Back to Journals » Infection and Drug Resistance » Volume 16
Distribution of ß-Lactamase Genes Among Multidrug-Resistant and Extended-Spectrum ß-Lactamase-Producing Diarrheagenic Escherichia coli from Under-Five Children in Ethiopia
Authors Zenebe T , Eguale T , Desalegn Z, Beshah D , Gebre-Selassie S, Mihret A, Abebe T
Received 28 August 2023
Accepted for publication 26 October 2023
Published 6 November 2023 Volume 2023:16 Pages 7041—7054
DOI https://doi.org/10.2147/IDR.S432743
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Tizazu Zenebe,1,2 Tadesse Eguale,3,4 Zelalem Desalegn,1 Daniel Beshah,5 Solomon Gebre-Selassie,1 Adane Mihret,1,6 Tamrat Abebe1
1Department of Microbiology, Immunology and Parasitology, Addis Ababa University, Addis Ababa, Ethiopia; 2Department of Medical Laboratory Science, Debre Berhan University, Debre Berhan, Ethiopia; 3Aklilu Lemma Institute of Pathobiology, Addis Ababa University, Addis Ababa, Ethiopia; 4Ohio State University, Global One Health LLC, Addis Ababa, Ethiopia; 5Department of Medical Laboratory, Tikur Anbessa Specialized Hospital, Addis Ababa University, Addis Ababa, Ethiopia; 6Bacterial and Viral Disease Research Directorate, Armauer Hansen Research Institute (AHRI), Addis Ababa, Ethiopia
Correspondence: Tizazu Zenebe, Department of Medical Laboratory Science, Debre Berhan University, P.O.Box 445, Debre Berhan, Ethiopia, Tel +251912372837, Email [email protected]
Purpose: Escherichia coli strains that produce extended-spectrum ß-lactamase (ESBL) and carbapenemase are among the major threats to global health. The objective of the present study was to determine the distribution of ß-lactamase genes among multidrug-resistant (MDR) and ESBL-producing Diarrheagenic E. coli (DEC) pathotypes isolated from under-five children in Ethiopia.
Patients and Methods: A cross-sectional study was conducted in Addis Ababa and Debre Berhan, Ethiopia. It was a health-facility-based study and conducted between December 2020 and August 2021. A total of 476 under-five children participated in the study. DEC pathotypes were detected by conventional Polymerase Chain Reaction (PCR) assay. After evaluating the antimicrobial susceptibility profile of the DEC strains by disk diffusion method, confirmation test was done for ESBL and carbapenemase production. ß-lactamase encoding genes were identified from phenotypically ESBLs and carbapenemase positive DEC strains using PCR assay.
Results: In total, 183 DEC pathotypes were isolated from the 476 under-five children. Seventy-nine (43%, 79/183) MDR-DEC pathotypes were identified. MDR was common among enteroaggregative E. coli (EAEC) (58%, 44/76), followed by enterotoxigenic E. coli (ETEC) (44%, 17/39)) and enteroinvasive E. coli (EIEC) (30%, 7/23). Phenotypically, a total of 30 MDR-DEC pathotypes (16.4%, 30/183) were tested positive for ESBLs. Few ETEC (5.1%, 2/39) and EAEC (2.6%, 2/76) were carbapenemase producers. The predominant β-lactamase genes identified was blaTEM (80%, 24/30) followed by blaCTX-M (73%, 22/30), blaSHV (60%, 18/30), blaNDM (13%, 4/30), and blaOXA-48 (13%, 4/30). Majority of the ß-lactamase encoding genes were detected in EAEC (50%) and ETEC (20%). Co-existence of different β-lactamase genes was found in the present study.
Conclusion: The blaTEM, blaCTX-M, blaSHV, blaNDM, and blaOXA-48, that are associated with serious and urgent threats globally, were detected in diarrheagenic E. coli isolates from under-five children in Ethiopia. This study also revealed the coexistence of the β-lactamase genes.
Keywords: Diarrheagenic E. coil, under-five children, ß-lactamase, carbapenemase, ESBL, multidrug resistance, Ethiopia
Introduction
Enterobacterales are Gram-negative bacteria that play a significant role in human disease.1 Among Enterobacterales, diarrheagenic Escherichia coli (DEC) is one of the main etiological agents of diarrheal disease, mainly in children.2 Six common DEC pathotypes: enterotoxigenic E. coli (ETEC), enteropathogenic E. coli (EPEC), Shiga toxin-producing E. coli (STEC), enteroinvasive E. coli (EIEC), enteroaggregative E. coli (EAEC), and diffusely adherent E. coli (DAEC), are associated with diarrhea.2 According to a systematic review of the global causes of mortality due to diarrheal diseases, more than 50% of all diarrheal death in under-five children are caused by EPEC, ETEC, rotavirus and calicivirus.3 The contribution of DEC to acute diarrhea incidence in children is greater than 30% in developing countries.4
The most commonly prescribed antimicrobials for infections caused by Enterobacterales include ß-lactam drugs, aminoglycosides, and fluoroquinolones.5,6 The ß-lactam drugs include penicillin, cephalosporins, carbapenems, and monobactams. In recent years, antimicrobial resistance (AMR) has become one of the top ten threats to health globally.7 AMR creates a huge clinical (high morbidity and mortality) and financial (increased healthcare costs) burden globally.8 Inappropriate use of antimicrobials, poor hygiene and sanitation, and poor infection prevention and control have contributed to AMR occurrence.9 Transmission of AMR traits between bacterial genera or species is mainly mediated by horizontal gene transfer through mobile genetic elements.10,11
Resistance to β-lactams among Enterobacterales occurs through different mechanisms.6 One of the primary resistance mechanisms to ß-lactam drugs in Enterobacterales is through inactivation of the antimicrobials by ß-lactamase.6 Currently, β-lactamase-producing Enterobacterales are the most serious and critical threats to the world.6 Gene mobilization mechanisms mediate the transfer of β-lactamases in Enterobacterales.12 β-lactamases (penicillinases, extended-spectrum cephalosporinase, carbapenemase, and oxacillinase) hydrolyze β-lactam antimicrobials.12 Many ß-lactamases are categorized into different classes based on their amino acid sequence, substrate, inhibitor profile, and variation in their active sites (using serine or require divalent zinc ions) for hydrolysis.12
Extended-spectrum ß-lactamase (ESBL) and carbapenemase are among the β-lactamases associated with serious and urgent threats globally.6,12,13 The most common ESBLs are categorized as class A of the Ambler classification and are capable of conferring resistance to penicillins, cephalosporins, extended-spectrum cephalosporins, and monobactams.12 The common and medically important types of ESBLs include TEM, SHV (sulfhydryl variable), and CTX-M (hydrolytic activity against cefotaxime).14,15 Currently, there are more than 183 TEM types, with the most common TEM-type found in E. coli6 and more than 178 SHV varieties, mainly in Enterobacterales.6,14 Carbapenems have been used to treat ESBL-producing bacteria and cephalosporin-resistant infections.12 Currently, the emergence and spread of carbapenemase-producing E. coli strains is of great concern globally.16 Carbapenemases are β-lactamases that hydrolyze penicillins, cephalosporins, monobactams, and carbapenems.13 Most commonly carbapenemases are grouped as class A (IMI, SME, and KPC), class B (NDM, VIM, IMP, and SIM) and Class D (OXA ß-lactamase).13 The KPC, NDM, and OXA-48 types are predominantly isolated from Enterobacterales, including E. coli in children.17–19
