Back to Journals » Infection and Drug Resistance » Volume 17
Disseminated Histoplasmosis Infection Diagnosed by Metagenomic Next-Generation Sequencing: A Case Report
Authors Qiang L, Deng X, Yang Y, Wang Z, Gai W
Received 24 November 2023
Accepted for publication 25 February 2024
Published 7 March 2024 Volume 2024:17 Pages 865—873
DOI https://doi.org/10.2147/IDR.S451564
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Zhi Ruan
Lei Qiang,1 Xianghui Deng,1 Yong Yang,1 Zhigan Wang,2 Wei Gai3
1Department of Critical Care Medicine, The Affiliated Changsha Central Hospital, Hengyang Medical School, University of South China, Changsha, People’s Republic of China; 2Department of Pathology, The Affiliated Changsha Central Hospital, Hengyang Medical School, University of South China, Changsha, People’s Republic of China; 3WillingMed Technology (Beijing) Co., Ltd, Beijing, People’s Republic of China
Correspondence: Xianghui Deng, Department of Critical Care Medicine, The Affiliated Changsha Central Hospital, Hengyang Medical School, University of South China, Changsha, People’s Republic of China, Email [email protected]
Abstract: Histoplasmosis is an endemic disease caused by Histoplasma capsulatum. This systemic disease can affect various organs beyond the lungs, such as the liver, spleen, adrenal gland, and lymph nodes. The clinical symptoms can range from asymptomatic to severe, life-threatening conditions, depending on the state of the patient’s immune system. This report describes a 40-year-old male who presented with reports of weight loss, low back pain, and progressively worsening movement disorder of the bilateral lower extremities for months. Computed tomography (CT) examination showed multiple lytic lesions of vertebral bodies, bilateral ribs, and pelvic bone, histopathological examination and tumor-related serum markers exclude tumors. mNGS was employed to identify H. capsulatum var. capsulatum as the etiological agent of the lesions in the bone biopsy. Through phylogenetic tree analysis, Histoplasma capsulatum var. Capsulatum (Hcc) was the main responsible pathogen, rarely reported in bone lesions. The patient underwent spinal surgery and was successfully treated with liposomal amphotericin B and itraconazole. Based on the diagnosis and treatment of this case, we discuss the epidemiologic status, clinical presentations, diagnostic criteria, and treatment guidelines of histoplasmosis to provide additional information about this disease. mNGS is utilized in this case, and it appears to be a reliable method for early and accurate diagnosis of this disease.
Keywords: metagenomic next generation sequencing, disseminated histoplasmosis, multifocal osteolytic lesions, diagnosis, Histoplasma capsulatum var. capsulatum
Introduction
Histoplasmosis is a systemic infectious disease caused by the fungus Histoplasma capsulatum. Based upon phenotypic traits including host, morphology, and pathogenicity, the genus Histoplasma has been classified into three separate varieties: H. capsulatum var. capsulatum (Hcc), H. capsulatum var. duboisii (Hcd), and H. capsulatum var. farciminosum (Hcf). Recently, through the use of molecular techniques, eight clades have been distinguished: North America clade 1 (Nam1), North America clade 2 (NAm 2), Latin America clade A (LAm A), Latin America clade B (LAm B), Australia, Netherlands, Eurasia, and Africa.1 Histoplasmosis is one of the most prevalent endemic fungal diseases and occurs in all continents in tropical and subtropical areas. Most cases are found in North America and Latin America. Areas around the Ohio and Mississippi River valleys have the highest exposure to H. capsulatum. The endemic region exhibits significant variations in the climate, soil conditions, and patient factors such as HIV infection, tumors, diabetes, and liver disease.2 Recent data show that over one-third of individuals in Central and South America have been exposed to Histoplasma. The highest rates of exposure are found in Central America and northern South America. Tourism and migration have increased the importance of imported histoplasmosis in nonendemic areas, with cases reported from France, Germany, Italy, Spain, and other European countries.3 In China, there is an increase in the number of cases in recent years with improved access to laboratory diagnosis, and the majority of cases have been reported in nine provinces and regions that are located along the Yangtze River.4
