Back to Journals » OncoTargets and Therapy » Volume 10
DNMT1 regulates human endometrial carcinoma cell proliferation
Received 12 December 2016
Accepted for publication 4 February 2017
Published 29 March 2017 Volume 2017:10 Pages 1865—1873
DOI https://doi.org/10.2147/OTT.S130022
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Ingrid Espinoza
Xinjing Wang, Bilan Li
Department of Gynecology, Shanghai First Maternity and Infant Hospital, Tongji Hospital, School of Medicine, Shanghai, People’s Republic of China
Abstract: Endometrial carcinoma (EC) is the most common gynecologic malignancy, but the molecular events involved in the development and progression of EC remain unclear. This study aimed to investigate the role of DNA methyltransferase 1 (DNMT1), a member of DNA methyltransferases, in EC. AN3CA cells were transfected with DNMT1 siRNA. The proliferation, cell cycle, and apoptosis of AN3CA cells were evaluated by Cell Counting Kit-8 (CCK-8) assay and flow cytometry. The expression of related genes was detected by polymerase chain reaction and Western blot analysis. Knockdown of DNMT1 inhibited the proliferation, induced apoptosis, and G0/G1 phase arrest of AN3CA cells. Furthermore, knockdown of DNMT1 upregulated the expression of nuclear factor kappa-B-inhibitor alpha (NF-κBIA) and Bax and downregulated the expression of Bcl-2 and CCND1/2 in AN3CA cells. In conclusion, this study provides the first evidence that knockdown of DNMT1 affects the expression of cell cycle- and apoptosis-associated proteins in EC cells, suggesting the potential of DNMT1 in EC therapy.
Keywords: DNMT1, endometrial carcinoma, NF-κB, Bcl-2, Bax, CCND1/2
Introduction
Endometrial carcinoma (EC) is one of the most common malignancies of the female genital tract. EC is the second most common form of gynecologic malignancy, with >189,000 new cases and 45,000 deaths worldwide each year.1,2 EC patients with advanced or recurrent tumor, regardless of histological subtype, have a poor prognosis. Radiation or hormone therapy could be used as an adjuvant treatment for the majority of women with advanced or recurrent EC.
It is well known that mutation or deletion of coding regions is responsible for gene inactivation. However, a new molecular mechanism of gene silencing has been discovered.3 DNA promoter hypermethylation is a key epigenetic mechanism for the silencing of many genes, including those involved in cell cycle regulation, DNA repair, and apoptosis.4 Hypermethylation of CpG islands may inhibit transcription by recruiting the methyl-CpG-binding domain proteins5 or by interfering with the recruitment and function of basal transcription factors or transcriptional coactivators.6 DNA methylation is modulated by DNA methyltransferases (DNMTs).7 The human genome includes four genes that code DNMTs (DNMT1, DNMT2, DNMT3A, and DNMT3B) with DNMT1 and DNMT3B being the more potent.8 DNMT1 is responsible for precise duplicating and maintaining the preexisting DNA methylation patterns after replication. DNMT1 could inhibit the transcription of tumor suppressor genes and facilitate tumorigenesis. Meanwhile, inhibition of DNMT1 activity could reduce the hypermethylation of repressive genes and reverse phenotypes of malignant tumor.9 Global DNA hypomethylation is associated with chromosomal instability and is an early event in the neoplastic progression of most human and animal cancers.10
DNMT1 silencing has been shown to inhibit cell proliferation and alter cell cycle progression through cell cycle arrest and apoptosis in cervical cancer.11 Apoptosis is a programmed cell death morphologically characterized by cell shrinkage, chromatin condensation, internucleosomal DNA fragmentation, and the formation of apoptotic bodies. Moreover, cell cycle progression and apoptosis are well regulated by a set of intracellular signal transduction pathways.12 DNMT1 inhibited the transcription of cell proliferation-related tumor suppressors, such as p27 and Bax, which are implicated in the development of several types of cancers.13,14 Several signals from the tumor microenvironment have been shown to trigger cell apoptosis processes via specific pathways involving Bcl-2, Cyclin-D1, and nuclear factor kappa-B (NF-κB), which are important regulators of cell fate in cancer cells.15
In this study, we aimed to investigate the role of DNMT1 in EC. We demonstrated that knockdown of DNMT1 inhibited cell proliferation and induced cell apoptosis by modulating the expression of Bcl-2, Bax, NF-κB-inhibitor alpha (NF-κBIA), and Cyclin (CCN)D1/2. These results suggest that DNMT1 plays an important role in the etiology and progression of EC.
