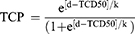Back to Journals » Journal of Hepatocellular Carcinoma » Volume 9
Does HCC Etiology Impact the Efficacy of Stereotactic Body Radiation Therapy for Hepatocellular Carcinoma? An Asian Liver Radiation Therapy Group Study
Authors Kim N, Cheng JCH, Ohri N, Huang WY, Kimura T, Zeng ZC , Lee VHF, Kay CS, Seong J
Received 14 June 2022
Accepted for publication 26 July 2022
Published 6 August 2022 Volume 2022:9 Pages 707—715
DOI https://doi.org/10.2147/JHC.S377810
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Manal Hassan
Nalee Kim,1,* Jason Chia-Hsien Cheng,2,* Nitin Ohri,3 Wen-Yen Huang,4 Tomoki Kimura,5 Zhao Chong Zeng,6 Victor Ho Fun Lee,7 Chul Seung Kay,8 Jinsil Seong9
1Department of Radiation Oncology, Samsung Medical Center, Sungkyunkwan School of Medicine, Seoul, Republic of Korea; 2Division of Radiation Oncology, Department of Oncology, National Taiwan University Hospital, Taipei City, Taiwan; 3Department of Radiation Oncology, Montefiore Medical Center, Albert Einstein College of Medicine, Bronx, New York; 4Department of Radiation Oncology, Tri-Service General Hospital, National Defense Medical Center, Taipei City, Taiwan; 5Department of Radiation Oncology, Hiroshima University Hospital, Hiroshima, Japan; 6Department of Radiation Oncology, Zhongshan Hospital, Fudan University, Shanghai, People’s Republic of China; 7Department of Radiation Oncology, The University of Hong Kong, Hong Kong; 8Department of Radiation Oncology, Jeju Halla Hospital, Jeju, Republic of Korea; 9Department of Radiation Oncology, Yonsei Cancer Center, Yonsei University College of Medicine, Seoul, Republic of Korea
*These authors contributed equally to this work
Correspondence: Jinsil Seong, Department of Radiation Oncology, Yonsei Cancer Center, Yonsei University College of Medicine, 50-1 Yonsei-ro, Seodaemun-gu, Seoul, 03722, Republic of Korea, Tel +82-2-2228-8095, Fax +82-2-2227-7823, Email [email protected]
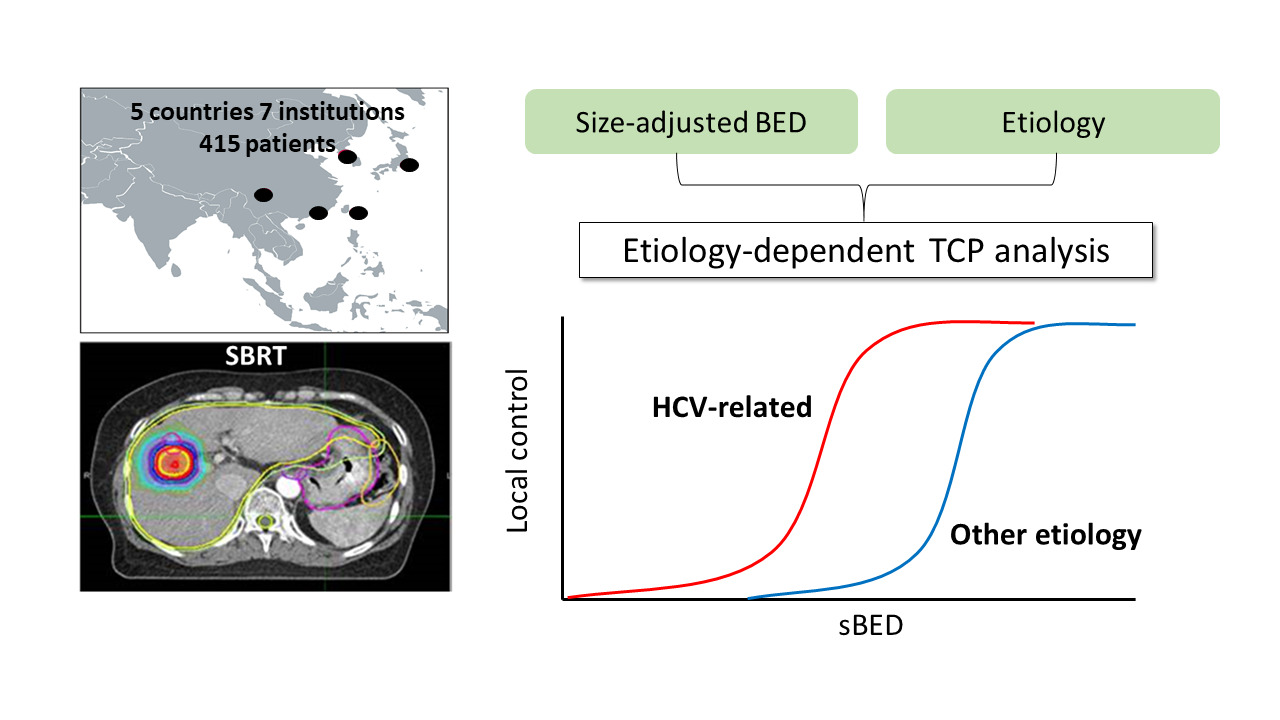
Background/Purpose: The Asian Liver Radiation Therapy Study Group has formed a large and detailed multinational database of outcomes following stereotactic body radiation therapy (SBRT) for hepatocellular carcinoma (HCC). Here, we explored the potential impact of HCC etiology on SBRT efficacy. Tumor control probability (TCP) models were established to estimate the likelihood of local control (LC).
Methods: Data from 415 patients who were treated with SBRT for HCC were reviewed. Cox proportional hazards models were used to identify key predictors of LC. TCP models accounting for biologic effective dose (BED) and tumor diameter were generated to quantify associations between etiology and LC.