Histoplasma exposure can lead to a nonspecific and wide range of clinical presentations.5 Fever, fatigue, weight loss, and anorexia are common symptoms. Therefore, it is prone to be confused with other diseases. While in most cases, it is asymptomatic or mild and self-limiting, less commonly, the infection can lead to progressive disseminated disease, with many organs becoming severely affected. The clinical type known as disseminated histoplasmosis is the most dangerous with a rapid progression and high mortality.6,7 Histoplasmosis should be confirmed based on a compatible clinical scenario and a positive culture or histopathology.8 Due to the lack of specificity in clinical manifestations, many cases are missed or misdiagnosed.9 The atypical histopathology and the low detection rate of the culture make an accurate diagnosis a challenge. Antigen and antibody detection is a fast and highly sensitive method that can also be used to diagnose,10 but antigen tests may show negative results in chronic infection due to the low fungal burden and the sensitivity and specificity of antibody tests are lower in immunocompromised patients.11 Early diagnosis is essential for improving mortality rates from histoplasmosis. Therefore, it’s essential to improve the accessibility of high-quality in vitro diagnostics to facilitate rapid detection. Compared with other laboratory diagnostics, metagenomic next-generation sequencing (mNGS), as a next-generation sequencing technology, can identify all nucleic acids present in a sample that includes the host and microbes by sequencing the total DNA or RNA to identify pathogens. Compared to other diagnostic technologies, the unbiased sampling and shorter detection period made this approach widely used to diagnose infectious diseases in recent years.12
We present a unique case of disseminated histoplasmosis caused by Histoplasma capsulatum var. capsulatum. The patient exhibited multiple osteolytic lesions as clinical symptoms. Early diagnosis was achieved through the use of mNGS.
Case Presentation
In August 2022, a 40-year-old man came to the emergency department with complaints of low back pain and weight loss for eight months, as well as a progressive movement disorder of bilateral lower limbs for a month. The patient was diagnosed with chronic hepatitis B over 20 years ago and did not receive antiviral therapy regularly. In 2017, he was diagnosed with pulmonary tuberculosis. Furthermore, he denied any recent travel, surgery, or trauma history.
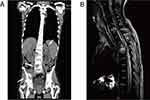
Upon admission, the patient was admitted with an average temperature of 36.3 °C, 20 breaths/min, normal blood pressure of 104/65 mmHg, raised heart rate of 117 beats/min, and normal oxygen saturation of 97% in room air. The physical examination showed the presence of multiple ulcers in his oral mucosa, T1-T8, L1-L5 spinous tenderness, and muscle strength of both lower extremities at grade 1–2. The laboratory tests revealed decreased hemoglobin (93 g/L; normal range, 120–160 g/L) and albumin (34 g/L; normal range, 40–55 g/L), and increased CRP level (28.20 ng/L; normal range, <5.00 ng/L). The tests conducted include HBV-DNA, HCV-RNA, HIV-1 antibody, EB-DNA, CMV-DNA, and anti-tuberculosis antibody returned negative. Peripheral chest and abdominal CT examination showed lytic lesions of C7, T1-5, T8, L2-3, and L5 vertebral bodies, bilateral ribs and pelvic bone, tissue mass shadow, and bilateral adrenal mass, considering the possibility of bone metastases (Figure 1A).
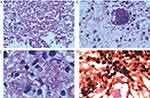
On the third day after admission, the patient developed dyspnea with fluctuant percutaneous SPO2. He was intubated and rapidly admitted to the ICU department, receiving mechanical ventilation. We kept administering anti-infective therapy using imipenem/cilastatin sodium (500 mg every 8 h), the patient developed a fever after admission, and the body temperature fluctuated to 38°C. The cause of the bone lesion in this patient was unknown, and the tumor-related serum markers were all negative. We encountered difficulties in diagnosing and treating the disease. Therefore, a bronchoscopy was conducted, bronchoalveolar lavage fluid (BALF), and blood samples were subjected to cultures and mNGS analysis on day 5 of hospitalization. Cultures were performed by experienced laboratory personnels following standard operating procedures, and mNGS was performed according to the standard protocol of Illumina sequencing on the NextSeq550 platform. We also performed a CT-directed needle biopsy of the pelvic bone lesion, and the tissue was also subjected to mNGS testing. A total of 8846 sequences reads of Histoplasma capsulatum was detected in tissue representing 99.7% of microbial reads and 1.78% of the nucleotide sequence coverage. Additionally, Histoplasma capsulatum was detected in both the blood (4 sequences) and bronchoalveolar lavage fluid (6 sequences). The biopsy of the pelvic bone lesion discovered some phagosomes in the cytoplasm that were suspected to be H. capsulatum. (Figure 2).
