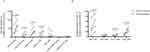Back to Journals » Journal of Inflammation Research » Volume 16
Disturbance of Adaptive Immunity System Was Accompanied by a Decrease in Plasma Short-Chain Fatty Acid for Patients Hospitalized During SARS-CoV-2 Infection After COVID-19 Vaccination
Authors Liang Z, Wang N, Fan C, Shang L, Zhang Y, Gao C, Luo J
Received 10 August 2023
Accepted for publication 7 November 2023
Published 14 November 2023 Volume 2023:16 Pages 5261—5272
DOI https://doi.org/10.2147/JIR.S434860
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Zhaojun Liang,1,2 Nan Wang,1,2 Chunxue Fan,1,2 Lili Shang,1,2 Yaping Zhang,1,2 Chong Gao,3 Jing Luo1,2
1Division of Rheumatology, Department of Medicine, the Second Hospital of Shanxi Medical University, Taiyuan, People’s Republic of China; 2Shanxi Key Laboratory for Immunomicroecology, the Second Hospital of Shanxi Medical University, Taiyuan, People’s Republic of China; 3Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA
Correspondence: Jing Luo, Email [email protected]
Introduction: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection can lead to disorders of immune function and a decrease in the diversity of intestinal flora. We aimed to explore the changes of circulating immune cell subsets and the plasma level of intestinal short-chain fatty acids (SCFAs) in patients with Coronavirus disease 2019 (COVID-19), further understanding the pathogenesis of COVID-19.
Methods: The study included 83 newly diagnosed COVID-19 patients and 39 non-COVID-19 controls. All have completed a full course of vaccination against SARS-CoV-2. The levels of peripheral lymphocyte subsets and plasma cytokines were detected by flow cytometry. Targeted metabolomics was used to explore the level of SCFAs in plasma.
Results: Compared with the non-COVID-19 group, COVID-19 patients showed a decrease in CD19+B cells, CD4+T cells, CD8+T cells, NK cells, CD4+CD8+T cells and CD4–CD8–T cells (all p< 0.001) and concomitantly an increase in sIL-2R, IL-6 and IL-10 (all p< 0.005). These alterations were more pronounced in those critical patients. In addition, COVID-19 patients had lower levels of propanoic acid (PA), butyric acid (BA), isobutyric acid (IBA) and isohexanoic acid (ICA) (all p< 0.01). Among them, the level of ICA is positively correlated with the absolute number of immune cells.
Conclusion: Our study suggests the immune cell subsets in COVID-19 patients who had completed vaccination were still severely disturbed and concomitantly lower SCFAs, especially in severe patients with poor prognosis. Lower levels of plasma SCFAs may contribute to lymphopenia in COVID-19. The potential relationship between plasma SCFAs and immune cell reduction provides a new direction for the treatment of COVID-19.
Keywords: coronavirus disease 2019, severe acute respiratory syndrome coronavirus 2, adaptive immunity, short-chain fatty acids
Introduction
Since December 2019, the spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has become a major public health threat affecting human health. Pneumonia is the main manifestation of SARS-CoV-2 infection. COVID-19 patients may have a fever, cough, dyspnea, and even multiple organ dysfunction. Now it is known that the imbalance of immune response is the essence of COVID-19, also one of the main reasons for the poor progress and prognosis of severe COVID-19 patients.1,2
According to research findings, SARS-CoV-2 can invade almost all immune cells, which leads to abnormal immune responses. SARS-CoV-2 can induce the over-activation of monocyte-macrophages and dendritic cells, as well as a large amount of apoptotic T lymphocytes, resulting in the imbalance of innate and adaptive immune responses.3 The cytokine storm generated after the over-activation of the immune system causes inflammation from local to systemic, which seriously threatens the life of patients.4 In addition, SARS-CoV-2 infection can also cause a decrease in the diversity of intestinal flora,5 especially a decrease in the abundance of SCFAs-producing intestinal flora. The gut microbiota produces SCFAs upregulates anti-inflammatory cytokines and downregulates pro-inflammatory cytokines through different mechanisms, maintaining mucosal homeostasis. Once this homeostasis is disrupted, normal lymphocyte differentiation will be inhibited.6 Therefore, it is of great value to fully understand the changes in immune function in COVID-19 patients to understand its pathophysiological process and find effective treatment methods.