Multidrug-resistant (MDR) is defined as developing acquired resistance to at least one antimicrobial in three or more antimicrobial categories or classes.20 Currently, there are reports of higher number of MDR Gram negative bacteria including members of Enterobacterales such as E. coli strains from clinical specimens.21–23 MDR E. coli strains including DEC pathotypes that produce ESBL and carbapenemase are emerging.24 High-risk groups are those contaminated with ESBL-producing E. coli strains and carbapenemase-producing E. coli strains.12 Understanding the level of the problem in a particular area could help not only for better management of high-risk group patients but also for better infection prevention measures. ESBL-producing Enterobacterales and carbapenemase-producing Enterobacterales are well characterized in different populations, including food handlers,25 from urine, blood, wounds, and sputum of infected individuals26–28 in Ethiopia. However, few studies have characterized β-lactamase genes originating from gastrointestinal sites, regardless of their role in AMR occurrence and spread.10 Moreover, horizontal gene transfer can be enhanced between members of Enterobacterales during inflammatory reactions, like in case of diarrhea.29 Globally, there are limited data on the molecular epidemiology of ß-lactamase genes in DEC pathotypes of children. ß-lactamase genes are not well characterized in either community-acquired or hospital-acquired diarrhea in children in Ethiopia. Moreover, no study has characterized ß-lactamase genes in MDR and ESBL-producing DEC (ESBL-DEC) pathotypes from under-five children in Ethiopia. This study aims to determine the distribution of β-lactamase genes among MDR and ESBL-DEC pathotypes isolated from under-five children in Ethiopia.
Materials and Methods
Study Setting
A cross-sectional study was conducted in Addis Ababa and Debre Berhan, Ethiopia (Figure 1). It was a health facility-based study and conducted between December 2020 and August 2021. Debre Berhan is located 130 km from Addis Ababa (capital city) and found in Amhara administrative region. Recent estimation reported 5.2 million and 95, 000 population for Addis Ababa and Debre Berhan, respectively.30 A total of five health centers (3 from Addis Ababa and 2 from Debre Berhan) were randomly selected for the study. A total of 391 children with diarrhea and 85 children attending a health facility for causes other than diarrhea were enrolled in the study. All the study participants were under-five children and with no antimicrobial treatment history (for the last three weeks). Standard structured questionnaire (S1 data) was used to collect socio-demographic data, including age, sex, occupation, and family income from parents (or guardians) of the study participants.
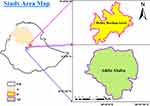 |
Figure 1 Map of the study area, Addis Ababa and Debre Berhan, Ethiopia. Note: Eth, Ethiopian Administration boundary; A, Amhara Regional state; D, Debre Berhan Town; Ad, Addis Ababa city. |
Bacterial Isolation and Identification
As described in detail in our previous work,30 standard bacteriological procedure was followed to isolate and identify the bacterial isolates. Briefly, stool samples collected from study participants in Cary-Blair transport media (Oxoid, UK) were transported (using cold chain) to Microbiology Laboratory. Fecal suspension was prepared and was inoculated onto MacConkey agar (Oxoid Ltd, UK) and incubated at 37 °C for 18–24 hours. A series of biochemical tests were used to identify the bacterial isolates.30,31 Presumptive E. coli isolates were stored at –80 °C in brain heart infusion broth containing 16% (v/v) glycerol until use.
Detection of DEC Pathotypes
Deoxyribonucleic acid (DNA) extraction was done by boiling method, and the concentration and purity of the extracted DNA was assessed by Nanodrop (Thermo Scientific). Each DEC pathotypes were identified based on specific virulence genes using conventional Polymerase Chain Reaction (PCR) assay. The detail procedure for the DNA extraction and PCR-based identification of the DEC pathotypes is described in our previous work.30
Antimicrobial Susceptibility Testing
Disk diffusion method was used to assess the antimicrobial resistance profile of the DEC pathotypes.32 A total of 12 different antimicrobials were evaluated against the DEC isolates.30 The test procedures and the interpretations were based on the Clinical and Laboratory Standards Institute (CLSI) guidelines.32 DEC isolates that were non-susceptible to at least one antimicrobial in three or more class of antimicrobials were defined as MDR bacterial isolates.20 This specific procedure was also described in our previous work.30
Phenotypic Detection of ESBLs and Carbapenemases Production
Isolates resistant to cefotaxime (30 µg) and ceftazidime (30 µg) were tested for ESBL production. Combination disk method and modified carbapenemase inactivation method were used for identifying ESBL and carbapenemase production, respectively.30,32 Control strains used during the test procedure were Klebsiella pneumoniae ATCC 700603 (ESBLs positive), E. coli ATCC 25922 (ESBLs negative), K. pneumoniae ATCC BAA-1705 (carbapenemase-positive), and K. pneumoniae ATCC BAA-1706 (carbapenemase-negative). The detailed procedure is described in our previous work.30
PCR Detection of ß-Lactamase Genes
A conventional PCR assay was conducted to identify the ß-lactamase genes (blaCTX-M, blaTEM, blaSHV, blaKPC, blaNDM, and blaOXA-48) at the Armauer Hansen Research Institute, Addis Ababa, Ethiopia as described previously33,34 with slight modification through optimization. The PCR assays were set in two separate PCR reactions, reaction 1 and reaction 2. Briefly, the first reaction targeted three ESBL genes (blaCTX−M, blaTEM, and blaSHV), and the second reaction targeted three carbapenemase genes (blaKPC, blaNDM, and blaOXA-48) in a separate tube. The sets of specific primers used to detect ESBL genes (blaCTX−M, blaTEM, and blaSHV)35,36 and carbapenemase genes (blaKPC, blaNDM, and blaOXA-48)17,37 are shown in Table 1. Platinum™ II Hot-Start PCR Master Mix (2X) (Thermo Fisher Scientific) was used as PCR master mix in all the PCR reactions. A final reaction volume of 20 µL, containing 10 µL of the PCR master mix, 1.2 µL of primer (each, 0.2 µM), 1.5 µL test DNA (template), 6.1 µL of nuclease-free water, was used in both PCR reactions. Initial denaturation at 94 °C for 15 min was set for the PCR thermal condition. It was followed by a total of 35 cycles of denaturation (92 °C), annealing (60 °C), and extension (72 °), each for 30s. And it had also a final extension of 5 min (at 72 °C). Agarose gel electrophoresis was used to separate the PCR products. UV transilluminator system (Gel Doc, Bio-Rad) was used to visualize the PCR products following the agarose gel electrophoresis procedure. In the agarose gel electrophoresis, 1.7% (w/v) agarose gel Tris Borate ethylene diamine tetra acetic acid (EDTA) buffer (pH 8.2), and ethidium bromide (10μg/mL) were used. Control strains including K. pneumoniae ATCC 700603 (ESBL-positive) and K. pneumoniae ATCC BAA-1705 (carbapenemase-positive) were used as quality control in the PCR assay.