Results: Cox models demonstrated that hepatitis C virus (HCV) infection was associated with favorable LC following SBRT (HR=0.52, 95% CI 0.04– 0.96, p=0.036). The 2-year LC rate for patients with HCV etiology was 88%, compared to 78% for other patients. Small tumor and high BED were also associated with favorable LC. TCP models demonstrated a 10– 20% absolute increase in predicted LC across the range of SBRT doses and tumor sizes.
Conclusion: We found a novel association between HCV status and LC after SBRT for HCC that warrants further exploration. If validated in other datasets, our findings could help clinicians tailor SBRT schedules.
Keywords: tumor control probability, hepatocellular carcinoma, stereotactic body radiation therapy, hepatitis C virus
Graphical Abstract:
Introduction
Liver-directed treatment options for unresectable hepatocellular carcinoma (HCC) include trans-arterial chemo/radioembolization, radiofrequency ablation, percutaneous ethanol injection, and stereotactic body radiation therapy (SBRT). SBRT can deliver ablative therapy with high conformality while sparing most of the uninvolved liver1,2 and has been demonstrated to yield local control (LC) rates ranging from 76% to 100%.3–8 SBRT may be utilized effectively in combination with other liver-directed treatments or as monotherapy.6,7,9,10
Despite growing evidence supporting SBRT as an effective local treatment for HCC, concrete treatment guidelines (ie, patient selection and dose prescription) have not yet been established. We previously reported that the use of a biologically effective dose (BED) above 100 Gy was associated with improved LC.11 In that analysis, HCC etiology, characterized as hepatitis B or C virus infection compared to others, was not identified as a prognostic factor. More detailed analysis of predictors’ tumor control probability (TCP) could further our understanding of SBRT efficacy and help clinicians balance the risks and benefits of various SBRT schedules.
Liver injury caused by chronic infection related to hepatitis B virus (HBV) or hepatitis C virus (HCV) accounts for 80% of HCC cases.12 Overall, 2–5% of the patients with cirrhosis caused by HBV or HCV infection develop HCC annually. Various chemoprevention methods for decreasing the incidence of HCC in these patients, based on direct and indirect mechanisms which cause HBV-related or HCV-related HCC, have been investigated.13,14 However, there are little data on the association between HCC etiologies and LC outcomes following SBRT.
Here, we perform a detailed analysis of predictors of LC following SBRT for HCC. Based on powerful associations between HCC etiology and LC, we present etiology-dependent TCP models.