In this study, we recruited 83 newly diagnosed COVID-19 patients, tested the levels of peripheral blood lymphocyte subsets and cytokines using flow cytometry (FCM), and analyzed their correlation with disease severity. Further, targeted metabolomics was used to detect the changes in SCFAs level in plasma to fully clarify the characteristics of adaptive immune cell distribution in COVID-19 patients and understand its pathogenesis.
Methods
Study Subjects
This study recruited eighty-three patients who were hospitalized in the Second Hospital of Shanxi Medical University due to SARS-CoV-2 infection from December 29, 2022, to January 6, 2023. The inclusion criteria included: SARS-CoV-2 reverse transcription polymerase chain reaction was positive, computerized tomography (CT) is characterized by the ground-glass lesions of the lung, and antibiotics and probiotics were not used within 3 months before hospitalization. The clinical classification is based on the diagnosis and treatment plan for COVID-19:7 1) moderate type: fever, cough, and shortness of breath, and the imaging shows characteristic pneumonia; 2) severe type: respiratory rate (RR) ≥ 30 per minute, oxygen saturation ≤ 93%, arterial partial pressure of oxygen (PaO2)/oxygen concentration (FiO2) ≤ 300 mmHg; 3) critical type: respiratory failure and mechanical ventilation are required, and shock or other organ failure are required to be treated in the intensive care unit. Thirty-nine age- and sex-matched community residents who have never been infected with SARS-CoV-2 and have not used antibiotics and probiotics in recent 3 months were recruited as controls. All subjects have completed a full course of vaccination against SARS-CoV-2. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). It was approved by the Ethics Committee of the Second Affiliated Hospital of Shanxi Medical University (number: 2022-KY-063). Written informed consent was obtained from all participants before the study.
Clinical and Laboratory Indices
Clinical parameters of patients were collected, including age, sex, duration of disease, clinical symptoms, body mass index (BMI), smoking history, chronic disease history, treatments, outcome, and so on. Laboratory data include blood routine examination [white blood cell (WBC), red blood cell (RBC), hemoglobin, platelet, neutrophile, lymphocyte, monocyte, eosinophil, basophilic] liver function test [alanine transaminase (ALT), aspartate aminotransferase (AST); serum total protein (TP); albumin (ALB), globulin (GLO)], renal function test [urea, creatinine (Cr), lactate dehydrogenase (LDH), hydroxybutyrate dehydrogenase (HBDH)], coagulation function test [prothrombin time (PT), prothrombin activity (PTA), fibrinogen (FIB), D-dimer], markers of myocardial damage [myoglobin, N-terminal pro-Brain natriuretic peptide (NT-proBNP, high-sensitivity cardiac troponin I (hs-cTnI)], procalcitonin (PCT), C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR).
Lymphocyte Subset Assay
Peripheral whole blood samples of patients were collected on an empty stomach in the morning within 24 hours of hospitalization. Lymphocyte subsets, including CD3+CD19– T cells, CD3–CD19+ B cells, CD3+CD4+ T cells, CD3+CD8+ T cells, CD3–CD16/CD56+ natural killer (NK) cells, CD4+CD8+ T cells, CD4–CD8– T cells, CD4+IFN-γ+ T-helper (Th) 1 cells, CD4+IL-4+ Th2 cells, CD4+IL-17+ Th17 cells, and CD4+CD25+Foxp3+ regulatory T (Treg) cells, were detected using BD-FACS-CANTO II flow cytometry (Becton, Dickinson and Co., Franklin Lakes, NJ, USA).
Cytokines Assay
Collect venous blood samples with a test tube containing separation gel, and retain plasma after centrifugation. Detection of cytokines soluble interleukin 2 receptor (sIL-2R), interleukin (IL)-2, IL-4, IL-6, IL-10, IL-17, tumor necrosis factor-α (TNF-α) and interferon-γ (IFN-γ) by human Th1/Th2/Th17 cytokine kit (Jiangxi Cellgene Biotechnology Co., Ltd China). The sample is processed according to the manufacturer’s specifications, and the data returned by FCAP ArrayTM v 3.0.1 software is the median fluorescence intensity (MFI) and concentration (pg/mL).
SCFAs Assay
The detection of SCFAs was performed on an AB Sciex Triple Quad™ 4500MD mass spectrometer coupling a JasperTM high performance liquid chromatography (HPLC) system.