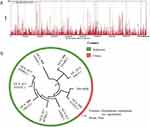
However, because the patient’s bones were badly damaged, we doubted whether it was a new variety of H. capsulatum. Due to the patient’s culture being negative. We were unable to perform the phenotypic identification by traditional methods. A phylogenetic analysis of the pathogen was done. First, we performed quality control on the original data offline to obtain valid data (Clean Data), and then use SPAdes assembly software to conduct assembly analysis to obtain scaffolds. The sequence similarity between the assembled scaffolds and the genomes of the existing target species was calculated, and the analysis results were visualized. Bowtie2 was used to compare clean data to the genome with the highest similarity above and determine the sample species. The similarity matrix results obtained from sequence alignment analysis were converted into a distance matrix. The distance matrix was used as the input file of FastME. The phylogenetic tree was constructed using NJ (adjacency method), and the online software ITOL was used to beautify the phylogenetic tree. The genome of Histoplasma capsulatum (9 strains) in the NCBI database and the country where the corresponding genome submission institution was located was used to construct a phylogenetic tree with the sample assembly sequence of this project. The results showed that the sample from the patient had the closest genetic relationship with H. capsulatum GCA_000313325.1 (Strain: Tmu) from Taiwan, China which was consistent with the genotyping results. The coverage depth map between our sample and the GCA _000313325.1 of the H. capsulatum genome showed consistency, and the genome corresponds to Histoplasma capsulatum var. capsulatum shown in Figure 3.
The clinical diagnosis was disseminated H. capsulatum infection. On the seventh day of hospitalization, we switched from imipenem/cilastatin sodium to an intravenous infusion of amphotericin B colloidal dispersion (200 mg once daily). After six days of antifungal treatment, the patient’s body temperature slowly got back to normal, and his general condition improved (Figure 4). During the third week of hospitalization, he was successfully weaned off the ventilator and transferred to the ward for rehabilitation. Intravenous administration of LAmB was replaced by an itraconazole tablet (200mg twice daily) for antifungal treatment. The patient had recurrent back pain and increased mobility impairment of both lower extremities after being transferred to the rehabilitation ward. A MRI scan shows further bone lesions of the thoracic spine and compression of the spinal cord (Figure 1B). Cervical and thoracic spinal surgery using the posterior approach was performed. He was discharged from our hospital one week after surgery in an improved condition. One month afterward, a follow-up of the patient, which was performed on an outpatient basis, the lesions had not progressed. The patient was pain-free and asymptomatic.
 |
Figure 4 The curve of body temperature, serum (1,3)-β-D-glucan test, and treatment course during hospitalization. |
Discussion
Histoplasmosis is an endemic disease caused by Histoplasma capsulatum. It is typically contracted through the inhalation of its spores in soil that has been contaminated with bird or bat droppings.13 This disease is present globally, but primarily endemic in the Americas, Southeast Asia, Africa, and other parts of the world.5,10 In China, histoplasmosis has been reported in areas where the Yangtze River flows.4 In a survey of histoplasmin skin test reactivity, 735 Chinese volunteers with no history of living abroad were tested, and 8.9% of the healthy volunteers from the Hunan provinces tested positive.14 A review analyzed 300 cases of histoplasmosis that were diagnosed in China, out of all the patients, 27.7% were from Yunnan, while 9.3% came from Jiangsu and Hunan, 8.7% were from Hubei and 7.3% were from Sichuan.4 In this case, no precise epidemiological exposure was found. Eventually, it was revealed that this patient lives in an area with a high reactivity rate of histoplasmin skin test in Hunan Province. Although histoplasmosis is rarely seen in immunocompetent individuals, it should always be included among differential diagnoses in patients presenting with symptoms from endemic regions.15 A careful epidemiological history study would help to clarify the diagnosis earlier.
