodological and reporting quality of systematic reviews on tuberculosis. Int J Tuberc Lung Dis. 2013;17:1160–1169. | ||
Norton-Mabus JC, Nelson DL. Reporting of randomized controlled trials in occupational therapy and speech therapy: evaluation using an expansion of the consort statement. Occup Particip Health. 2008;28:64–71. | ||
Ntala C, Birmpili P, Worth A, Anderson NH, Sheikh A. The quality of reporting of randomised controlled trials in asthma: a systematic review. Prim Care Respir J. 2013;22:417–424. | ||
Panic N, Leoncini E, De Belvis G, Ricciardi W, Boccia S. Evaluation of the endorsement of the preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement on the quality of published systematic review and meta-analyses. PLoS One. 2013;8(12):e83138. | ||
Parsons NR, Hiskens R, Price CL, Achten J, Costa ML. A systematic survey of the quality of research reporting in general orthopaedic journals. J Bone Joint Surg. 2011;93:1154–1159. | ||
Patel MX, Collins S, Hellier J, Bhatia G, Murray RM. The quality of reporting of phase II and III trials for new antipsychotics: a systematic review. Psychol Med. 2014;45(3):467–479. | ||
Piggott M, McGee H, Feuer D. Has CONSORT improved the reporting of randomized controlled trials in the palliative care literature? A systematic review. Palliat Med. 2004;18:32–38. | ||
Péron J, Pond GR, Gan HK, et al. Quality of reporting of modern randomized controlled trials in medical oncology: a systematic review. J Natl Cancer Inst. 2012;104:982–989. | ||
Peters JPM, Hooft L, Grolman W, Stegeman I. Reporting quality of systematic reviews and meta-analyses of otorhinolaryngologic articles based on the PRISMA statement. PLoS One. 2015;10:1–11. | ||
Plint AC, Moher D, Morrison A, et al. Does the CONSORT checklist improve the quality of reports of randomised controlled trials? A systematic review. Med J Aust. 2006;185:263–267. | ||
Prady SL, Richmond SJ, Morton VM, MacPherson H. A systematic evaluation of the impact of STRICTA and CONSORT recommendations on quality of reporting for acupuncture trials. PLoS One. 2008;3(2):e1577. | ||
Pratoomsoot C, Sruamsiri R, Dilokthornsakul P, Chaiyakunapruk N. Quality of reporting of randomised controlled trials of herbal interventions in ASEAN plus six countries: a systematic review. PLoS One. 2015;10(1):e108681. | ||
Rao A, Brück K, Methven S, et al. Quality of reporting and study design of CKD cohort studies assessing mortality in the elderly before and after STROBE: a systematic review. PLoS One. 2016;11:1–16. | ||
Rice DB, Kloda LA, Shrier I, Thombs BD. Reporting completeness and transparency of meta-analyses of depression screening tool accuracy: a comparison of meta-analyses published before and after the PRISMA statement. J Psychosom Res. 2016;87:57–69. | ||
Rios LP, Odueyungbo A, Moitri MO, Rahman MO, Thabane L. Quality of reporting of randomized controlled trials in general endocrinology literature. J Clin Endocrinol Metab. 2008;93:3810–3816. | ||
Rikos D, Dardiotis E, Tsivgoulis G, Zintzaras E, Hadjigeorgiou GM. Reporting quality of randomized-controlled trials in multiple sclerosis from 2000 to 2015, based on CONSORT statement. Mult Scler Relat Disord. 2016;9:135–139. | ||
Schwarz F, Iglhaut G, Becker J. Quality assessment of reporting of animal studies on pathogenesis and treatment of peri-implant mucositis. Clin Periodontol. 2012;39(suppl 12):63–72. | ||
Scott P, Ott F, Egger M, Low N. Completeness of reporting in randomized controlled trials of 3 vaccines. Pediatr Infect Dis J. 2012;31:1286–1294. | ||
Shawyer AC, Pemberton J, Kanters D, Alnaqi AAA, Flageole H. Quality of reporting of the literature on gastrointestinal reflux after repair of esophageal atresia-tracheoesophageal fistula. J Pediatr Surg. 2015;50:1099–1103. | ||
Shea B, Boers M, Grimshaw JM, Hamel C, Bouter LM. Does updating improve the methodological and reporting quality of systematic reviews? BMC Med Res Methodol. 2006;6:1–7. | ||
Shea B, Bouter LM, Grimshaw JM, et al. Scope for improvement in the quality of reporting of systematic reviews. From the Cochrane Musculoskeletal Group. J Rheumatol. 2006;33:9–15. | ||
Stevely A, Dimairo M, Todd S, et al. An investigation of the shortcomings of the CONSORT 2010 statement for the reporting of group sequential randomised controlled trials: a methodological systematic review. PLoS One. 2015;10:1–20. | ||
Strech D, Soltmann B, Weikert B, Bauer M, Pfennig A. Quality of reporting of randomized controlled trials of pharmacologic treatment of bipolar disorders: a systematic review. J Clin Psychiatry. 2011;72:1214–1221. | ||