The venous blood was collected in the test tube containing heparin sodium and centrifuged at 3000 r for 15 minutes. 25ul sample, quality control sample and standard sample are added into the EP tube respectively. Add 10 μL 1μg/mL IS aqueous solution, 10 μL 1M EDC and 10 μL 1M O-BHA, and then mix the sample at 1000 rpm for 60 minutes. Add 50 μL Milli-Q water and 400 μL ethyl acetate to each sample and mix well. Take 200 μL upper solution, dry for 20 minutes, and reconstituted with 100 μL Milli-Q water. The injection volume is 5 μL for LC-MS analysis.
The analytes were separated on a Kinetex Evo C18 analytical column (100×2.1 mm 2.6μm; Phenomenex), which were maintained at 40°C using a mobile phase of 0.1% aqueous formic acid and methanol at a flow rate of 0.6 mL/min. The volume of injection was 5μL and the analytical run time was 7.5 minutes. The gradient elution was 0–1.0 min, 45% B; 1.0–5.0 min, 45–80% B; 5.0–6.5 min, 80% B; 6.5–6.7 min, 80–45% B; and 6.7–7.5 min, 45% B. Under mass spectrometry conditions, the electrospray ionization interface (ESI) in the positive ion mode with multiple reaction monitoring (MRM) was used for detection using the following parameters: ion source temperature = 500°C; ion spray voltage = 4500 V; curtain gas (nitrogen) = 30 psi; atomizing gas (GS 1) = 60 psi; auxiliary gas (GS 2) = 60 psi.
Statistical Analysis
SPSS 25.0 (IBM Corp., Armonk, NY, USA) or GraphPad Prism 9 (GraphPad Software Inc., San Diego, CA, USA) was used for statistical analysis. Normally distributed variables were expressed as means ± standard deviation (M ± SD), and nonparametric variables were expressed as medians and interquartile range (IQR). Chi-square test, Mann–Whitney U-test or independent-sample T test was used to compare two groups, Kruskal–Wallis test or one-way ANOVA was used for ex-post hoc comparisons compare of various indicators among moderate, severe, and critical. Spearman correlation test was used to evaluate the correlation between indicators. P < 0.05 was considered statistically significant.
Results
Characteristics of COVID-19 Group and Non-COVID-19 Group
A total of 83 COVID-19 patients (35 females; mean age: 75.27 ± 10.55 years) and 39 non-COVID-19 people (20 females; mean age: 70.33 ± 8.80 years) were included in this study (Table 1). In the COVID-19 group, 49 (59.0%) patients had cardiovascular diseases, 24 (28.9%) had endocrine diseases, 9 (10.8%) had respiratory diseases, 3 (3.6%) had kidney diseases, 14 (16.9%) had autoimmune diseases, and 4 (4.8%) had malignant tumors, which were consistent with those in the non-COVID-19 group (p>0.05). Compared with the non-COVID-19 group, the COVID-19 group exhibited lower levels of WBC (p=0.008), RBC (p=0.017), platelet (p<0.001), lymphocytes (p<0.001), monocytes (p=0.002), eosinophils (p<0.001) and basophils (p<0.001). Liver and kidney function indicators such as ALT (p=0.014), AST (p<0.001), LDH (p<0.001), and HBDH (p<0.001) were higher in the COVID-19 group, while TP (p=0.007) and ALB (p=0.001) were decreased. Coagulation function indexes such as FIB (p<0.001) and D-dimer (p=0.019) and inflammatory indexes ESR (p=0.001) and CRP (p=0.001) were significantly increased in the COVID-19 group.
 |
Table 1 Comparisons of the Demographic, Clinical, and Laboratory Features Between the COVID-19 Group and the Non-COVID-19 Group |
Lymphocyte Subsets in Peripheral Blood Were Reduced in COVID-19 Patients
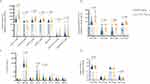
To compare the changes in immune function between the COVID-19 group and the non-COVID-19 group, we detected the absolute numbers of lymphocyte subsets using flow cytometry. Compared with the non-COVID-19 group, COVID-19 group showed that the absolute number of peripheral lymphocyte subsets was significantly reduced, including CD3+ T cells (p<0.001), CD19+ B cells (p<0.001), CD4+ T cells (p<0.001), CD8+ T cells (p<0.001), NK cells (p<0.001), CD4+CD8+ T cells (p<0.001) and CD4–CD8– T cells (p<0.001) (Figure 1A). Further analysis of CD4+ T lymphocyte subsets showed that the absolute number of Th1, Th2, Th17 and Treg cells in the COVID-19 group was significantly lower than that in the non-COVID-19 group (both p<0.001) (Figure 1B), and the ratio of Th1/Th2 decreased (p=0.033), and Th2/Treg increased (p<0.001) (Figure 1D). In addition, we also measured the lymphocyte-related cytokines (Figure 1C). The results showed that the levels of sIL-2R (p=0.010), IL-6 (p=0.040) and IL-10 (p<0.001) in the COVID-19 group were significantly higher than those in the non-COVID-19 group.