Materials and methods
Cell culture and transfection
The human EC cell lines HEC-1B, Kle, Ishikawa, and AN3CA were obtained from the Chinese Academy of Sciences Committee Type Culture Collection (Shanghai, People’s Republic of China). Cells were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM)/F12 (11030; Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS; 16000-44; Gibco) in a humidified atmosphere containing 5% CO2 at 37°C. DNMT1 siRNA and its control siRNA negative control (NC) were purchased from Sangon Technologies (Shanghai, People’s Republic of China) and transfected into cells using Lipofectamine 2000 (Thermo Fisher Scientific) according to the manufacturer’s protocol.
Quantitative reverse transcription polymerase chain reaction (RT-PCR)
Total RNA was extracted from the cells using TRIzol reagent (Thermo Fisher Scientific) and reverse-transcribed into cDNA using the one-step PrimeScript RT Reagent Kit (TaKaRa Bio Inc., Kusatsu, Japan). cDNA was then used for real-time quantitative polymerase chain reaction (qPCR) using SYBR Premix Ex Taq on an Eppendorf RealPlex Mastercycler. The primers used were as follows: NF-κBIA forward 5′-CTCCGAGACTTTCGAGGAAATAC-3′, reverse 5′-GCCATTGTAGTTGGTAGCCTTCA-3′; β-actin forward 5′-TCATGGGTGTGAACCATGTGAA-3′, reverse 5′-GGCATGGACTGTGGTCATGAG-3′; p65 forward 5′-CCTGGAGCAGGCTATCAGTC-3′, reverse 5′-ATCTTGAGCTCGGCAGTGTT-3′; Bcl-2 forward 5′-CGCGACTCCTGATTCATT-3′, reverse 5′-TGCATTCTTGGAGGG-3′; Bax forward 5′-CCAAGAAGCTGAGCGAGTGTC-3′, reverse 5′-TGAGGACTCCAGCCACAAAGA-3′; CCND1 forward 5′-ATGTTCGTGGCCTCTAAGATGA-3′, reverse 5′-CAGGTTCCACTTGAGCTTGTTC-3′; CCND2 forward 5′-AAGAATTCCTCCTCAATAGCCTGCAGCAGTA-3′, and reverse 5′-GCGGGATATCGACCTGTGAGAATTCGAT-3′. Relative mRNA levels were calculated using the 2(−ΔCt) method, and data were obtained in triplicate from three independent experiments.
Western blot analysis
Cells were washed with ice-cold phosphate-buffered saline (PBS) and lysed in ice-cold RIPA Lysis Buffer (Thermo Fisher Scientific) on ice for 30 min. Total protein was measured using the Bio-Rad protein assay reagent according to the manufacturer’s protocol. Proteins (20 mg) were separated on 10% polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes. The membranes were blocked with 5% bovine serum albumin (BSA)/PBS for 1 h and then incubated overnight at 4°C with primary antibodies against DNMT1 (1:1,000 dilution; Abcam, Cambridge, UK), p65 (1:500 dilution; Sangon Technologies), NF-κBIA (1:500 dilution; Sangon Technologies), Caspase-2 ((1:1,000 dilution; Sangon Technologies), Bcl-2 (1:1,000 dilution; Sangon Technologies), Bax (1:500 dilution; Sangon Technologies), CCND1 (1:1,000 dilution; Sangon Technologies), CCND2 (1:1,000 dilution; Sangon Technologies), and GAPDH (1:1,000 dilution; Cell Signaling Technology, Danvers, MA, USA). The membranes were washed three times with PBS and then incubated with peroxidase-linked secondary antibody (1:1,000 dilution) for 1 h at room temperature. The signals were developed using an electro-chemi-luminescence kit (Pierce™, Thermo Fisher Scientific) and analyzed using the TotalLab software.