 |
Table 1 Target ß-Lactamase Encoding Genes for PCR Assay and Primer Profiles |
Statistical Analysis
SPSS version 20 software program was used for the analysis of the data. Descriptive statistics (including frequency and proportion) were used. Chi-square test, bivariate and multivariate analyses were performed. For the multivariate logistic regression analysis (final model), independent variables were identified with p < 0.25. A p < 0.05 was considered as statistical significance. The results were interpreted using crude odds ratio (COR) and adjusted odds ratio (AOR).
Results
Characteristics of the Study Population and DEC Pathotypes
Of the total participants, 58% (274/476) were male, and 60% (288/476) of the study participants were in the age range of 25–59 months (Table 2). DEC was detected in 38% (183/476) of study participants. The pathotypes detected in the study were EPEC, ETEC, EIEC, EAEC, STEC, DAEC, and hybrid strains. From the total DEC, 104 (56.8%, 104/183) and 79 (43.2%, 79/183) were found in males and females, respectively. The majority (59.6%, 109/183) of the DEC were detected in children aged 25–59 months, followed by 13–24 months (23.5%, 43/183) and 0–12 months (16.9%, 31/183). Of the total DEC detected, 76 (41.5%), and 107 (58.5%, 107/183) were from Debre Berhan and Addis Ababa, respectively.
 |
Table 2 Factors Associated with ESBLs Producing DEC Pathotype in Under-Five Children in Addis Ababa and Debre Berhan, Ethiopia |
Antibiogram of DEC Pathotypes
Two to seven DEC pathotypes were resistant to various antimicrobials (Figure 2). As it can be seen in Figure 2 all antimicrobials (except carbapenems) tested in the present study were associated with four or more DEC pathotypes. DEC isolates were resistant to ampicillin (95%), tetracycline (91%), gentamicin (28%), trimethoprim-sulfamethoxazole (42%), and chloramphenicol (26%). Significant proportions of DEC isolates were resistant to ciprofloxacin (15%), ceftazidime (16%), cefotaxime (16%), cefepime (4%), meropenem, and ertapenem (2%).
Ampicillin and tetracycline resistance were high among DEC strains from children with- and without diarrhea from both study areas, Addis Ababa and Debre Berhan (Table 3). Seventy-nine (43%) of the DEC pathotypes were MDR (Table 4). MDR to three or more antimicrobials was commonly detected in EAEC (58%, 44/76), ETEC (44%, 17/39), and EIEC (30%, 7/23) pathotypes. Rate of MDR among isolates from Addis Ababa and Debre Berhan, and children with- and without diarrhea were not statistically different (p > 0.05).
 |
Table 4 Resistance Antibiogram of DEC Pathotypes Isolated from Under-Five Children in Debre Berhan and Addis Ababa, Ethiopia |
Phenotypic ESBL and Carbapenemase Production
All the ESBL-DEC and carbapenemase-producing DEC strains were MDR. The proportion of ESBL-DEC was 38% (30/79) of total MDR-DEC strains. Carbapenemase-producing DEC pathotypes were 2.2% (4/183). All the three STEC isolates (100%), 19.7% (15/76) EAEC, 15.3% (6/39) ETEC, 10.7% (3/28) EPEC, 8.7% (2/23) EIEC, and 7.7% EPEC/EAEC (1/13, a hybrid strain) were ESBL producers. Except for two ETECs (5.1%) and two EAECs (2.6%), carbapenemase production was not observed in any of the other DEC pathotypes. Sample image of ESBL-producing strains is available in S1 Figure. Phenotypically, 67% (20/30) of the ESBL-DEC strains were from Addis Ababa and the remaining 33% (10/30) were from Debre Berhan. Carbapenemase-producing DEC strains were not detected in Debre Berhan. Aged 25–59 months, mother/guardian being self-employed, and others (farming, doing paid work, and non-regular businesses) were associated with the acquisition of ESBL-DEC pathotypes. DEC isolated from children aged 25–59 months were more likely (AOR = 2.827, CI = 1.576, 5.071) to be ESBL-producing than those isolated from children aged <12 months. Children whose mothers or guardians were self-employed (AOR = 2.159, CI = 1.018, 4.579) and others (farming, paid work, and small businesses) (AOR = 1.769, CI = 1.07, 2.922) were twice as likely to get ESBL-DEC pathotypes.
Distribution of ß-Lactamase Genes
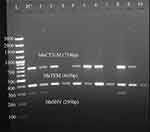
ß-lactamase genes were detected in all phenotypically ESBL-DEC strains. The five common ß-lactamase genes tested in the present study, blaCTX-M, blaTEM, and blaSHV, blaNDM and blaOXA−48, were detected among the ESBL-DEC strains. The predominant ß-lactamase gene was blaTEM (80%, 24/30), followed by blaCTX-M (73%, 22/30), and blaSHV (60%, 18/30) of ESBL-DEC pathotypes (Table 5). All the three common ß-lactamase genes were detected in 33% (10/30) of ESBL-DEC strains in combination (S1 Table). The other ß-lactamase genes detected in the present study were blaNDM (13%, 4/30) and blaOXA-48 (13%, 4/30). Fifty percent (15/30) of ß-lactamase genes were detected in EAEC, 20% (6/30) in ETEC, 10% (3/30) in EPEC, 10% (3/30) in STEC, 6.7% (2/30) in EIEC, and 3.3% (1/30) in EPEC/EAEC DEC strains (Table 5). Nearly 67% (20/30) of ß-lactamase genes were detected in DEC pathotypes isolated from Addis Ababa, whereas 33% (10/30) were from DEC pathotypes isolated from Debre Berhan. All the two carbapenemase encoding genes, blaNDM and blaOXA-48, co-existed among 13.3% of ESBL-DEC pathotypes (4/30). In the present study, blaKPC was not detected in any of the tested DEC pathotypes. Sample image of gel electrophoresis for PCR β-lactamase gene products is presented in Figure 3 and is also available in S2 Figure.
 |
Table 5 Distribution of ß-Lactamase Genes Among DEC Isolates from Under-Five Children in Addis Ababa and Debre Berhan, Ethiopia |
Discussion
Diarrhea and pneumonia are among the common childhood diseases in Ethiopia.38 The diarrheal burden is higher in under-five children39 and with a significant mortality rate40 in the country. There is 63.8% of an overall prevalence of antimicrobial use, and most treatments are empiric (96.7%) in Ethiopia.41 Periodic antibiogram profiling of pathogenic bacteria in such areas could help patient management decisions and outcomes. In a country with high prevalence of diarrheal disease, characterizing the distribution of β-lactamase genes in DEC strains will contribute to inform policies and monitor impact of local and global control strategies.