Methods
Study Population
We retrospectively reviewed the data of 519 patients from seven institutions who were treated with SBRT between January 1, 2010 and December 31, 2016. Tumor with vascular invasion were excluded from the study because initial analyses demonstrated that vascular invasion was related to a high risk of local failure, with no evidence of a dose–response relationship when treating tumors with vascular invasion (Supplementary Figure 1). This study was performed in accordance with the provisions of the 1975 Declaration of Helsinki, which are ethical principles for medical research involving human subjects. Also, the study was approved by the Institutional Review Boards of every participating institution (Supplementary Table 1). The requirement for informed consent was waived due to the retrospective nature of the study. All confidential patient information is protected, and detailed information has been removed to ensure anonymity.
Stereotactic Body Radiation Therapy
Details about the techniques of SBRT used for the current cohort have been described previously.7,11 In summary, either shallow breathing or breath holding was used for respiratory management. Gross tumor volume was defined in multiphase contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI). Individualized dose prescriptions and pre-treatment image guidance were used for all patients. The planning target volume was covered by an isodose of 70–95% with various SBRT planning techniques among institutions. Detailed information on prescription dose was summarized in Supplementary Table 2.
Follow-Up Evaluation
The primary study endpoint was local tumor progression, which was scored by the treating physicians based on serial imaging studies. Local tumor progression was defined based on the modified Response Evaluation Criteria in Solid Tumors. Contrast-enhanced lesions within the planning target volume observed in either dynamic contrast-enhanced CT (70.5%) or MRI (29.5%) were considered as tumor progression. Time to local tumor progression was defined from the date of SBRT initiation to the date of local failures or last follow-up.
TCP Modeling
First, the BED was calculated for each patient using a standard α/β ratio of 10 Gy. As in previous analyses examining outcomes following SBRT for lung cancer, size-adjusted BED (sBED) was defined as BED minus 10 times the maximal tumor diameter, in centimeters.15,16 This variable was incorporated into a standard TCP model:
d: sBED; TCD50: the dose required to achieve 50% tumor control; k: a fitting constant equal to 25 divided by the slope of the TCP curve at a dose equal to the TCD50.17
Patient data were sorted into four groups of equal size based on sBED, and the actuarial 2-year LC rate for each group was calculated. The TCP model was fitted to these data points using least-squares optimization. We utilized a bootstrap resampling method (5000 iterations) to characterize the distributions of model parameters and to formulate 95% confidence bounds for the TCP curve.18 After statistical analyses demonstrated a powerful association between HCV etiology and LC, we conducted TCP modeling separately for patients with HCV versus patients with other HCC etiologies.
Statistical Analysis
The Pearson chi-squared or Fisher’s exact test was used to compare categorical variables, along with the Mann–Whitney U-test for continuous variables stratified by HCV infection. LC rates were estimated using the Kaplan–Meier method. Univariable and multivariable Cox proportional hazards models were utilized to identify predictors of LC in the entire cohort and in patient subgroups. All analyses were performed using MATLAB (The Mathworks, Natick, MA, USA) and R software (version 4.0.2; R Foundation for Statistical Computing, Vienna, Austria).
Results
In total, 415 patients met inclusion criteria for the current study. Patient, tumor, and treatment details are summarized in Table 1. The median age was 67 years (interquartile range [IQR], 59–76). The underlying liver diseases were HBV infection in 227 of the patients (54.7%) and HCV infection in 125 of the patients (30.1%). Regarding SBRT planning, the median gross tumor volume and planning target volume were 16.6 cm3 (IQR, 3.9–50.2) and 52.2 cm3 (IQR, 22.2–101.7), respectively. With a total dose of 48 Gy (IQR, 40.0–54.0), the median BED and sBED were 100 Gy (IQR, 80.0–116.0) and 70.0 Gy*cm (IQR, 46.0–92.5), respectively. There was no strong correlation among variables except for sBED, BED, and tumor size (Supplementary Figure 2).
 |
Table 1 Patient and Tumor Characteristics |
After a median follow-up duration of 26.5 months (IQR, 14.7–43.7) following SBRT initiation for patients without local failure, 73 patients (17.6%) developed local failure. Median time to local progression for those patients was 9.1 months (IQR, 4.4–17.7). For all patients, the 2-year LC rate was 81.6% (95% confidence interval [CI], 77.5–85.8). In addition, the 2-year overall survival rate was 75.8% (95% CI, 71.4–80.4) and the median survival duration was 46.0 months (95% CI, 50.6–51.6, Supplementary Figure 3).