Cell proliferation assay
Cell Counting Kit-8 (CCK-8; Beyotime Institute of Biotechnology) was used for the detection of cell proliferation. In 96-well plates, 103 cells/well were incubated in DMEM for 48 h prior to the addition of 10 μL of CCK-8 solution. The mixture was incubated at 37°C for 4 h, and then, the absorbance in each well was measured using an enzyme-linked immunosorbent assay reader (Bio-Rad Laboratories, Hercules, CA, USA) at 450 nm.
Cell cycle analysis
Cells were seeded at a density of 2×105 cells/mL in six-well plates. After overnight incubation, the cells were exposed to 100 nM of DNMT1 siRNA for 48 h. The cells were fixed in 70% ethanol for 16 h at 4°C, and the fixed cells were washed with PBS and resuspended in 1 mL of propidium iodide (PI) staining solution for incubation at 37°C for 15 min. The stained cells were analyzed to determine the fraction of cell population in various cell cycle stages using a flow cytometer (BD FACSCanto II; BD Biosciences, Franklin Lakes, NJ, USA).
Apoptosis analysis
Cells were seeded at a density of 2×105 cells/mL in six-well plates. After overnight incubation, the cells were exposed to 100 nM of DNMT1 siRNA for 48 h. The cells were fixed in 70% ethanol for 16 h at 4°C, and the fixed cells were washed with PBS and subjected to Annexin V-fluorescein isothiocyanate (FITC)/PI staining using Annexin V-FITC/PI Apoptosis Detection Kit (Sangon Technologies). The stained cells were analyzed to determine the fraction of cell population in various cell cycle stages using a flow cytometer (BD FACSCanto II).
Statistical analysis
Data were shown as the mean ± SD, and the differences were evaluated using one-way analysis of variance (ANOVA) for three-group comparisons and Student’s t-tests for two-group comparisons. All statistical analyses were performed using the SPSS 13.0 software package. P<0.05 was considered to be statistically significant.
Results
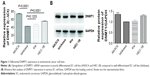
DNMT1 expression is high in poorly differentiated EC cell lines
In four EC cell lines, DNMT1 mRNA and protein expression levels were higher in poorly differentiated EC cell lines (AN3CA and HEC-1B) than in well-differentiated EC cell lines (Ishikawa and Kle) (Figure 1A and B). We selected AN3CA and HEC-1B cell lines for following experiments. Compared to siRNA NC-transfected cells, the mRNA level of DNMT1 in DNMT1 siRNA-transfected cells was significantly decreased (P<0.01) (Figure 2A and B). Moreover, the protein level of DNMT1 was significantly decreased in DNMT1 siRNA-transfected cells (Figure 2C and D). These data indicate that DNMT1 siRNA could effectively knockdown the expression of DNMT1 in EC cells.
Knockdown of DNMT1 inhibits the proliferation of EC cells
To understand the role of DNMT1 in EC cells, we examined the effect of DNMT1 knockdown on EC cell proliferation. CCK-8 assay showed that cell proliferation was significantly inhibited in AN3CA and HEC-1B cells transfected with DNMT1 siRNA compared to the cells transfected with siRNA NC (Figure 2E and F). These data suggest that DNMT1 promotes the proliferation of EC cells.