Histoplasma infection can lead to a nonspecific and wide range of clinical presentations.5 Histoplasma capsulatum var. capsulatum caused infection generally involves the lungs, tongue, palate, and buccal mucosa.16 It can also cause endogenous endophthalmitis and cutaneous nerves17,18 but hardly shows cutaneous or bone lesions. Case reports of bone lesions in the literature have focused on the H. capsulatum var. duboisii (Hcd) which is restricted endemic to sub-Saharan Africa and infections caused by H. capsulatum var. duboisii (Hcd) typically lead to clinical manifestations such as lesions in the skin, lymph nodes, subcutaneous tissues, and bones.19 Previous literature reported that Hcd could cause extensive bone destruction, significantly affecting the skull, ribs, and vertebrae.20 However, we have only found one reported case of Hcc-disseminated infection with multifocal osteolytic lesions.21 Additionally, there have been two anecdotal reports of skeletal involvement with H. capsulatum, which include septic arthritis, osteomyelitis, and carpal tunnel syndrome.22,23 In our case, the patient presents with typical clinical manifestations of Hcd infection, which include oral ulcers, mediastinal and abdominal lymphadenopathy, and extensive lytic lesions of the thoracic and lumbar spine. By phylogenetic analysis, the results showed that the sample has the closest genetic relationship with H. capsulatum GCA_000313325.1 (Strain: Tmu) from Taiwan, China. And the genome corresponds to H. capsulatum var. capsulatum. According to Oladele et al, the symptoms of the disease vary depending on different factors. These factors include the immune status of patients, the amount of fungal particles inhaled (especially for Hcc), and the strength of the infective strain.24 In this case, the cause of osteolytic lesions in our patient remains unclear.
According to the consensus of the European Organization for Research and Treatment of Cancer/ Mycosis Study Group (EORTC ⁄ MSG) in 2008, Histoplasmosis is a disease that is diagnosed when Histoplasma capsulatum is detected in a culture or confirmed through histopathology of bone marrow, blood, or other infected sites. Disseminated histoplasmosis can be diagnosed when Histoplasma capsulatum is confirmed from blood and bone marrow, or the fungus is confirmed from multiple non-contiguous organs in the organism.25 However, Histoplasmosis mold form requires 4–6 weeks to grow in culture, in the literature review of histoplasmosis from 2001 to 2019 in the Chinese mainland, only 8.6% of cases were diagnosed by culture.26 In our case, the patient’s three times cultures were negative. It may delay patient treatment.27 Serology tests can also be used to diagnose.10 After the initial infection, antibodies can be detected within 4 to 8 weeks, therefore, it’s used primarily to diagnose subacute and chronic forms of the disease.11,27 Moreover, antibody titers can remain elevated for months or even years after successful therapy, it may be difficult to distinguish between sub-acute or inactive infections, chronic active forms, and relapses.11 The Histoplasma galactomannan detection is also widely used in diagnosis. The urine and serum samples are commonly used in this test. Compared to traditional methods such as culture, galactomannan testing can provide faster results, which can help with early diagnosis and treatment. However, the test may cross-react with other fungal infections, such as Aspergillus, resulting in false-positive results, and due to the higher cost, this technology is not widely used in resource-limited countries.28 Compared with other laboratory diagnostics, The use of molecular methods provides the benefit of high analytical specificity with a shorter turnaround period. mNGS, as a next-generation sequencing technology, by sequencing the total DNA or RNA, the presence of pathogens can be identified in a specimen that contains both the host and microbes. This method surpasses the constraints of conventional pathogen detection, offering wider coverage and greater sensitivity while being less influenced by past use of antimicrobial medication and the duration of treatment.29 In comparison to culture or clinical diagnosis of histoplasmosis, the sensitivity of molecular assays in published studies has varied from 67% to 100%.30,31 Some studies also showed that the sensitivity of molecular assays is 33 and 87%.32,33 Histoplasmosis can be diagnosed through a combination of clear clinical presentation, positive culture results, or a typical histopathological examination. Due to a lack of specificity in clinical manifestations, patients are prone to be missed or misdiagnosed during their initial visit. In our case, the patient’s typical clinical features are multiple bone lesions in the spine and pelvis. Histoplasma capsulatum-caused spinal infection mimicked Pott’s disease,34 and the bone lesion may be misdiagnosed as a metastatic spinal tumor.35 Misdiagnosed and overlooked cases can result in insufficient treatment and ultimately lead to patient fatalities. Clinicians in non-endemic regions may lack adequate knowledge of histoplasmosis, leading to underdiagnosis or misdiagnosis. In such circumstances, mNGS can serve as a supplementary diagnostic tool, enhancing the accuracy of the diagnosis. Despite its benefits, mNGS has certain limitations. For instance, samples can be contaminated by environmental organisms, which may cause inaccurate outcomes. And the detection ability of fungi with cell walls and intracellular bacteria is relatively low. In addition, the approach used does not provide clear proof of the connection between pathogens and the advancement of disease, and it’s unable to identify clinical infection and colonization status.36,37 Moreover, the absence of standards for experimental protocols and data interpretation is a considerable challenge and requires collaboration among clinicians, clinical microbiologists, and medical administration.38 Currently, mNGS testing should be a supplement but not a replacement for traditional microbiological testing methods in diagnosing infectious diseases.