Univariate Cox proportional hazards models exploring predictors of LC are shown in Table 2. As expected, tumor size and BED were identified as potential predictors of LC. Unexpectedly, HCV infection was associated with a risk reduction of nearly 50% compared to other HCC etiologies (HR compared to HBV = 0.52, 95% CI 0.04–0.96, p=0.036). Kaplan–Meier curves for LC for patients grouped by tumor size, BED, and HCC etiology are shown in Figure 1. The 2-year LC rate for patients with HCC etiology was 88%, compared to only 78% for other patients. Patient characteristics for etiology of HCC are detailed and compared in Supplementary Table 3. Although patients with HCV-related HCC were older, had decreased liver function, smaller tumor size, and target volume compared to those with non-HCV-related (either HBV-related or non-viral) HCC, there was no difference in sBED between two groups (median 70 Gy vs 73 Gy, p=0.334). In subsequent analysis based on underlying HCV infection status, sBED ≥70 Gy was related to LC in the HCV-related group and not in the non-HCV-related group (Supplementary Table 4).
 |
Table 2 Prognostic Factors for Local Control |
 |
Table 3 Projected 2-Year Local Control Rates for Common Stereotactic Body Radiation Therapy (SBRT) Schedules and Selected Tumor Diameters, Based on Etiology of Hepatocellular Carcinoma |
 |
Figure 1 Kaplan–Meier curves for local tumor control following SBRT stratified by (A) tumor diameter, (B) biologically effective dose (BED), and (C) etiology. |
Based on the powerful association between LC after SBRT and HCC etiology observed in our dataset, we performed TCP modeling separately for patients with HCV-related HCC and for other patients. In patients with HCV infection, our bootstrap resampling technique yielded median optimal values of 140 and −240 Gy for k and TCD50, respectively. In patients with other HCC etiologies, median optimal values of 70 for k and −10 Gy for TCD50 were obtained, suggesting that HCV-related HCC is associated with favorable LC and a flatter dose–response curve. TCP modeling results are depicted in Figure 2, with HCV infection conferring a 10–20% absolute increase in predicted LC across the range of SBRT doses and tumor sizes included in this dataset (Table 3).
Discussion
Using a large, multinational database, we detected a powerful and unexpected association between HCC etiology and LC following SBRT, with favorable outcomes observed in patients with HCV infection compared to other patients. HCV etiology was associated with approximately 50% relative risk reduction and 10–20% absolute risk reduction for local recurrence following SBRT. Our findings, if validated in other datasets, could have broad implications in the implementation of SBRT for HCC.
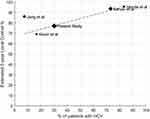
To our knowledge, this is the first report demonstrating an association between HCV infection and favorable LC following SBRT for HCC. Reasons why this relationship has not been identified previously could include limited sample sizes in published analyses of outcomes following SBRT for HCC, small subsets of patients with or without HCV in those series, and lack of adjustment for other important prognostic factors, such as tumor size and BED. In previous reports from our group, patients with HBV and HCV infection were grouped together, masking the association between HCV infection and favorable LC.7,11 To put our findings into context, we reviewed data from the HyTEC analysis of outcomes following SBRT for liver tumors.19 Four HCC studies reported prevalence of HCV infection,20–23 and there appears to be an association between HCV prevalence and estimated 2-year LC rate (Figure 3).