COVID-19 Results in the Blocked Synthesis of SCFAs in Plasma
As an important metabolite of intestinal microorganisms, SCFAs play an important role in intestinal and immune homeostasis. We used targeted metabonomics to detect the concentration of SCFAs in the plasma of the COVID-19 group and non-COVID-19 group (Figure 2). The level of SCFAs in the plasma of COVID-19 patients was low, especially propanoic acid (PA) (p=0.004), butyric acid (BA) (p<0.001), isobutyric acid (IBA) (p<0.001) and isocaproic acid (ICA) (p<0.001).
Characteristics of COVID-19 Patients with Moderate, Severe and Critical
According to the severity of the disease, we divided COVID-19 patients into three clinical types: moderate (n=34, 17 females; mean age: 70.53 ± 11.14 years), severe (n=33, 12 females; mean age: 76.55 ± 9.68 years) and critical (n=16, 6 females; mean age: 80.54 ± 7.14 years) (Table 2). There is no gender difference among the three groups (p=0.483), and there is no significant difference in the disease duration (p=0.757), BMI (p=0.620), and the proportion of patients with chronic diseases (both p>0.1). Critical and severe patients were older than moderate (p=0.001, p=0.031, respectively), and the smoking rate of severe patients was higher than that of moderate (p=0.014). The neutrophil count of critical patients was significantly higher than that of severe (p=0.043), but the lymphocyte count was the lowest among the three groups, although there was no statistical difference. There were similar concentrations of ALT and AST among the three groups, but TP and ALB were significantly decreased in the critical, and far lower than those in the moderate patients (p=0.008, p=0.002, respectively). The urea level of critical patients was higher than that of moderate (p=0.010) and severe (p=0.007), but there was no difference in Cr. In coagulation function, PT and d-dimer in critical patients were significantly higher than those in moderate (p=0.029, p=0.037, respectively) and severe (p=0.005, p=0.021, respectively), and PTA was significantly reduced (p=0.007). In addition, myoglobin (p=0.016), NT-proBNP (p=0.001), and hs-cTnI (p=0.006) in critical patients are significantly higher than those in moderate, which may be related to the presence of viral myocarditis or cardiac failure. The level of PCT in critical patients was significantly higher than that in moderate (p=0.043), and ESR and CRP also showed an increasing trend.
 |
Table 2 Comparison of Demographic, Clinical and Laboratory Features of COVID-19 Patients with Different Clinical Types |
Immune Impairment is More Serious in Critical COVID-19 Patients
We analyzed the levels of lymphocyte subsets and related cytokines in moderate, severe and critical COVID-19 patients (Figure 3). The results showed that the damage to immune function in critical patients was more serious. Compared with moderate patients, the absolute number of CD3+ T cells in severe (p=0.005) and critical (p=0.036) was significantly lower. The level of CD19+ B cells in severe patients was significantly lower than that in moderate (p=0.001). There was no statistical difference in the absolute number of CD4+ T cells and CD8+ T cells among the three groups, but it was the lowest in the critical patients. CD4+CD8+ T cells in critical patients were significantly lower than those in moderate (p=0.026), and CD4–CD8– T cells were significantly lower than those in severe (p=0.043). In the CD4+ T cell subsets, the absolute number of Th1 and Treg cells and the ratio of Th1/Th2 in critical patients were significantly lower than those in moderate (p=0.009, p=0.039, p=0.007, respectively). In terms of cytokines, IL-6 was significantly increased in severe patients, and sIL-2R and IL-10 showed an increasing trend.