A previous study reported a large proportion of ampicillin (85%) and tetracycline (67%)-resistant enteric pathogens in Ethiopia.42 In line with this, large proportions of ampicillin-resistant (95%) and tetracycline-resistant (91%) DEC strains were found in the present study. Most antimicrobials (98%) prescribed in Ethiopia are from the national essential medicines list43 and almost all (except tetracycline) the antimicrobials tested in the present study were among these lists. In the present study, a low resistance rate to Amoxicillin-Clavulanate, third- and fourth-generation cephalosporins, and carbapenems was observed. However, the presence of strains resistant to third- and fourth-generation cephalosporin and carbapenem in the present study could alert health personnel to timely measures. The Ethiopian essential medicine list grouped the 3rd-generation cephalosporin and carbapenems as the watch and reserve group antimicrobials, respectively.44 Resistance to both groups of antimicrobials could lead to the death of the patient or no treatment options. Thus, protection and prioritization from the misuse of these drugs must be given attention at the right time. The antimicrobial resistance rate did not differ between Addis Ababa and Debre Berhan (p > 0.05) for all the antimicrobials tested in this study. This could be due to the inappropriate use of antimicrobials without significant differences between urban and rural communities in Ethiopia.45
The human gut serves as a major conduit for the development and environmental spread of MDR organisms.46 A recent study conducted in Ethiopia42 and other area23 reported a higher rate of MDR (≥71%) among Gram-negative bacterial pathogens. The MDR rate in the present study was 43%, which was lower than report from Iran (78.1%)47 and China (66.7%).48 This inconsistency may be due to differences in contributing factors, variations in DEC pathotypes, and geographical variations. However, some studies have reported MDR-DEC strains,49,50 which is in agreement with the present study. A higher rate of MDR was observed in EAEC (58%), ETEC (44%), and EIEC (30%) in this study. Abbasi et al47 also reported high rates of MDR in EAEC (82%), ETEC (67%) and EIEC from central Iran (100%). The leading cause of travellers’ diarrhea is ETEC followed by EAEC; EAEC can also cause severe chronic diarrhea.2 EIEC is also associated with shigellosis.2 Antimicrobial treatment is required when infections associated with EIEC, ETEC, and EAEC are severe or prolonged.2 However, the present findings showed that EIEC, ETEC, and EAEC developed resistance to the most commonly prescribed antimicrobials, which could be a challenge in treating resistant strains in the study area.
Globally, ESBL- and carbapenemase-producing Enterobacterales are considered as a serious threat to health.46 Prevalence of ESBL (17.1–67.3%) - and Carbapenemase-producing Enterobacterales (2.4–7.7%) were reported in Ethiopia.25,28,51 However, there have been no reports of ESBL-DEC and carbapenemase-producing DEC strains in Ethiopia. The present study identified 16.4% ESBL- and 2.2% carbapenemase-producing DEC strains. The presence of ESBL-DEC strains in the gut may result not only in high dissemination of the resistant trait to non-resistant bacterial strains10 but will also be a problem in patient management. Bacterial infections with ESBL-producing Gram-negative bacteria are associated with high mortality rates in Ethiopia.52 Intervention is needed for timely control of the spread of resistant strains.
ESBL-DEC strains differed between children with and without diarrhea in the present study. This difference could be due to the small sample size of non-diarrheic children participated in the study compared with diarrheic children, which is a limitation of the present study. Inflammatory reaction occurred in the gut (e.g. diarrhea) that could mediate gene transfer among bacterial strains could explain the higher ESBL-DEC strains in diarrheic children.29 Mandal et al reported the prevalence of ESBLs in ETEC (18.32%), EPEC (10.9%), EAEC (6.8%), and EIEC (1.57%) among diarrheic children in India.53 In the present study, the prevalence of ESBLs in the ETEC, EPEC, and EIEC groups was consistent with Mandal et al report.53 Among the DEC pathotypes, EAEC (19.7%) and ETEC (15.3%) showed a high rate of ESBLs compared to other pathotypes (p < 0.001) in the present study. This high rate could be associated with their high prevalence in the study area compared with other pathotypes. Since EAEC is a commonly emerging pathogen, and ETEC is most prevalent in low-income countries,2 the presence of resistant strains of both pathogens requires attention. Regular assessment of antimicrobial resistance profile may help to treat infections caused by EAEC and ETEC in a given area.
The association of socio-demographic factors with phenotypic ESBL-producing bacterial strains varies among different studies.51,54,55 Lower educational level (mothers) and drinking tap water (children) were associated with ESBL-producing bacterial strains in previous study done in Ethiopia.51 According to the present findings, children at the age range of 25 −59 months were less likely to develop ESBL-DEC than those aged <1 year, which is consistent with another report.55 However, those with age greater than or equal to one year was found significantly associated with ESBL-producing Enterobacterales in other study.54 This discrepancy may be due to other factors included in the analysis. Additionally, children whose mothers/guardians were self-employed or engaged in other occupations (farming, paid work, and non-regular businesses) were more likely to be positive for ESBL-DEC. Exposure of farmers to animals and workers in non-regular settings (high workload or low income) may contribute to the acquisition of ESBL-DEC compared to employed mothers or caretakers who have less exposure.
Currently, E. coli strains carrying ß-lactamase-encoding genes including blaCTX-M, blaSHV, and blaTEM are distributed in human, animal, food and the environment.56 These common ß-lactamase-encoding genes have also been reported from E. coli isolates in sepsis patients in Ethiopia.27,28 In agreement with these reports, the present study also found β-lactamase-encoding genes (blaCTX-M, blaSHV, and blaTEM) in DEC pathotypes. The β-lactamase-encoding genes detected in the present study were blaTEM (80%), blaCTX-M (73%), and blaSHV (60%). In India, 86.1% blaCTX-M, 68% blaSHV, and 52% blaTEM were detected in children with diarrhea53 which is in-line with the present study. The findings from other studies conducted in Iran47 and Ghana57 are also in line with those of the present study. However, Monira et al58 reported 39% blaCTX-M, 26% blaTEM, and 12% blaSHV in children from Bangladesh. The higher prevalence of β-lactamase genes in the present study may be due to difference in risk factors contributing to emergence of such genes. This study revealed the presence of DEC strains carrying ß-lactamase-encoding genes that circulate in the community and could be a threat to effective patient management.