In keeping with practice patterns as SBRT for HCC was established,19,24 a wide range of SBRT schedules was utilized to treat patients in our dataset. We employed TCP modeling to visualize the relationship between SBRT parameters and likelihood of local disease control. Because hepatitis C infection was identified as a powerful favorable prognostic factor with respect to LC, we derived models separately for patients with HCV-related HCC and other patients, demonstrating a 10–20% absolute difference in predicted 2-year LC rate based on HCC etiology. Validation studies using other large datasets will be required to optimize our TCP model formulation, which was extrapolated from NSCLC SBRT series,15,16 for characterizing outcomes following SBRT for HCC. Previous studies employing TCP modeling for HCC have been limited by small sample sizes (<100 patients).21,25,26 If validated in additional cohorts, our etiology- and sBED-based TCP models may help clinicians in selecting patient-specific SBRT schedules to optimize the risk/benefit ratio.
HCV infection has not been established as a favorable prognostic factor in other HCC treatment settings. A meta-analysis of large studies employing sorafenib for treating advanced HCC did not indicate that hepatitis B or C infection influences treatment efficacy.27 In a large series of patients treated with radiofrequency ablation for HCC, HCV infection was not associated with local disease control and was associated with reduced long-term survival rates.28 A meta-analysis of nearly 5000 HCC patients who underwent surgery found that either HBV- or HCV-related HCC was a poor prognostic factor, and there was no difference in outcomes between HBV-related HCC and HCV-related HCC.29 Integrative genomic analysis from the Cancer Genome Atlas Research Network has demonstrated that HCV-related HCC is associated with CDKN2A promoter silencing and TERT promoter mutations.30 Furthermore, HCV-related HCC demonstrated better survival outcomes than non-viral or HBV-related HCC did when treated with atezolizumab with bevacizumab.31 Recently, non-viral HCC, mostly related to non-alcoholic steatohepatitis, showed decreased immune response and survival outcomes after immune checkpoint inhibitor.32,33 Considering the notion that large fractional dose of SBRT elicits immune-mediated cell death, improved LC outcomes of HCV-related HCC in the current cohort might stem from immune response.34,35 These and other potential mechanisms for differential radiosensitivity among HCC patients warrant further study.
Limitations of our study include absence of central review for defining local failures and limited follow-up duration for many patients. As in most prior studies examining LC following SBRT and implementing TCP modeling, we did not formally account for the competing risk of mortality occurring before local disease recurrence in our statistical methods. This is the first report of TCP models using sBED in HCC. We found that current TCP as a function of sBED predicted 2-year LC more accurately than TCP as a function of BED in the current data (Supplementary Figure 4). As mentioned previously, the TCP model formulation used in this analysis was initially developed using NSCLC data, and validation studies are needed to validate its use in HCC and characterize optimal model parameters. In addition, further study with detailed information on etiology of HCC including not only viral-related but also non-alcoholic steatohepatitis could reveal the radiosensitivity according to the etiology of HCC.
In conclusion, we have identified a novel association between HCV infection and favorable LC outcomes following SBRT for HCC. Also, current etiology-dependent TCP modeling hypothetically provided the size-adjusted dose–response relationship according to HCV status in patients treated with SBRT. Our TCP models require further validation with an external dataset including multiple events to confirm its usefulness in clinical practice. We hope our TCP models could be used as a reference for decision-making by physicians before planning SBRT.
Abbreviations
BED, biologically effective dose; CT, computed tomography; HBV, hepatitis B virus; HCV, hepatitis C virus; HR, hazard ratio; LC, local control; MRI, magnetic resonance imaging; SBRT, stereotactic body radiation therapy; TCP, tumor control probability.