Comparison of SCFAs in COVID-19 Patients with Moderate, Severe and Critical
To determine whether the level of SCFAs is related to the severity of the disease, we compared the plasma concentrations of SCFAs in COVID-19 patients with different clinical types (Figure 4). From the results, we can see that there is no statistical difference among the three groups in these SCFAs, but the concentration of PA and BA in severe patients is lower than that in moderate, and the concentration of PA, BA and caproic acid (CA) in critical patients is the highest in the three groups.
 |
Figure 4 Comparison of SCFAs in plasma among moderate, severe and critical COVID-19 patients. Data were presented as median (IQR) and were analyzed by the Kruskal–Wallis test. |
Correlation Analysis of SCFAs and Immune Cells in COVID-19 Patients
To clarify the relationship between SCFAs and immune cells, we conducted a correlation analysis between them (Figure 5). PA, BA, 2-methylbutyric acid (2-MBA), IBA and CA have no significant correlation with immune cells and related cytokines. ICA was positively correlated with CD3+ T cells (r=0.421, p<0.001), CD4+ T cells (r=0.370, p=0.001), CD8+ T cells (r=0.460, p<0.001), CD4+CD8+T cells (r=0.301, p=0.006), Th1 cells (r=0.401, p<0.001), Th2 cells (r=0.370, p<0.001), Treg cells (r=0.358, p<0.001), and IL-6 (r=0.309, p=0.004).
 |
Figure 5 Correlation heat map of SCFAs and immune cells and cytokines in COVID-19 patients. * = p<0.05, ** = p<0.01, *** = p<0.001 by Spearman correlation test. |
Changes of Immune Cells in COVID-19 Patients after Treatment
In this study, 4 patients were followed up, and peripheral lymphocyte subsets were detected after 1 week of treatment (Figure 6). After a week of standard treatment, the absolute number of CD3+ T cells (p=0.006), CD19+ B cells (p=0.016), CD4+ T cells (p=0.001) and CD8+ T cells (p=0.025) in 4 patients increased significantly after treatment. Th17 cells (p=0.012) and Treg cells (p=0.048) were mainly elevated in CD4+ T lymphocyte subsets.
Discussion
The normal functioning of the immune system is crucial for the body to resist viral infection. Innate immunity is the first line of defense against infection. In the early stage of SARS-CoV-2 infection, effector cells involved in innate immunity can be rapidly mobilized, effectively controlling the number and scale of pathogens.8 In COVID-19 patients, the epithelial cells of the nasopharyngeal mucosa present neutrophil and monocyte infiltration, while infected epithelial cells secrete chemokines such as CXCL1, CXCL3, CXCL6, CXCL15, CXCL16, and CXCL17.9 Inflammatory macrophages in the lungs of critical patients can further promote the recruitment and differentiation of neutrophils and monocytes, ultimately leading to the excessive inflammatory response.10 In our study, we observed a decrease in NK cells, monocytes, eosinophils, and basophils in patients with COVID-19, which may be related to the presence of immune failure in the patient.11 SARS-CoV-2 infection leads to depletion of lymphocyte and suppresses of immune function, which may be a potential immunological mechanism for the occurrence and development of COVID-19.12
Not only innate immunity, once infected with SARS-CoV-2, adaptive immunity mediated by T and B cells also quickly exerts protective effects.13 An adaptive immune response usually occurs four days after infection. If the body does not produce an effective adaptive antiviral response in a timely manner, the innate immune response will strengthen, leading to the uncontrolled release of inflammatory cytokines.14 It takes longer for elderly and chronic disease patients to produce an effective adaptive immune response. Relying on early infection to strengthen the innate antiviral immune response can lead to the occurrence of cytokine storms, increasing the risk of severe illness.15,16
Vaccination has a significant protective effect on the entire population, especially the elderly and prepares for the rapid memory response of subsequent exposure to induce neutralizing antibodies.17 Mass vaccination of COVID-19 vaccine has become a key measure for countries to fight against the epidemic.18 In addition to traditional inactivated vaccine and adenovirus vector vaccine, mRNA vaccine has also received global attention.19 After inoculation of the COVID-19 vaccine, innate immunity and adaptive immunity were widely activated, including a significant increase in the proportion of CD16+ monocytes, the activation of CD4+ T cells and CD8+ T cells, and the level of neutralizing antibodies.20 For the elderly COVID-19 patients who are likely to develop severe and critical diseases, the protection rate of 2–3 doses of inactivated vaccine still reaches 90.15%.21 Although vaccination can significantly reduce the severity rate and mortality rate of COVID-19 patients,22 their immune function is still damaged to a certain extent, mainly manifested by the reduction of lymphocytes and the increase of cytokines.23 In this study, COVID-19 patients not only had a decrease in innate immune cells, but also had a significant decrease in adaptive immune cells, including CD19+ B cells, CD4+ T cells, and CD8+ T cells. CD3+ T cells, CD4+CD8+ T cells, and CD4–CD8– T cells showed lower levels in critical patients.