In the present study, the predominant β-lactamase was blaTEM which is contrary to other reports in Ethiopia from Enterobacterales in patients with sepsis27,28 where blaCTX-M is more prevalent. blaCTX-M has also been reported as the predominant ß-lactamase in extraintestinal invasive E. coli in Ethiopia.59 This discrepancy may be due to differences in the types of bacterial strains and cases involved in the analysis. In a study conducted in Iran among under-five children60 and in Ghana on diarrheic patients,47 blaTEM was found to be the predominant β-lactamase gene. EAEC was the predominant MDR strain with a higher prevalence of blaTEM in the present study. The DEC pathotypes may have contributed to the epidemiology of common β-lactamase genes. Except TEM-1 and TEM-2 variants, all blaTEM are ESBLs and show activity against cefotaxime.14 It could contribute not only to the patient management problem but also to the spread of resistant traits to other strains that could result in no drugs for use.44
In the present study, blaTEM was detected in 83% (5/6) ETEC, 73% (13/15) EAEC, 67% (2/3) STEC, in all EPEC (3/3), EIEC (2/2), and EPEC/EAECs (1/1). This finding is in agreement with a study conducted in Iran47 in which blaTEM was detected in all EAEC (9/9), EPEC (5/5), ETEC (2/2), and EIEC (1/1) isolates. blaCTX-M from EPEC (0/3) and EIEC (0/2), and blaTEM from EPEC/EAEC (0/1) were not detected in the present study. blaSHV was detected from ETEC (2/6) and EIEC (2/2) in the present study, in contrast to the report by Abbasi et al,47 whereas blaSHV was not detected from EIEC and ETEC. This discrepancy may be due to variations in the acquisition of antimicrobial resistance determinants at different selection pressures.61
In Norway, data from 2007 to 2014 show an increasing number of carbapenemase-producing Enterobacterales cases yearly.62 Recent data showed an increased detection of carbapenemase-producing Gram negative bacteria from clinical isolates in Ethiopia.63,64 A systematic review and meta-analysis showed 5.4% pooled prevalence of carbapenemase-producing Enterobacterales, with high prevalence in Central Ethiopia.65 Recent data reports blaKPC, blaNDM, and blaOXA-48 from clinical isolates of Enterobacterales in Ethiopia.66,67 Carbapenemase encoding genes, blaNDM, and blaOXA−48 are becoming the dominant carbapenemase variants in E. coli strains.62 In another study, carbapenemase genes, including blaNDM-1, were identified in the DEC strains.68 Carbapenemase-producing DEC, blaOXA-48 (13%), and blaNDM (13%) were found in the present study. Carbapenemase blaOXA-48 reported in different studies included 31% in Egypt,69 57% in Burkina Faso,70 29% in Kenya,71 and 33% in Uganda.18 The higher prevalence in these studies compared to the present study may be due to the source of the samples and geographical differences. In studies conducted in China48 and Egypt,69 blaNDM has been detected in DEC isolated from children. Both blaOXA-48 and blaNDM hydrolyze penicillin and carbapenem, while blaNDM hydrolyzes cephalosporins and extended-spectrum cephalosporins.12 The presence of carbapenemase-producing strains in the present study could indicate problems that lead to a lack of drugs in the reserve antimicrobial group for use.44
In the present study, 33% of the DEC pathotypes contained all three common ß-lactamase encoding genes (blaCTX-M, blaSHV, and blaTEM) in combination (co-occurrence), which as reported in Nigeria.72 Co-occurrence of carbapenemase genes was also identified in this study. The carbapenemase encoding genes, blaNDM−1 and blaOXA−48 were isolated from clinical isolates of Klebsiella pneumonia (48%) in Egypt.17 The co-occurrence of β-lactamase genes in a single strain showed the incidence of MDR-DEC strains, rate of dissemination of resistant determinants, and emergence of resistant strains in the study area. Mobile genetic elements such as plasmid and integrons11,73 could play vital roles in the spread of the resistance traits and the occurrence of MDR. Ssekatawa et al18 reported 14.8% blaKPC in DEC and extra-intestinal pathogenic E. coli in Uganda. Dembele et al also reported70 low prevalence of blaKPC64 in EAEC and aEPEC. In the present study, blaKPC was not found in DEC strains isolated from under-five children. Prolonged hospital stay, invasive devices, lack of immune-competency, history of antimicrobial therapy could contribute to the acquisition of KPC-producing bacteria.74 The presence of these risk factors or exposure to them will determine the epidemiology of KPC-related infections,74 and thereby dissemination or spread of blaKPC among bacterial strains. In a study done by Han et al, the prevalence of blaKPC among Enterobacterales varied among strains and it was higher in K. pneumoniae (64.5%) but lower in E. coli (2.7%).19 Thus, the lack of blaKPC in the present study may be due to differences in bacterial strains, types of cases, and risk factor exposures that contribute to selection pressure.
Conclusions
DEC with a multidrug resistance profile and ß-lactamase-encoding genes (blaCTX-M, blaSHV, and blaTEM, blaNDM-1 and blaOXA-48) that are associated with serious and urgent global threats were found in under-five children in Ethiopia. The coexistence of β-lactamase genes suggests the severity of this problem. The presence of MDR ß-lactamase, including carbapenemase-producing DEC, in the gut of under-five children could reveal the presence of risk for emergence of MDR bacterial strains in the area. Therefore, there is a need for the timely control of the dissemination of such resistant strains. Antibiogram profile-based drug prescriptions and health facility-based surveillance could help manage and control ESBL- and carbapenemase-producing DEC-related infections.
Abbreviations
AMR, Antimicrobial resistance; ATCC, American Type Culture Collection; CLSI, Clinical and Laboratory Standards Institute guidelines; DAEC, diffusely adherent E. coli; DEC, Diarrheagenic E. coli; DNA, Deoxyribonucleic acid; EAEC, Enteroaggregative E. coli; EIEC, Enteroinvasive E. coli; EPEC, Enteropathogenic E. coli; ESBL-DEC, ESBL-producing DEC; ESBLs, extended-spectrum ß-lactamase; MDR, Multidrug-resistant; PCR, polymerase-chain reaction; STEC, Shiga toxin-producing E. coli.
Data Sharing Statement
All the information is presented in the main manuscript, and there are no remaining data or materials.
Ethical Approval and Informed Consent
The study was approved at institutional and national level, by Institutional Review Board of College of Health Science, Addis Ababa University (PN_025/20/DMIP), and Ethiopian National Research Review Committee (Ref.No_RED/1.14/9428/21), respectively. Informed consent (verbal and written) was obtained from parents (or guardians). The study was conducted in accordance with the Declaration of Helsinki.
Acknowledgments
We thank our colleagues in Bacteriology and Molecular Biology Laboratory of Armauer Hansen Research Institute for their significant collaboration for the laboratory investigations. We also thank Addis Ababa University for their support.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agreed to be accountable for all aspects of the work.