The most characteristic features of disseminated histoplasmosis are rapid progression and aggressiveness, emphasizing the need for prompt and efficient medical care. Based on guidelines from the Infectious Diseases Society of America, for moderately severe to severe infection, it is recommended to use liposomal amphotericin B at a dosage of 3.0 mg/kg per day for 1–2 weeks. This should be followed by oral itraconazole 200 mg twice daily for a minimum of 12 months.23 In this case, our patient received a daily dose of 200mg of amphotericin B colloidal dispersion as a treatment after the disease was diagnosed, and his body temperature slowly got back to normal after one week. We monitored the serum (1,3)-β-D-glucan (BDG) level during antifungal therapy and noted changes over time (Figure 4). The indices of serum BDG significantly increased after switching to itraconazole given orally, we suspect that the blood level of itraconazole did not reach the target in a short time, resulting in aggravated infection. As the itraconazole administration continued, the BDG level gradually decreased, the patient’s general condition improved, and the treatment was considered effective. Based on the guidelines, it is recommended to acquire the blood levels of itraconazole to ensure sufficient drug exposure,23 monitoring the BDG level simultaneously may assist in customizing medication for the unique requirements of severe patients, but it still needs further study.
Conclusion
In conclusion, this case report describes an atypical presentation of H. capsulatum var. capsulatum infection with multiple osteolytic lesions in a male by utilizing mNGS for early diagnosis. Our understanding of the distribution of H. capsulatum var. capsulatum in China is still incomplete. The mechanism by which H. capsulatum causes disease has not yet been fully elucidated. mNGS appears to be a reliable method for early and precise diagnosis made through clinical symptoms. Increased disease awareness and availability of diagnostic tests in non-endemic areas will help us redraw the map of this infection.
Data Sharing Statement
The data presented in the study are deposited in the SRA (https://www.ncbi.nlm.nih.gov/sra/) repository, accession number PRJNA 992918.
Ethics Statement
Written informed consent was obtained from the individual for the publication of any potentially identifiable images or data included in this article.
Acknowledgments
We thank WillingMed Technology (Beijing) Co., Ltd for providing metagenomic NGS analysis of samples and assistance with data analysis and discussion in this study.
Funding
The authors state no funding is involved.