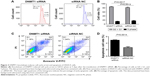
Knockdown of DNMT1 induces cell cycle arrest at G0/G1 phase in EC cells
To explore the possible mechanisms by which DNMT1 promotes EC cell proliferation, the effect of DNMT1 silencing on cell cycle progression of AN3CA cells was analyzed by flow cytometry. The results showed that knockdown of DNMT1 in AN3CA cells induced an increase in the percentage of cells in G0/G1 phase (from 82.07%±0.80% in the siRNA NC group to 85.77%±0.29% in the DNMT1 siRNA group, P<0.05), parallel with a decrease in the percentage of cells in S phase (from 17.17%±0.59% in siRNA NC group to 13.87%±0.40% in DNMT1 siRNA group, P<0.05) (Figure 3A and B). These results suggest that DNMT1 knockdown inhibits EC cell proliferation through inducing G0/G1 cell cycle arrest.
Knockdown of DNMT1 induces apoptosis in EC cells
Furthermore, we examined the effect of DNMT1 silencing on the apoptosis of AN3CA cells. Flow cytometry of AN3CA cells stained with Annexin-V-FITC showed that the proportion of early apoptotic cells was increased in AN3CA cells transfected with DNMT1 siRNA (4.93%±0.20%) compared to that in cells transfected with siRNA NC (2.66%±0.45%) (Figure 3C and D). Our results suggest that DNMT1 inhibits the apoptosis of EC cells.
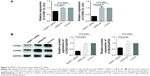
Knockdown of DNMT1 alters the expression of cell cycle- and apoptosis-related proteins in EC cells
To understand the molecular mechanism by which DNMT1 modulates EC cell cycle and apoptosis, we detected the expression of a variety of proteins involved in cell cycle and apoptosis. qPCR and Western blot analyses showed that NF-κBIA and Caspase-2 were upregulated and p65 was downregulated in DNMT1 siRNA-transfected AN3CA cells (Figure 4A–C). Moreover, Bax was upregulated and Bcl-2 was downregulated in AN3CA cells after the knockdown of DNMT1 (Figure 4D and E). These results reveal that DNMT1 regulates these apoptosis-related genes to promote the apoptosis of EC cells.
Furthermore, we found that mRNA and protein levels of CCND1 and CCND2 were significantly decreased in DNMT1 siRNA-transfected AN3CA cells compared to that in siRNA NC-transfected cells (Figure 5A and B). These data indicate that DNMT1 upregulates CCND1 and CCND2 to enhance the proliferation of EC cells.
Discussion
In recent years, numerous studies have shown an association between cell cycle regulation and cancer and inhibition of cell cycle has become an appreciated target for the management of cancer.16 Our study showed that knockdown of DNMT1 exerted strong growth inhibitory effects on human EC cells by the perturbation of G1–S phase transition. CCNDs are crucially involved in G1–S phase transition of cell cycle progression, and their overexpression is associated with various cancers.17,18 Indeed, we found that DNMT1 knockdown led to a decreased expression of CCND1 and CCND2 in EC cells, which may explain how DNMT1 promotes EC cell proliferation. A study demonstrated that hypermethylation of CCND was associated with the loss of mRNA expression and tumor development in several kinds of cancer, such as prostate cancer.19 A recent study showed that DNMT1 protein interacted with β catenin and regulated Wnt signaling in colorectal cancer cells.20 CCND1 and CCND2 are the downstream targets of Wnt/β catenin signaling and the important effectors to enhance cell cycle progression. Therefore, it is possible that the knockdown of DNMT1 decreased the stability and activity of β catenin and downregulated the expression of CCND1 and CCND2, leading to cell cycle arrest. Further exploration of the mechanism of action of DNMT1 in regulating cell cycle progression and apoptosis will deepen our understanding of the role of DNMT1 in tumorigenesis.