Funding
This work was funded by Addis Ababa University with grant number VPRTTPY-0402018.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Kang E, Crouse A, Chevallier L, Pontier SM, Alzahrani A, Silué N. Enterobacteria and host resistance to infection. Mammal Geno. 2018;29(7):558–576. doi:10.1007/s00335-018-9749-4
2. Croxen MA, Law RJ, Scholz R, Keeney KM, Wlodarska M, Finlay BB. Recent advances in understanding enteric pathogenic Escherichia coli. Clin Microbiol Rev. 2013;26(4):822–880. doi:10.1128/CMR.00022-13
3. Lanata CF, Fischer-Walker CL, Olascoaga AC, Torres CX, Aryee MJ, Black RE. Global causes of diarrheal disease mortality in children, <5 years of age: a systematic review. PLoS One. 2013;8(9):e72788. doi:10.1371/journal.pone.0072788
4. Miliwebsky E, Schelotto F, Varela G, Luz D, Chinen I, Piazza RMF. Human diarrheal infections: diagnosis of diarrheagenic Escherichia coli pathotypes. In: Torres AG, editor. Escherichia Coli in the Americas. Switzerland: Springer International Publishing; 2016:343–369.
5. Delgado-Valverde M, Sojo-Dorado J, Pascual A, et al. Clinical management of infections caused by multidrug-resistant Enterobacteriaceae. Ther Adv Infect Dis. 2013;1(2):49–69. doi:10.1177/2049936113476284
6. Bush K, Bradford PA. Epidemiology of beta Lactamase-Producing Pathogens. Clin Microbiol Rev. 2020;33(2):e00047–19. doi:10.1128/CMR.00047-19
7. World Health Organization. Ten Threats to Global Health in 2019. World Health Organization 2019; 2019. Available from: https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019.
8. Prestinaci F, Pezzotti P, Pantosti A. Antimicrobial resistance: a global multifaceted phenomenon. Pathog Glob Health. 2015;109(7):309–318. doi:10.1179/2047773215Y.0000000030
9. World Health Organization. World Health Organization/ Antimicrobial Resistance; 2021 Available from: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance.
10. Casals-Pascual C, Vergara A, Vila J. Intestinal microbiota and antibiotic resistance: perspectives and solutions. Hum Micro J. 2018;9(11):11–15. doi:10.1016/j.humic.2018.05.002
11. Uddina TM, Chakrabortya AJ, Khusrob A, et al. Antibiotic resistance in microbes: history, mechanisms, therapeutic strategies and future prospects. J Infect Public Health. 2021;14(12):1750–1766. doi:10.1016/j.jiph.2021.10.020
12. Bush K. Past and Present Perspectives on -Lactamases. Antimicrob Agents Chemother. 2018;62(10):e01076–18. doi:10.1128/AAC.01076-18
13. Queenan AM, Bush K. Carbapenemases: the Versatile -Lactamases. Clin Microbiol Rev. 2007;20(3):440–458. doi:10.1128/CMR.00001-07
14. Ghafourian S, Sadeghifard N, Soheili S, Sekawi Z. Extended spectrum beta-lactamases: definition, classification and epidemiology. Curr Issues Mol Biol. 2015;17:11–22.
15. Shaikh S, Fatima J, Shakil S, Rizvi SM, Kamal MA. Antibiotic resistance and extended spectrum beta-lactamases: types, epidemiology and treatment. Saudi J Biol Sci. 2015;22(1):90–101. doi:10.1016/j.sjbs.2014.08.002
16. Liang W-J, Liu H-Y, Duan G-C, et al. Emergence and mechanism of carbapenem-resistant Escherichia coli in Henan, China, 2014. J Infect Public Health. 2018;11(3):347–351. doi:10.1016/j.jiph.2017.09.020
17. El-Domany RA, El-Banna T, Sonbol F, Abu-Sayedahmed SH. Co-existence of NDM-1 and OXA-48 genes in Carbapenem Resistant Klebsiella pneumoniae clinical isolates in Kafrelsheikh, Egypt. Afr Health Sci. 2021;21(2):489–496. doi:10.4314/ahs.v21i2.2
18. Ssekatawa K, Byarugaba DK, Nakavuma JL, et al. Carbapenem resistance profile pathogenic Escherichia coli in Uganda. Research Square. 2020.
19. Han R, Shi Q, Wu S, et al. Dissemination of Carbapenemases (KPC, NDM, OXA-48, IMP, and VIM) among carbapenem-resistant Enterobacteriaceae isolated from adult and children patients in China. Front Cell Infect Microbiol. 2020;10(314). doi:10.3389/fcimb.2020.00314
20. Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–281. doi:10.1111/j.1469-0691.2011.03570.x
21. Alemayehu T. Prevalence of multidrug-resistant bacteria in Ethiopia: a systematic review and meta-analysis. J Glob Anti Resist. 2021;26:133–139. doi:10.1016/j.jgar.2021.05.017
22. Mirzaei B, Babaei R, Bazgir ZN, Goli HR, Keshavarzi S, Amiri E. Prevalence of Enterobacteriaceae spp. and its multidrug-resistant rates in clinical isolates: a two-center cross-sectional study. Mol Biol Rep. 2021;48(1):665–675. doi:10.1007/s11033-020-06114-x
23. Sadeghi H, Khoei SG, Bakht M, et al. A retrospective cross-sectional survey on nosocomial bacterial infections and their antimicrobial susceptibility patterns in hospitalized patients in northwest of Iran. BMC Res Notes. 2021;14(1):88. doi:10.1186/s13104-021-05503-0
24. Chellapandi K, Dutta TK, Sharma I, Mandal SD, Kumar NS, Ralte L. Prevalence of multi drug resistant enteropathogenic and enteroinvasive Escherichia coli isolated from children with and without diarrhea in Northeast Indian population. Ann Clin Microbiol Antimicrob. 2017;16(1):49. doi:10.1186/s12941-017-0225-x
25. Amare A, Eshetie S, Kasew D, Moges F, Algammal AM. High prevalence of fecal carriage of Extended-spectrum beta-lactamase and carbapenemase-producing Enterobacteriaceae among food handlers at the University of Gondar, Northwest Ethiopia. PLoS One. 2022;17(3):e0264818. doi:10.1371/journal.pone.0264818
26. Tadesse S, Mulu W, Genet C, Kibret M, Belete MA, Mascellino MT. Emergence of high prevalence of extended-spectrum beta lactamase and carbapenemase-producing Enterobacteriaceae species among patients in Northwestern Ethiopia Region. Biomed Res Int. 2022;2022:1–9. doi:10.1155/2022/5727638
27. Legese MH, Asrat D, Aseffa A, Swedberg G, Mihret A, Swedberg G. Molecular epidemiology of extended-spectrum beta-lactamase and AmpC producing Enterobacteriaceae among sepsis patients in Ethiopia: a prospective multicenter study. Antibiotics. 2022;11(131):131. doi:10.3390/antibiotics11020131
28. Seman A, Mihret A, Sebre S, et al. Prevalence and molecular characterization of extended spectrum β-lactamase and carbapenemase-producing Enterobacteriaceae isolates from bloodstream infection suspected patients in Addis Ababa, Ethiopia. Infect Drug Resist. 2022;15:1367–1382. doi:10.2147/IDR.S349566
29. Stechera B, Denzlera R, Maiera L, et al. Gut inflammation can boost horizontal gene transfer between pathogenic and commensal Enterobacteriaceae. PNAS. 2012;109(4):1269–1274. doi:10.1073/pnas.1113246109
30. Zelelie TZ, Eguale T, Yitayew B, et al. Molecular epidemiology and antimicrobial susceptibility of diarrheagenic Escherichia coli isolated from children under age five with and without diarrhea in Central Ethiopia. PLoS One. 2023;18(7):e0288517. doi:10.1371/journal.pone.0288517