Disclosure
WG is employed by WillingMed Technology (Beijing) Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Kasuga T, White TJ, Koenig G, et al. Phylogeography of the fungal pathogen Histoplasma capsulatum. Mol Ecol. 2003;12(12):3383–3401. doi:10.1046/j.1365-294X.2003.01995.x
2. Colombo AL, Tobón A, Restrepo A, Queiroz-Telles F, Nucci M. Epidemiology of endemic systemic fungal infections in Latin America. Med Mycol. 2011;49(8):785–798. doi:10.3109/13693786.2011.577821
3. Ashbee HR, Evans EGV, Viviani MA, et al. Histoplasmosis in Europe: report on an epidemiological survey from the European confederation of medical mycology working group. Med Mycol. 2008;46(1):57–65. doi:10.1080/13693780701591481
4. Pan B, Chen M, Pan W, Liao W. Histoplasmosis: a new endemic fungal infection in China? Review and analysis of cases. Mycoses. 2013;56(3):212–221. doi:10.1111/myc.12029
5. Wheat LJ, Azar MM, Bahr NC, Spec A, Relich RF, Hage C. Histoplasmosis. Infect Dis Clin North Am. 2016;30(1):207–227. doi:10.1016/J.IDC.2015.10.009
6. Xiong XF, Fan LL, Kang M, Wei J, Cheng DY. Disseminated histoplasmosis: a rare clinical phenotype with difficult diagnosis. Respirol Case Rep. 2017;5(3):e00220. doi:10.1002/rcr2.220
7. Pagliarone MJ, Macedo LD, Faria JM, et al. Disseminated histoplasmosis in a non-HIV patient: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol. 2020;130(3):e114. doi:10.1016/j.oooo.2020.04.022
8. Thompson GR, Le T, Chindamporn A, et al. Global guideline for the diagnosis and management of the endemic mycoses: an initiative of the European Confederation of Medical Mycology in cooperation with the International Society for Human and Animal Mycology. Lancet Infect Dis. 2021;21(12):e364–e374. doi:10.1016/S1473-3099(21)00191-2
9. Kallen ME, Khalil A, DeRosa PA, Baer MR. Disseminated histoplasmosis mimicking hematologic malignancy in a patient with human immunodeficiency virus. EJHaem. 2022;3(2):545–546. doi:10.1002/jha2.389
10. Araúz AB, Papineni P. Histoplasmosis. Infect Dis Clin North Am. 2021;35(2):471–491. doi:10.1016/j.idc.2021.03.011
11. Richer SM, Smedema ML, Durkin MM, et al. Improved diagnosis of acute pulmonary histoplasmosis by combining antigen and antibody detection. Clinl Infect Dis. 2016;62(7):896–902. doi:10.1093/cid/ciw007
12. Vasconcellos IC, Dalla Lana DF, Pasqualotto AC. The role of molecular tests in the diagnosis of disseminated histoplasmosis. J Fungi. 2020;6(1):1. doi:10.3390/jof6010001
13. Avva K, Wu B, Cler L. Disseminated histoplasmosis: a rare cause of pancytopenia in an immunocompromised patient case presentation. Cureus. 2022;14(6). doi:10.7759/cureus.25966
14. Zhao B, Xia X, Yin J. Epidemiological investigation of Histoplasma capsulatum infection in China. Chin Med J. 2001;114(7):743–746.
15. Antinori S. Histoplasma capsulatum: more widespread than previously thought. Am J Trop Med Hyg. 2014;90(6):982–983. doi:10.4269/ajtmh.14-0175
16. de Souza BC, Munerato MC. Oral manifestation of histoplasmosis on the palate. An Bras Dermatol. 2017;92(5):107–109. doi:10.1590/abd1806-4841.20175751
17. Grazia Angiusa A, Anna Viviani M, Muratori S, Cusini M, Brignolo L, Alessi E. Disseminated histoplasmosis presenting with cutaneous lesions in a patient with acquired immunodeficiency syndrome. J Eur Acad Dermatol Venereol. 1998;10(2):182–185.