B lymphocytes exert immune effects by proliferating and differentiating into plasma cells to produce specific antibodies.24 In the early stage, T cells play a key role in durable antiviral protection.25 We further analyzed CD4+ T lymphocyte subsets and found that Th1, Th2, Th17 and Treg cells in COVID-19 patients were significantly reduced, of which Th1 and Treg cell reduction was more serious in critical patients. As an immunosuppressive cell subset, the decrease in the number of Treg promotes the occurrence of cytokine storms and inflammatory reactions.26 Cytokine storms are an important cause of death in severe and critical illnesses. Many studies have found that there are higher levels of IL-2, IL-7, IL-10, granulocyte colony-stimulating factor (G-CSF), IFN-γ, Inducible protein-10 (IP-10), monocyte chemokine protein-1 (MCP-1), macrophage inflammatory protein-1A (MIP-1A), and TNF-α in the plasma of severe and critical COVID-19 patients.4 In our study, we also found significant increases in sIL-2R, IL-6, and IL-10 in COVID-19 patients, with the increase in IL-6 being more pronounced in severe patients.
The gastrointestinal tract is the largest immune organ in the human body. The intestinal flora and intestinal mucosa together constitute an immune barrier in the intestine. It has been reported that SARS-CoV-2 infection can change the structure of intestinal flora, mainly manifested in the reduction of beneficial symbiotic bacteria and the enrichment of conditionally pathogenic bacteria. Compared with the control group, COVID-19 patients had lower levels of intestinal bacteria such as Eubacteriaceae, Fecal Bacilliaceae, Rosebacilliaceae, Bifidobacteriaceae and Trichospiriaceae.5 In addition, the composition of intestinal flora in COVID-19 patients was analyzed by shotgun metagenome sequencing technology,27 and the data showed that the main characteristics of patients with COVID-19 were the loss of Bifidobacterium adolescentis, Ruminococcus bromii and Faecalibacterium prausnitzii, and the enrichment of Bacteroides ovatus, Bacteroides dorei, and Bacteroides thetaiotaomicron. Another study also confirmed that compared with the healthy controls, the microbial diversity in the mouth and feces of COVID-19 patients was significantly reduced.28 SCFAs are important metabolites of intestinal microorganisms, which can regulate the functions of natural immune cells such as macrophages, neutrophils, and dendritic cells and participate in immune responses. Studies have shown that SCFAs can enhance immune cell function and improve mitochondrial homeostasis, contributing to the adjuvant treatment of autoimmune diseases.29 SCFAs, especially BA, can promote the production of IL-22 by CD4+ T cells and innate lymphoid cells (ILC) by activating G-protein coupled receptor 41 (GPR41) and inhibiting histone deacetylase, thereby alleviating colitis in mice.30 For COVID-19, SCFAs, the active metabolite of intestinal symbiotic bacteria, can not only reduce the pathway of COVID-19 entering the human body by reducing the expression of angiotensin-converting enzyme 2 (ACE2) but also enhance antiviral immunity.31 SCFAs can reduce the transcription levels of important genes that control virus entry and replication, including retinoic acid-inducible gene 1 (RIG1), transmembrane protease serine 2 (TMPRSS2), and interferon (IFN) receptors while maintaining intestinal cell permeability.32 They can also regulate the differentiation of T and B cells and antigen-specific adaptive immunity.33 Our data show that plasma SCFAs in COVID-19 patients are significantly lower than those in non-COVID-19 patients, which may be related to a decrease in the bacterial population that produces SCFAs. Moreover, we have found that there is a trend towards an increase in plasma SCFAs in critical patients, which may be related to the severe damage to liver function in patients, resulting in the inability of SCFAs to undergo oxidative decomposition in the liver and their massive release into the blood. Interestingly, we found no significant correlation between the concentration of plasma SCFAs and the absolute number of immune cells, except for ICA. However, there are currently few reports on the role of plasma ICA in regulating immune cell levels.
However, the limitations of this study should be pointed out. The first is the limited sample size of the study. Secondly, most patients did not receive further interviews from us. In addition, as an observational study, we cannot indicate whether SCFAs in the plasma directly lead to changes in lymphocyte subsets in patients. Therefore, we should conduct further multicenter studies to determine the changes in the intestinal flora and their impact on immune cells in COVID-19 patients. This may help to provide a new target for the treatment of COVID-19.