Grand Support
This work was supported by the Dong-A research fund (Grant number 2018-31-0904) and an Accuray research grant.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Nitin Ohri reports personal fees from Merck, personal fees from AstraZeneca, personal fees from Genentech, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Heimbach JK, Kulik LM, Finn RS., et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358–380. doi:10.1002/hep.29086
2. Choi SH, Seong J. Stereotactic body radiotherapy: does it have a role in management of hepatocellular carcinoma? Yonsei Med J. 2018;59(8):912–922. doi:10.3349/ymj.2018.59.8.912
3. Bujold A, Massey CA, Kim JJ, et al. Sequential Phase I and II trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma. J Clin Oncol. 2013;31(13):1631–1639. doi:10.1200/jco.2012.44.1659
4. Cárdenes HR, Price TR, Perkins SM, et al. Phase I feasibility trial of stereotactic body radiation therapy for primary hepatocellular carcinoma. Clin Transl Oncol. 2010;12(3):218–225. doi:10.1007/s12094-010-0492-x
5. Kim JW, Kim DY, Han KH, Seong J. Phase I/II trial of helical IMRT-based stereotactic body radiotherapy for hepatocellular carcinoma. Dig Liver Dis. 2019;51(3):445–451. doi:10.1016/j.dld.2018.11.004
6. Kim N, Kim HJ, Won JY, et al. Retrospective analysis of stereotactic body radiation therapy efficacy over radiofrequency ablation for hepatocellular carcinoma. Radiother Oncol. 2019;131:81–87. doi:10.1016/j.radonc.2018.12.013
7. Kim N, Cheng J, Jung I, et al. Stereotactic body radiation therapy vs. radiofrequency ablation in Asian patients with hepatocellular carcinoma. J Hepatol. 2020;73(1):121–129. doi:10.1016/j.jhep.2020.03.005
8. Rim CH, Kim HJ, Seong J. Clinical feasibility and efficacy of stereotactic body radiotherapy for hepatocellular carcinoma: a systematic review and meta-analysis of observational studies. Radiother Oncol. 2019;131:135–144. doi:10.1016/j.radonc.2018.12.005
9. Shen PC, Chang WC, Lo CH, et al. Comparison of stereotactic body radiation therapy and transarterial chemoembolization for unresectable medium-sized hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2019;105(2):307–318. doi:10.1016/j.ijrobp.2019.05.066
10. Sapisochin G, Barry A, Doherty M, et al. Stereotactic body radiotherapy vs. TACE or RFA as a bridge to transplant in patients with hepatocellular carcinoma. An intention-to-treat analysis. J Hepatol. 2017;67(1):92–99. doi:10.1016/j.jhep.2017.02.022
11. Kim N, Cheng J, Huang WY, et al. Dose-response relationship in stereotactic body radiation therapy for hepatocellular carcinoma: a pooled analysis of an asian liver radiation therapy group study. Int J Radiat Oncol Biol Phys. 2021;109(2):464–473. doi:10.1016/j.ijrobp.2020.09.038
12. Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16(10):589–604. doi:10.1038/s41575-019-0186-y
13. Papatheodoridis GV, Chan HL, Hansen BE, Janssen HL, Lampertico P. Risk of hepatocellular carcinoma in chronic hepatitis B: assessment and modification with current antiviral therapy. J Hepatol. 2015;62(4):956–967. doi:10.1016/j.jhep.2015.01.002
14. Hoshida Y, Fuchs BC, Bardeesy N, Baumert TF, Chung RT. Pathogenesis and prevention of hepatitis C virus-induced hepatocellular carcinoma. J Hepatol. 2014;61(2):S79–S90. doi:10.1016/j.jhep.2014.07.010
15. Ohri N, Tomé W, Kalnicki S, Garg M. Stereotactic body radiation therapy for stage I non-small cell lung cancer: the importance of treatment planning algorithm and evaluation of a tumor control probability model. Pract Radiat Oncol. 2018;8(2):e33–e39. doi:10.1016/j.prro.2017.10.002
16. Ohri N, Werner-Wasik M, Grills IS, et al. Modeling local control after hypofractionated stereotactic body radiation therapy for stage I non-small cell lung cancer: a report from the elekta collaborative lung research group. Int J Radiat Oncol Biol Phys. 2012;84(3):e379–e384. doi:10.1016/j.ijrobp.2012.04.040
17. Okunieff P, Morgan D, Niemierko A, Suit HD. Radiation dose-response of human tumors. Int J Radiat Oncol Biol Phys. 1995;32(4):1227–1237. doi:10.1016/0360-3016(94)00475-z