31. Cheesbrough M. District Laboratory Practice in Tropical Countries.
32. CLSI. Clinical and Laboratory Standards Institute Antimicrobial Susceptibility Testing Standards M02, M07, and M11.
33. Mohammed Y, Gadzama GB, Zailani SB, Aboderin AO. Characterization of ExtendedSpectrum Beta-lactamase from Escherichia coli and Klebsiella Species from North Eastern Nigeria. J Clin Diagno Res. 2016;10(2):DC07–DC10. doi:10.7860/JCDR/2016/16330.7254
34. Poirela L, Walshb TR, Cuvilliera V, Nordmann P. Multiplex PCR for detection of acquired carbapenemase genes. Diagn Microbiol Infect Dis. 2011;70(1):119–123. doi:10.1016/j.diagmicrobio.2010.12.002
35. Ramachandran A, Shanthi M, Sekar U. Detection of blaCTX-M extended spectrum beta-lactamase producing salmonella enterica serotype Typhi in a Tertiary care centre. J Clin Diagno Res. 2017;11(9):DC21–DC4. doi:10.7860/JCDR/2017/30150.10637
36. Ungo-Kore H, Bulus M, Nuhu T, et al. Genotypic detection of extended spectrum beta lactamases from selected bacterial isolates in the specialist hospital Sokoto, Nigeria. J Appl Sci Environ Manage. 2019;23(8):1573–1577.
37. Ecea G, Tunc E, Otlu B, Aslan D, Ece C. Detection of blaOXA-48 and clonal relationship in carbapenem resistant K. pneumoniae isolates at a tertiary care center in Western Turkey. J Infect Public Health. 2018;11(5):640–642. doi:10.1016/j.jiph.2018.04.003
38. IVAC. pneumonia & Diarrhea Progress Report 2020. International Vaccine Access Center (IVAC). 2020. Available from: https://resourcecentre.savethechildren.net/pdf/ivac_pdpr_2020.pdf/.
39. Wolde D, Tilahun GA, Kotiso KS, Medhin G, Eguale T. The burden of diarrheal diseases and its associated factors among under-five children in Welkite Town: a community based cross-sectional study. Int J Public Health. 2022;67:1604960. doi:10.3389/ijph.2022.1604960
40. Yalew M, Arefaynie M, Bitew G, Amsalu ET, Kefale B. Time to under-five mortality and its predictors in rural Ethiopia: cox-gamma shared frailty model. PLoS One. 2022;17(4):e0266595. doi:10.1371/journal.pone.0266595
41. Fentie AM, Degefaw Y, Asfaw G, et al. Multicentre point- prevalence survey of antibiotic use and healthcare- associated infections in Ethiopian hospitals. BMJ Open. 2022;12(2):e054541. doi:10.1136/bmjopen-2021-054541
42. Beyene AM, Gezachew M, Mengesha D, Yousef A, Gelaw B, Clegg S. Prevalence and drug resistance patterns of Gram-negative enteric bacterial pathogens from diarrheic patients in Ethiopia: a systematic review and meta-analysis. PLoS One. 2022;17(3):e0265271. doi:10.1371/journal.pone.0265271
43. Demoz GT, Kasahun GG, Hagazy K, et al. Prescribing Pattern of Antibiotics Using WHO Prescribing Indicators Among Inpatients in Ethiopia: a Need for Antibiotic Stewardship Program. Infect Drug Resist. 2020;Volume 13:2783–2794. doi:10.2147/IDR.S262104
44. MOH/EFDA. Ethiopian Essential Medicines List, Sixth Edition, September 2020: ministry of Health/Ethiopian Food and Drug Authority; 2020 Available from: http://efmhaca.hcmisonline.org/wp-content/uploads/2020/12/EML-sixth-edition.pdf.
45. Gebeyehu E, Bantie L, Azage M, Ciccozzi M. Inappropriate use of antibiotics and its associated factors among urban and rural communities of Bahir Dar City Administration, Northwest Ethiopia. PLoS One. 2015;10(9):e0138179. doi:10.1371/journal.pone.0138179
46. Wallace MJ, Fishbein RS, Dantas G. Antimicrobial resistance in enteric bacteria: current state and next-generation solutions. Gut Micro. 2020;12(1):e1799654. doi:10.1080/19490976.2020.1799654
47. Abbasi E, Mondanizadeh M, Belkum A, Ghaznavi-Rad E. Multi-drug-resistant diarrheagenic Escherichia coli pathotypes in pediatric patients with gastroenteritis from central Iran. Infect Drug Resist. 2020;13:1387–1396. doi:10.2147/IDR.S247732
48. Zhou Y, Zhu X, Hou H, et al. Characteristics of diarrheagenic Escherichia coli among children under 5 years of age with acute diarrhea: a hospital based study. BMC Infect Dis. 2018;18(63). doi:10.1186/s12879-017-2936-1
49. Sirous M, Hashemzadeh M, Keshtvarz M, et al. Molecular characterization and antimicrobial resistance of enteropathogenic Escherichia coli in Children from Ahvaz, Iran. Jundishapur J Microbiol. 2020;13(7):e100877. doi:10.5812/jjm.100877
50. Shah MS, Shah AA, Eppinger M, Ahmed S, Hameed A, Hasan F. Multidrug-resistant diarrheagenic E. coli pathotypes are associated with ready-to-eat salad and vegetables in Pakistan. J Korean Soc Appl Biol Chem. 2015;58(2):267–273. doi:10.1007/s13765-015-0019-9
51. Tola MA, Abera NA, Gebeyehu YM, Tullu KD, Tullu KD. High prevalence of extended-spectrum beta lactamase-producing Escherichia coli and Klebsiella pneumoniae fecal carriage among children under five years in Addis Ababa, Ethiopia. PLoS One. 2021;16(10):e0258117. doi:10.1371/journal.pone.0258117
52. Tufa TB, Fuchs A, Tufa TB, et al. High rate of extended-spectrum beta lactamase-producing gram-negative infections and associated mortality in Ethiopia: a systematic review and metaanalysis. Antimicrob Resist Infect Control. 2020;9(128):1.