NF-κB family of transcription factors include five members, such as p50, p52, RelA (also known as p65), RelB, and c-Rel, which regulate the expression of various genes involved in inflammatory, antiapoptotic, and immune responses. The activation of NF-κB in cancer cells promotes cancer development and progression, and NF-κB has been thought to be a target for cancer therapy. NF-κB promotes cell survival by inhibiting apoptosis.21 Our results demonstrate a decreased expression of p65 in EC cells with subsequent increased expression of NF-κBIA after the knockdown of DNMT1. We postulate that the loss of DNMT1-mediated downregulation of NF-κB activity could lead to the inhibition of cell growth and induction of apoptosis. We confirmed that DNMT1 knockdown significantly upregulated the expression of Caspase-2 in EC cells, which may contribute to apoptosis.
Members of the Bcl-2 family of proteins are the critical regulators of apoptotic pathway.22 Bcl-2 is an upstream effector in the apoptotic pathway and is a potent suppressor of apoptosis.23 Bcl-2 is found at inappropriately high levels in more than half of all human tumors. Bcl-2 has been shown to form a heterodimer with the proapoptotic member Bax and neutralize its proapoptotic effects. Therefore, the ratio of Bax/Bcl-2 is a crucial factor in determining whether cells will undergo apoptosis under conditions that promote cell death.24 In our study, a decrease in Bcl-2 protein expression was observed in DNMT1 siRNA-transfected EC cells, while the protein expression of Bax was upregulated; hence, the ratio of Bax to Bcl-2 was altered in favor of apoptosis. Our results suggest that the upregulation of Bax and the downregulation of Bcl-2 may be another molecular mechanism through which knockdown of DNMT1 induces apoptosis.
Consistent with our results in EC cells with DNMT1 silencing, we obtained similar results in AN3CA cells treated with 5-aza-dC, the DNA methyl-transferase inhibitor.25 Notably, a previous study reported that DNMT1 was overexpressed in endometrial tumor tissues and DNMT1 expression level was correlated with tumor progression.26 These data suggest the potential of DNMT1 as a new target for EC treatment. However, in vivo studies on animal models of EC are needed before clinical trials on DNMT1 inhibitors.
Conclusion
DNMT1 is aberrantly overexpressed in aggressive and poorly differentiated EC cells. Knockdown of DNMT1 significantly inhibited EC cell proliferation, mostly by inducing cell cycle arrest at G0/G1 phase and inducing apoptosis. Knockdown of DNMT1 could downregulate CCNDs and NF-κB, decrease Bcl-2/Bax ratio, and upregulate Caspase-2. Our study suggests that DNMT1 is a potential therapeutic target for EC.
Acknowledgment
This study was sponsored by Natural Science Foundation of Shanghai (No. 16ZR1427100), the National Natural Science Foundation of China (No. 81602280 and No. 81602281).
Disclosure
The authors report no conflicts of interest in this work.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. | ||
Tsikouras P, Bouchlariotou S, Vrachnis N, et al. Endometrial cancer: molecular and therapeutic aspects. Eur J Obstet Gynecol Reprod Biol. 2013;169(1):1–9. | ||
Chomet PS. Cytosine methylation in gene-silencing mechanisms. Curr Opin Cell Biol. 1991;3(3):438–443. | ||
Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet. 2002;3(6):415–428. | ||
Bogdanović O, Veenstra GJC. DNA methylation and methyl-CpG binding proteins: developmental requirements and function. Chromosoma. 2009;118(5):549–565. | ||
Ballestar E, Esteller M. Methyl-CpG-binding proteins in cancer: blaming the DNA methylation messenger. Biochem Cell Biol. 2005;83(3):374–384. | ||