18. Deasy JO, Chao KS, Markman J. Uncertainties in model-based outcome predictions for treatment planning. Int J Radiat Oncol Biol Phys. 2001;51(5):1389–1399. doi:10.1016/s0360-3016(01)02659-1
19. Ohri N, Tomé WA, Méndez Romero A, et al. Local control after stereotactic body radiation therapy for liver tumors. Int J Radiat Oncol Biol Phys. 2018. doi:10.1016/j.ijrobp.2017.12.288
20. Honda Y, Kimura T, Aikata H, et al. Stereotactic body radiation therapy combined with transcatheter arterial chemoembolization for small hepatocellular carcinoma. J Gastroenterol Hepatol. 2013;28(3):530–536. doi:10.1111/jgh.12087
21. Jang WI, Kim MS, Bae SH, et al. High-dose stereotactic body radiotherapy correlates increased local control and overall survival in patients with inoperable hepatocellular carcinoma. Radiat Oncol. 2013;8:250. doi:10.1186/1748-717x-8-250
22. Kwon JH, Bae SH, Kim JY, et al. Long-term effect of stereotactic body radiation therapy for primary hepatocellular carcinoma ineligible for local ablation therapy or surgical resection. Stereotactic radiotherapy for liver cancer. BMC Cancer. 2010;10:475. doi:10.1186/1471-2407-10-475
23. Sanuki N, Takeda A, Oku Y, et al. Stereotactic body radiotherapy for small hepatocellular carcinoma: a retrospective outcome analysis in 185 patients. Acta Oncol. 2014;53(3):399–404. doi:10.3109/0284186x.2013.820342
24. Rajyaguru DJ, Borgert AJ, Smith AL, et al. Radiofrequency ablation versus stereotactic body radiotherapy for localized hepatocellular carcinoma in nonsurgically managed patients: analysis of the national cancer database. J Clin Oncol. 2018;36(6):600–608. doi:10.1200/jco.2017.75.3228
25. Lausch A, Sinclair K, Lock M, et al. Determination and comparison of radiotherapy dose responses for hepatocellular carcinoma and metastatic colorectal liver tumours. Br J Radiol. 2013;86(1027):20130147. doi:10.1259/bjr.20130147
26. Cheung MLM, Kan MWK, Yeung VTY, et al. Analysis of hepatocellular carcinoma stereotactic body radiation therapy dose prescription method using uncomplicated tumor control probability model. Adv Radiat Oncol. 2021;6(5):100739. doi:10.1016/j.adro.2021.100739
27. Jackson R, Psarelli EE, Berhane S, Khan H, Johnson P. Impact of viral status on survival in patients receiving sorafenib for advanced hepatocellular cancer: a Meta-Analysis of Randomized Phase III Trials. J Clin Oncol. 2017;35(6):622–628. doi:10.1200/jco.2016.69.5197
28. Kim YS, Lim HK, Rhim H, et al. Ten-year outcomes of percutaneous radiofrequency ablation as first-line therapy of early hepatocellular carcinoma: analysis of prognostic factors. J Hepatol. 2013;58(1):89–97. doi:10.1016/j.jhep.2012.09.020
29. Zhou Y, Si X, Wu L, Su X, Li B, Zhang Z. Influence of viral hepatitis status on prognosis in patients undergoing hepatic resection for hepatocellular carcinoma: a meta-analysis of observational studies. World J Surg Oncol. 2011;9:108. doi:10.1186/1477-7819-9-108
30. Ally A, Balasundaram M, Carlsen R. Comprehensive and integrative genomic characterization of hepatocellular carcinoma. Cell. 2017;169(7):1327–1341.e23. doi:10.1016/j.cell.2017.05.046
31. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–1905. doi:10.1056/NEJMoa1915745
32. Inada Y, Mizukoshi E, Seike T, et al. Characteristics of immune response to tumor-associated antigens and immune cell profile in patients with hepatocellular carcinoma. Hepatology. 2019;69(2):653–665. doi:10.1002/hep.30212
33. Pfister D, Núñez NG, Pinyol R, et al. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature. 2021;592(7854):450–456. doi:10.1038/s41586-021-03362-0
34. Lee BM, Seong J. Radiotherapy as an immune checkpoint blockade combination strategy for hepatocellular carcinoma. World J Gastroenterol. 2021;27(10):919–927. doi:10.3748/wjg.v27.i10.919
35. Boustani J, Grapin M, Laurent PA, Apetoh L, The MC. 6th R of radiobiology: reactivation of anti-tumor immune response. Cancers. 2019;11(6). doi:10.3390/cancers11060860
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.