53. Mandal A, Sengupta A, Kumar A, et al. Molecular epidemiology of extended-spectrum β-Lactamase–producing Escherichia coli pathotypes in diarrheal children from low socioeconomic status communities in Bihar, India: emergence of the CTX-M Type. Infect Dis. 2017;10:1–11.
54. Birgy A, Cohen R, Levy C, Bidet P, Courroux C, Benani M. Community faecal carriage of extended-spectrum beta-lactamase-producing Enterobacteriaceae in French children. BMC Infect Dis. 2012;12(315). doi:10.1186/1471-2334-12-315
55. Tellevik MG, Blomberg B, Kommedal Ø, Maselle SY, Langeland N, Moyo SJ. High prevalence of faecal carriage of ESBLProducing Enterobacteriaceae among children in Dar es Salaam, Tanzania. PLoS One. 2016;11(12):e0168024. doi:10.1371/journal.pone.0168024
56. Bastidas-Caldes C, Romero-Alvarez D, Valdez-Vélez V, et al. Extended-spectrum beta-lactamases producing Escherichia coli in South America: a systematic review with a one health perspective. Infect Drug Resist. 2022;15:5759–5779. doi:10.2147/IDR.S371845
57. Dela H, Egyir B, Majekodunmi AO, et al. Diarrhoeagenic E. coli occurrence and antimicrobial resistance of Extended Spectrum Beta-Lactamases isolated from diarrhoea patients attending health facilities in Accra, Ghana. PLoS One. 2022;17(5):e0268991. doi:10.1371/journal.pone.0268991
58. Monira S, Shabnam SA, Ali SI, et al. Multi-drug resistant pathogenic bacteria in the gut of young children in Bangladesh. Gut Pathog. 2017;9(19):1–8. doi:10.1186/s13099-017-0170-4
59. Negeri AA, Mamo H, Gurung JM, et al. Antimicrobial resistance profiling and molecular epidemiological analysis of extended spectrum β-Lactamases produced by extraintestinal invasive Escherichia coli isolates from Ethiopia: the presence of international high-risk clones ST131 and ST410 Revealed. Front Microbiol. 2021;12(706846). doi:10.3389/fmicb.2021.706846
60. Amin M, Sirous M, Javaherizadeh H, et al. Antibiotic resistance pattern and molecular characterization of extended-spectrum β-lactamase producing enteroaggregative Escherichia coli isolates in children from southwest Iran. Infect Drug Resist. 2022;11:1097–1104. doi:10.2147/IDR.S167271
61. Aslam B, Wang W, Arshad MI, et al. Antibiotic resistance: a rundown of a global crisis. Infect Drug Resist. 2018;11:1645–1658. doi:10.2147/IDR.S173867
62. Samuelsen Ø, Overballe-Petersen S, Bjørnholt JV, et al. Molecular and epidemiological characterization of carbapenemase producing Enterobacteriaceae in Norway, 2007 to 2014. PLoS One. 2017;12(11):e0187832. doi:10.1371/journal.pone.0187832
63. Shibabaw A, Sahle Z, Metaferia Y, et al. Epidemiology and prevention of hospital-acquired carbapenem-resistant Enterobacterales infection in hospitalized patients, Northeast Ethiopia. IJID Region. 2023;7:77–83. doi:10.1016/j.ijregi.2023.02.008
64. Beshah D, Desta AF, Woldemichael GB, et al. High burden of ESBL and carbapenemase producing gram-negative bacteria in bloodstream infection patients at a tertiary care hospital in Addis Ababa, Ethiopia. PLoS One. 2023;18(6):e0287453. doi:10.1371/journal.pone.0287453
65. Alemayehu E, Fiseha T, Gedefie A, et al. Prevalence of carbapenemase-producing Enterobacteriaceae from human clinical samples in Ethiopia: a systematic review and meta-analysis. BMC Infect Dis. 2023;23(1):277. doi:10.1186/s12879-023-08237-5
66. Tekele SG, Teklu DS, Legese MH, et al. Multidrug-resistant and carbapenemase-producing Enterobacteriaceae in Addis Ababa, Ethiopia. Biomed Res Int. 2021;2021:1–10. doi:10.1155/2021/9999638
67. AwokeI T, Teka B, Aseffa A, et al. Detection of blaKPC and blaNDM carbapenemase genes among Klebsiella pneumoniae isolates in Addis Ababa, Ethiopia: dominance of blaNDM. PLoS One. 2022;17(4):e0267657. doi:10.1371/journal.pone.0267657
68. Zhou H, Zhang K, Chen W, et al. Epidemiological characteristics of carbapenem-resistant Enterobacteriaceae collected from 17 hospitals in Nanjing district of China. Antimicrob Resist Infect Control. 2020;9(15). doi:10.1186/s13756-019-0674-4
69. El-Shaer S, Abdel-Rhman SH, Barwa R, Hassan R. Genetic characterization of extendedspectrum β-Lactamase- and carbapenemase producing Escherichia coli isolated from Egyptian hospitals and environments. PLoS One. 2021;16(7):e0255219.
70. Dembele R, Soulama I, Kaboré AD, et al. Molecular characterization of carbapenemaseProducing Escherichia coli and Salmonella in children with diarrhea in rural Burkina Faso. Research Square; 2020.
71. Ndukui JG, Gikunju JK, Aboge GO, Mwanik I, Maina JN, Mbaria JM. Molecular characterization of ESBLs and QnrS producers from selected Enterobacteriaceae strains isolated from commercial poultry production systems in Kiambu County, Kenya. Microbiol Insight. 2022;15:1–8. doi:10.1177/11786361211063619
72. Nwafia IN, Ohanu ME, Ebede SO, Ozumba UC. Molecular detection and antibiotic resistance pattern of extended-spectrum beta-lactamase producing Escherichia coli in a Tertiary Hospital in Enugu, Nigeria. Ann Clin Microbiol Antimicrob. 2019;18(41). doi:10.1186/s12941-019-0342-9
73. Ahmadian L, Haghshenas MR, Mirzaei B, Bazgir ZN, Goli HR. Distribution and molecular characterization of resistance gene cassettes containing class 1 integrons in Multi-Drug Resistant (MDR) clinical isolates of pseudomonas aeruginosa. Infect Drug Resist. 2020;13:2773–2781. doi:10.2147/IDR.S263759
74. Nordmann P, Cuzon G, Naas T. The real threat of Klebsiella pneumoniae carbapenemase producing bacteria. Lancet Infect Dis. 2009;9(4):228–236. doi:10.1016/S1473-3099(09)70054-4
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.