In summary, this study indicates that immune function in COVID-19 patients after vaccination is still severely disrupted. The levels of plasma SCFAs are related to the level of some lymphocyte subsets and play an important role in immune regulation. It may be used as an immune modulator in the adjuvant treatment of COVID-19.
Data Sharing Statement
The research article data used to support the findings of this study are available from the corresponding author upon request.
Ethics Approval
The study was approved by the Ethics Committee of the Second Affiliated Hospital of Shanxi Medical University (number: 2022-KY-063).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Scientific Research Project of Health commission of Shanxi Province (2019044), the Research Project Supported by Shanxi Scholarship Council of China (2020-191), the Science and Technology Innovation Project of Shanxi Province (2020SYS08), the Project of Central Guides Local Science and Technology Development Funds (YDZJSX2022C031) and the Foundation of Shanxi Key Laboratory for immunomicroecology (202104010910012).
Disclosure
The authors declare that there is no conflict of interest regarding the publication of this article.
References
1. Zhang B, Zhou X, Zhu C, et al. Immune Phenotyping Based on the Neutrophil-to-Lymphocyte Ratio and IgG Level Predicts Disease Severity and Outcome for Patients With COVID-19. Front Mol Biosci. 2020;7:157. doi:10.3389/fmolb.2020.00157
2. Liang W, Liang H, Ou L, et al.; China Medical Treatment Expert Group for COVID-19. Development and Validation of a Clinical Risk Score to Predict the Occurrence of Critical Illness in Hospitalized Patients With COVID-19. JAMA Intern Med. 2020;180(8):1081–1089. doi:10.1001/jamainternmed.2020.2033
3. Shah VK, Firmal P, Alam A, Ganguly D, Chattopadhyay S. Overview of immune response during SARS-CoV-2 infection: lessons from the past. Front Immunol. 2022;11:1949. doi:10.3389/fimmu.2020.01949
4. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi:10.1016/S0140-6736(20)30183-5
5. Zuo T, Zhang F, Lui GCY, et al. Alterations in gut microbiota of patients with COVID-19 during time of hospitalization. Gastroenterology. 2020;159(3):944–955.e8. doi:10.1053/j.gastro.2020.05.048
6. Martin-Gallausiaux C, Marinelli L, Blottière HM, Larraufie P, Lapaque N. SCFA: mechanisms and functional importance in the gut. Proc Nutr Soc. 2021;80(1):37–49. doi:10.1017/S0029665120006916
7. National Health Commission of the People’s Republic of China. Diagnosis and treatment plan for COVID-19 (trial version 10). Chin J Clin Infect Dis. 2023;16(1):1–9. doi:10.3760/cma.j.issn.1674-2397.2023.01.001
8. Schultze JL, Aschenbrenner AC. COVID-19 and the human innate immune system. Cell. 2021;184(7):1671–1692. doi:10.1016/j.cell.2021.02.029
9. Chua RL, Lukassen S, Trump S, et al. COVID-19 severity correlates with airway epithelium-immune cell interactions identified by single-cell analysis. Nat Biotechnol. 2020;38(8):970–979. doi:10.1038/s41587-020-0602-4
10. Liao M, Liu Y, Yuan J, et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat Med. 2020;26(6):842–844. doi:10.1038/s41591-020-0901-9
11. Arunachalam PS, Wimmers F, Mok CKP, et al. Systems biological assessment of immunity to mild versus severe COVID-19 infection in humans. Science. 2020;369(6508):1210–1220.