Okano M, Bell DW, Haber DA, Li E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell. 1999;99(3):247–257. | ||
Okano M, Xie S, Li E. Cloning and characterization of a family of novel mammalian DNA (cytosine-5) methyltransferases. Nat Genet. 1998;19(3):219–220. | ||
Bestor TH. The DNA methyltransferases of mammals. Hum Mol Genet. 2000;9(16):2395–2402. | ||
Figueiredo JC, Grau MV, Wallace K, et al. Global DNA hypomethylation (LINE-1) in the normal colon and lifestyle characteristics, dietary and genetic factors. Cancer Epidemiol Biomarkers Prev. 2009;18(4):1041–1049. | ||
Zhang Y, Chen F-Q, Sun Y-H, Zhou S-Y, Li T-Y, Chen R. Effects of DNMT1 silencing on malignant phenotype and methylated gene expression in cervical cancer cells. J Exp Clin Cancer Res. 2011;30:98. | ||
Eom H-J, Choi J. p38 MAPK activation, DNA damage, cell cycle arrest and apoptosis as mechanisms of toxicity of silver nanoparticles in Jurkat T cells. Environ Sci Technol. 2010;44(21):8337–8342. | ||
Peng D-F, Kanai Y, Sawada M, et al. DNA methylation of multiple tumor-related genes in association with overexpression of DNA methyltransferase 1 (DNMT1) during multistage carcinogenesis of the pancreas. Carcinogenesis. 2006;27(6):1160–1168. | ||
Sowińska A, Jagodzinski PP. RNA interference-mediated knockdown of DNMT1 and DNMT3B induces CXCL12 expression in MCF-7 breast cancer and AsPC1 pancreatic carcinoma cell lines. Cancer Lett. 2007;255(1):153–159. | ||
Gupta S, Afaq F, Mukhtar H. Involvement of nuclear factor-kappa B, Bax and Bcl-2 in induction of cell cycle arrest and apoptosis by apigenin in human prostate carcinoma cells. Oncogene. 2002;21(23):3727–3738. | ||
Porter TR, Richards FM, Houlston RS, et al. Contribution of Cyclin D1 (CCND1) and E-cadherin (CDH1) polymorphisms to familial and sporadic colorectal cancer. Oncogene. 2002;21(12):1928–1933. | ||
Casimiro MC, Di Sante G, Ju X, et al. Cyclin D1 promotes androgen-dependent DNA damage repair in prostate cancer cells. Cancer Res. 2016;76(2):329–338. | ||
Rahal OM, Pabona JMP, Kelly T, et al. Suppression of Wnt1-induced mammary tumor growth and lower serum insulin in offspring exposed to maternal blueberry diet suggest early dietary influence on developmental programming. Carcinogenesis. 2013;34(2):464–474. | ||
Henrique R, Costa LV, Cerveira N, et al. Hypermethylation of Cyclin D2 is associated with loss of mRNA expression and tumor development in prostate cancer. J Mol Med. 2006;84(11):911–918. | ||
Song J, Du Z, Ravasz M, Dong B, Wang Z, Ewing RM. A protein interaction between β-catenin and Dnmt1 regulates wnt signaling and DNA methylation in colorectal cancer cells. Mol Cancer Res. 2015;13(6):969–981. | ||
Bours V, Bonizzi G, Bentires-Alj M, et al. NF-κB activation in response to toxical and therapeutical agents: role in inflammation and cancer treatment. Toxicology. 2000;153(1–3):27–38. | ||
Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281(5381):1322–1326. | ||
Liu Z, Ding Y, Ye N, Wild C, Chen H, Zhou J. Direct activation of bax protein for cancer therapy. Med Res Rev. 2016;36(2):313–341. | ||
Kroemer G. The proto-oncogene Bcl-2 and its role in regulating apoptosis. Nat Med. 1997;3(6):614–620. | ||
Li BL, Lu W, Lu C, et al. CpG island hypermethylation-associated silencing of microRNAs promotes human endometrial cancer. Cancer Cell Int. 2013;13(1):44. | ||
Ikeda S, Imura J, Suzuki K. Protein expression, mRNA expression and gene amplification of DNA methyltransferase 1 in endometrial tumor tissues. Mol Clin Oncol. 2013;1(3):423–429. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.