Tan WK, Wigley J, Shantikumar S. The reporting quality of systematic reviews and meta-analyses in vascular surgery needs improvement: a systematic review. Int J Surg. 2014;12:1262–1265. | ||
Thabane L, Chu R, Cuddy K, Douketis J. What is the quality of reporting in weight loss intervention studies? A systematic review of randomized controlled trials. Int J Obes. 2007;31:1554–1559. | ||
Tunis AS, McInnes MDF, Hanna R, Esmail K. Association of study quality with completeness of reporting: have completeness of reporting and quality of systematic reviews and meta-analyses in major radiology journals changed since publication of the PRISMA statement? Radiology. 2013;269:413–426. | ||
Turner L, Shamseer L, Altman DG, et al. Consolidated standards of reporting trials (CONSORT) and the completeness of reporting of randomised controlled trials (RCTs) published in medical journals. Cochrane Database Syst Rev. 2012;11:MR000030. | ||
Vigna-Taglianti F, Vineis P, Liberati A, Faggiano F. Quality of systematic reviews used in guidelines for oncology practice. Ann Oncol. 2006;17:691–701. | ||
Walleser S, Hill SR, Bero LA. Characteristics and quality of reporting of cluster randomized trials in children: reporting needs improvement. J Clin Epidemiol. 2011;64:1331–1340. | ||
Wang G, Mao B, Xiong ZY, et al. The quality of reporting of randomized controlled trials of traditional Chinese medicine: a survey of 13 randomly selected journals from mainland China. Clin Ther. 2007;29:1456–1467. | ||
Wang P, Xu Q, Sun Q, Fan FF, Guo XR, Guo F. Assessment of the reporting quality of randomized controlled trials on the treatment of diabetes mellitus with traditional Chinese medicine: a systematic review. PLoS One. 2013;8(7):e70586. | ||
Wangge G, Klungel OH, Roes KC, de Boer A, Hoes AW, Knol MJ. Room for improvement in conducting and reporting non-inferiority randomized controlled trials on drugs: a systematic review. PLoS One. 2010;5(10):e13550. | ||
Weingärtner V, Dargatz N, Weber C, et al. Patient reported outcomes in randomized controlled cancer trials in advanced disease: a structured literature review. Expert Rev Clin Pharmacol. 2016;9:821–829. | ||
Weir CR, Staggers N, Laukert T. Reviewing the impact of computerized provider order entry on clinical outcomes: the quality of systematic reviews. Int J Med Inform. 2012;81(4):219–231. | ||
Wen J, Ren Y, Wang L, et al. The reporting quality of meta-analyses improves: a random sampling study. J Clin Epidemiol. 2008;61:770–775. | ||
Willis B, Quigley M. The assessment of the quality of reporting of meta-analyses in diagnostic research: a systematic review. BMC Med Res Methodol. 2011;11:163–163. | ||
Yao AC, Khajuria A, Camm CF, Edison E, Agha R. The reporting quality of parallel randomised controlled trials in ophthalmic surgery in 2011: a systematic review. Eye. 2014;28:1341–1349. | ||
Zafar A, Khan GI, Siddiqui MA. The quality of reporting of diagnostic accuracy studies in diabetic retinopathy screening: a systematic review. Clin Experiment Ophthalmol. 2008;36(6):537–542. | ||
Zhang Z-W. Epidemiology, quality, and reporting characteristics of systematic reviews and meta-analyses of observational studies published in Chinese journals. BMJ Open. 2015;63:446–455. | ||
Zhao X, Zhen Z, Guo J, et al. Assessment of the reporting quality of placebo-controlled randomized trials on the treatment of type 2 diabetes with traditional Chinese medicine in mainland China: a PRISMA-compliant systematic review. Medicine. 2016;95(3): e2522. | ||
Zheng SL, Chan FT, Maclean E, Jayakumar S, Nabeebaccus AA. Reporting trends of randomised controlled trials in heart failure with preserved ejection fraction: a systematic review. Open Heart. 2016;3(2):e000449. | ||
Zhong Y, Zhou W, Jiang H, et al. Quality of reporting of two-group parallel randomized controlled clinical trials of multi-herb formulae: a survey of reports indexed in the Science Citation Index Expanded. Eur J Integr Med. 2011;3(4):e309–e316. | ||
Zintzaras E, Kitsios GD, Papathanasiou AA, et al. Randomized trials of dopamine agonists in restless legs syndrome: a systematic review, quality assessment, and meta-analysis. Clin Ther. 2010;32(2):221–237. | ||
Zintzaras E, Papathanasiou AA, Ziogas DC, Voulgarelis M. The reporting quality of studies investigating the diagnostic accuracy of anti-CCP antibody in rheumatoid arthritis and its impact on diagnostic estimates. BMC Musculoskelet Disord. 2012;13:113. | ||
Ziogas DC, Zintzaras E. Analysis of the quality of reporting of randomized controlled trials in acute and chronic myeloid leukemia, and myelodysplastic syndromes as governed by the CONSORT statement. Ann Epidemiol. 2009;19(7):494–500. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.