18. Gonzales CA, Scott IU, Chaudhry NA, et al. Endogenous endophthalmitis caused by Histoplasma capsulatum var. capsulatum: a case report and literature review. Ophthalmology. 2000;107(4):725–729. doi:10.1016/S0161-6420(99)00179-7
19. Develoux M, Amona FM, Hennequin C. Histoplasmosis Caused by Histoplasma capsulatum var. duboisii: a Comprehensive Review of Cases from 1993 to 2019. Clinl Infect Dis. 2021;73(3):E543–E549. doi:10.1093/cid/ciaa1304
20. Loulergue P, Bastides F, Baudouin V, et al. Literature review and case histories of Histoplasma capsulatum var. duboisii infections in HIV-infected patients. Emerg Infect Dis. 2007;13(11):1647–1652. doi:10.3201/eid1311.070665
21. Mathews DM, John R, Verghese V, et al. Histoplasma capsulatum infection with extensive lytic bone lesions mimicking LCH. J Trop Pediatr. 2016;62(6):496–499. doi:10.1093/tropej/fmw040
22. Jones RC, Goodwin RAJ. Histoplasmosis of bone. Am J Med. 1981;70(4):864–866. doi:10.1016/0002-9343(81)90544-1
23. Wheat LJ, Freifeld AG, Kleiman MB, et al. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 Update by the Infectious Diseases Society of America. Clinl Infect Dis. 2007;45(7):807–825. doi:10.1086/521259
24. Oladele RO, Ayanlowo OO, Richardson MD, Denning DW, Vinetz JM. Histoplasmosis in Africa: an emerging or a neglected disease? PLoS Negl Trop Dis. 2018;12(1):e0006046. doi:10.1371/journal.pntd.0006046
25. De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clinl Infect Dis. 2008;46(12):1813–1821. doi:10.1086/588660
26. Lv X, Jiang M, He R, Li M, Meng J. Clinical features and endemic trend of histoplasmosis in China: a retrospective analysis and literature review. Clin Respir J. 2020;14(4):307–313. doi:10.1111/crj.13125
27. Swartzentruber S, Rhodes L, Kurkjian K, et al. Diagnosis of acute pulmonary histoplasmosis. Clinl Infect Dis. 2009;49(12):1878–1882. doi:10.1086/648421
28. Abdallah W, Myint T, Larue R, et al. Diagnosis of histoplasmosis using the MVista Histoplasma galactomannan antigen qualitative lateral flow-based immunoassay: a multicenter study. Open Forum Infect Dis. 2021;8(9). doi:10.1093/ofid/ofab454
29. Azar MM, Loyd JL, Relich RF, Wheat LJ, Hage CA. Current concepts in the epidemiology, diagnosis, and management of histoplasmosis syndromes. Semin Respir Crit Care Med. 2020;41(1):13–30. doi:10.1055/s-0039-1698429
30. da Silva RM, da Silva Neto JR, Santos CS, et al. Fluorescent in situ hybridization of pre-incubated blood culture material for the rapid diagnosis of histoplasmosis. Med Mycol. 2015;53(2):160–164. doi:10.1093/mmy/myu080
31. Tang YW, Li H, Durkin MM, et al. Urine polymerase chain reaction is not as sensitive as urine antigen for the diagnosis of disseminated histoplasmosis. Diagn Microbiol Infect Dis. 2006;54(4):283–287. doi:10.1016/j.diagmicrobio.2005.10.008
32. Brilhante RSN, Guedes GMDM, Riello GB, et al. RYP1 gene as a target for molecular diagnosis of histoplasmosis. J Microbiol Methods. 2016;130:112–114. doi:10.1016/j.mimet.2016.09.006
33. Muraosa Y, Toyotome T, Yahiro M, Watanabe A, Shikanai-Yasuda MA, Kamei K. Detection of Histoplasma capsulatum from clinical specimens by cycling probe-based real-time PCR and nested real-time PCR. Med Mycol. 2016;54(4):433–438. doi:10.1093/mmy/myv106
34. Boukassa L, Braice Ngackosso O, Brice Kinata Bambino S, et al. Cranial and spinal locations of Histoplasma Capsulatum var. duboisii in Brazzaville, Congo. Iran J Neurosurg. 2019:63–69. doi:10.32598/irjns.5.2.63
35. Liu B, Qu L, Zhu J, Yang Z, Yan S. Histoplasmosis mimicking metastatic spinal tumour. J Int Med Res. 2017;45(4):1440–1446. doi:10.1177/0300060517708530
36. Stratton CW, Schutzbank TE, Tang YW. Use of metagenomic next-generation sequencing in the clinical microbiology laboratory: a step forward, but not an end-all. J Mol Diagn. 2021;23(11):1415–1421. doi:10.1016/j.jmoldx.2021.09.003
37. Gu W, Miller S, Chiu CY. Clinical metagenomic next-generation sequencing for pathogen detection. Ann Rev Pathol. 2018;14:319–338. doi:10.1146/annurev-pathmechdis
38. Diao Z, Han D, Zhang R, Li J. Metagenomics next-generation sequencing tests take the stage in the diagnosis of lower respiratory tract infections. J Adv Res. 2022;38:201–212. doi:10.1016/j.jare.2021.09.012
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.