12. Guo Z, Zhang Z, Prajapati M, Li Y. Lymphopenia Caused by Virus Infections and the Mechanisms Beyond. Viruses. 2021;13(9):1876. doi:10.3390/v13091876
13. Priyanka P, Choudhary OP, Singh I. Protective immunity against COVID-19: unravelling the evidences for humoral vs. cellular components. Travel Med Infect Dis. 2021;39:101911. doi:10.1016/j.tmaid.2020.101911
14. Karki R, Kanneganti TD. Innate immunity, cytokine storm, and inflammatory cell death in COVID-19. J Transl Med. 2022;20(1):542. doi:10.1186/s12967-022-03767-z
15. Wang D, Hu B, Hu C, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi:10.1001/jama.2020.1585
16. Datta PK, Liu F, Fischer T, Rappaport J. SARS-CoV-2 pandemic and research gaps: understanding SARS-CoV-2 interaction with the ACE2 receptor and implications for therapy. Theranostics. 2020;10(16):7448–7464. doi:10.7150/thno.48076
17. DiPiazza AT, Graham BS, Ruckwardt TJ. T cell immunity to SARS-CoV-2 following natural infection and vaccination. Biochem Biophys Res Commun. 2021;538:211–217. doi:10.1016/j.bbrc.2020.10.060
18. Choudhary OP, Choudhary P, Singh I. India’s COVID-19 vaccination drive: key challenges and resolutions. Lancet Infect Dis. 2021;21(11):1483–1484. doi:10.1016/S1473-3099(21)00567-3
19. Chopra H, Choudhary OP. mRNA vaccines as an armor to combat the infectious diseases. Travel Med Infect Dis. 2023;52:102550. doi:10.1016/j.tmaid.2023.102550
20. Yin J, Zhao Y, Huang F, et al. Immune response and homeostasis mechanism following administration of BBIBP-CorV SARS-CoV-2 inactivated vaccine. Innovation. 2023;4(1):100359. doi:10.1016/j.xinn.2022.100359
21. Fu Z, Liang D, Zhang W, et al. Host protection against Omicron BA.2.2 sublineages by prior vaccination in spring 2022 COVID-19 outbreak in Shanghai. Front Med. 2023:1–14. doi:10.1007/s11684-022-0977-3
22. Robinson ML, Morris CP, Betz JF, et al. Impact of SARS-CoV-2 variants on inpatient clinical outcome. medRxiv. 2022. doi:10.1101/2022.02.02.22270337
23. Yu K, He J, Wu Y, et al. Dysregulated adaptive immune response contributes to severe COVID-19. Cell Res. 2020;30(9):814–816. doi:10.1038/s41422-020-0391-9
24. Wang Y, Liu J, Burrows PD, Wang JY. B Cell Development and Maturation. Adv Exp Med Biol. 2020;1254:1–22. doi:10.1007/978-981-15-3532-1_1
25. Diao B, Wang C, Tan Y, et al. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19). Front Immunol. 2020;11:827. doi:10.3389/fimmu.2020.00827
26. Wang H, Wang Z, Cao W, Wu Q, Yuan Y, Zhang X. Regulatory T cells in COVID-19. Aging Dis. 2021;12(7):1545–1553. doi:10.14336/AD.2021.0709
27. Zhang F, Wan Y, Zuo T, et al. Prolonged Impairment of Short-Chain Fatty Acid and L-Isoleucine Biosynthesis in Gut Microbiome in Patients With COVID-19. Gastroenterology. 2022;162(2):548–561.e4. doi:10.1053/j.gastro.2021.10.013
28. Ren Z, Wang H, Cui G, et al. Alterations in the human oral and gut microbiomes and lipidomics in COVID-19. Gut. 2021;70(7):1253–1265. doi:10.1136/gutjnl-2020-323826
29. Duscha A, Gisevius B, Hirschberg S, et al. Propionic Acid Shapes the Multiple Sclerosis Disease Course by an Immunomodulatory Mechanism. Cell. 2020;180(6):1067–1080.e16. doi:10.1016/j.cell.2020.02.035
30. Yang W, Yu T, Huang X, et al. Intestinal microbiota-derived short-chain fatty acids regulation of immune cell IL-22 production and gut immunity. Nat Commun. 2020;11(1):4457. doi:10.1038/s41467-020-18262-6
31. Brown JA, Sanidad KZ, Lucotti S, et al. Gut microbiota-derived metabolites confer protection against SARS-CoV-2 infection. Gut Microbes. 2022;14(1):2105609. doi:10.1080/19490976.2022.2105609
32. Pascoal LB, Rodrigues PB, Genaro LM, et al. Microbiota-derived short-chain fatty acids do not interfere with SARS-CoV-2 infection of human colonic samples. Gut Microbes. 2021;13(1):1–9. doi:10.1080/19490976.2021.1874740
33. Yao Y, Cai X, Fei W, Ye Y, Zhao M, Zheng C. The role of short-chain fatty acids in immunity, inflammation and metabolism. Crit Rev Food Sci Nutr. 2022;62(1):1–12. doi:10.1080/10408398.2020.185467
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.