Back to Journals » International Journal of Nanomedicine » Volume 18
Discovery of Aptamers and the Acceleration of the Development of Targeting Research in Ophthalmology
Authors Cao J , Zhang F, Xiong W
Received 21 April 2023
Accepted for publication 19 June 2023
Published 2 August 2023 Volume 2023:18 Pages 4421—4430
DOI https://doi.org/10.2147/IJN.S418115
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yan Shen
Jiamin Cao, Feng Zhang, Wei Xiong
Department of Ophthalmology, Third Xiangya Hospital, Central South University, Changsha, People’s Republic of China
Correspondence: Feng Zhang; Wei Xiong, Department of Ophthalmology, the Third Xiangya Hospital, Central South University, Changsha, Hunan, 410013, People’s Republic of China, Email [email protected]; [email protected]
Abstract: Aptamers are widely applied to diagnosis and therapy because of their targeting. However, the current progress of research into aptamers for the treatment of eye disorders has not been well-documented. The current literature on aptamers was reviewed in this study. Aptamer-related drugs and biochemical sensors have been evaluated for several eye disorders within the past decade; S58 targeting TGF-β receptor II and pegaptanib targeting vascular endothelial growth factor (VEGF) are used to prevent fibrosis after glaucoma filtration surgery. Anti-brain-derived neurotrophic factor aptamer has been used to diagnose glaucoma. The first approved aptamer drug (pegaptanib) has been used to inhibit angiogenesis in age-related macular degeneration (AMD) and diabetic retinopathy (DR), and its efficacy and safety have been demonstrated in clinical trials. Aptamers, including E10030, RBM-007, AS1411, and avacincaptad pegol, targeting other angiogenesis-related biomarkers have also been discovered and subjected to clinical trials. Aptamers, such as C promoter binding factor 1, CD44, and advanced end products in AMD and DR, targeting other signal pathway proteins have also been discovered for therapy, and biochemical sensors for early diagnosis have been developed based on aptamers targeting VEGF, connective tissue growth factor, and lipocalin 1. Aptamers used for early detection and treatment of ocular tumors were derived from other disease biomarkers, such as CD71, nucleolin, and high mobility group A. In this review, the development and application of aptamers in eye disorders in recent years are systematically discussed, which may inspire a new link between aptamers and eye disorders. The aptamer development trajectory also facilitates the discovery of the pathogenesis and therapeutic strategies for various eye disorders.
Keywords: aptamer, ophthalmology, targeting therapy, early diagnosis
Introduction
Ophthalmology is an important clinical specialty that focuses on the physiological and pathological processes of the eye, which may become diseased as a result of internal and external factors. Several approaches have been used to increase the understanding of eye disease and provide effective therapeutic strategies. In recent years, as the development of targeting research in ophthalmology, several proteins have been discovered to participate and play key roles in the disease processes. By enhancing the function of proteins such as vascular endothelial growth factor (VEGF), the course of eye disorders may be modified; for example1 Using targeting to discover the pathogenesis of eye disorders is another research hotspot; it facilitates a better understanding of the mechanism of disease and contributes to the development of new treatment strategies.2 Therefore, aptamers were developed to be used for targeting research and edited for various deformations and combinations with other elements.
Aptamers have been widely used for their ability to bind to different targets and their functions as chemical antibodies.2 Since their discovery in 1990, aptamers have also been used in ophthalmology to investigate disease mechanisms and for diagnosis and therapy.3 The binding of aptamers to various targets has been demonstrated and applied in disease research. In the field of ophthalmology, aptamers are mainly used for targeting therapy. Various aptamers have been discovered and applied to nanomedicine. Therefore, a review of the current research status of aptamers in ophthalmology has become necessary.
In the present review, the current research developments of aptamers and their characteristics for targeting in eye disease are introduced. The application of aptamers, encompassing their development, targeting, and mechanism of action in ophthalmology, over the past 10 years has been discussed.
Characteristics of Aptamers
Aptamers are short single-stranded ribonucleotides or deoxyribonucleotides.4 The first aptamer was composed of ribonucleotides, and deoxyribonucleotides were subsequently added.5 According to McKeague, approximately 68.8% of aptamers comprised ribonucleotides from 1990 to 2007, after which the proportion dropped to 29.5%.6 Aptamers have a high affinity toward their target and their equilibrium dissociation constants may reach nanomolar levels with the Kd value of aptamers mainly ranging from 1–100nM; several factors such as the template length and selection condition affect this constant.6 Aptamers recognize various types of targets and undergo various modifications that facilitate connections between aptamers and other carriers, giving aptamers broader biosensor applications than antibodies.2 These characteristics make aptamers popular for diagnostic innovation and drug development; for example, aptamer-based biosensors have been used in the diagnosis of diabetes, and aptamer-siRNA has been used for cancer therapy.7,8 Other characteristics such as cellular uptake, stability, and low cost are advantages for therapeutic application. However, some characteristics limit the application of aptamers, such as their size determining the high rapid clearance rate for the weights of aptamers lower than the renal filtration threshold, and their single-strand nucleic acid structure being easily degenerated by nuclease.9 To overcome these limitations, aptamers have been modified by the chemical group or coupled drug carrier.10 For example, Judith found that aptamers conjugating with polyethylene glycol have an extended circulatory half-life and a reduced rate of urinary elimination.11 When methyl was added to KH1C12.O2, its stability was extended to 24h while its affinity was not affected.12 Similarly, hydrophobic F bases improved nuclease resistance ability, and aptamers coupled with F and paclitaxel (Sgc8-F-PTX) had significantly higher stability than Sgc8, perhaps because F bases could bind with albumin to form a stable complex.13,14
Systematic Evolution of Ligands by Exponential Enrichment (SELEX)
Aptamer development was supported by SELEX. The initial single-stranded random oligonucleotides were used to incubate positive and negative targets, resulting in the amplification of a pool of single-stranded random oligonucleotides with high affinity. After several rounds of selection, aptamers with high binding ability were obtained and identified (Figure 1). In this process, the positive targets included proteins, cells, or tissues,15–17 and several variations of the SELEX approach were developed to suit different targets and improve processes.18 In addition, aptamers collected through SELEX always need to be further optimized and modified, and characteristics of aptamers such as affinity, specificity, stability, and internalization have been evaluated for later application.19
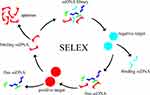 |
Figure 1 Process of Systematic Evolution of Ligands by Exponential Enrichment. |
Characteristics of Target-Based Research in Ophthalmology
As the primary research structures, the eyeball, ocular adnexa, and orbital cavity include epithelial, bone, vascular, muscle, nervous, and other tissues.20 These may undergo biological processes common to a range of tissues in other parts of the body, and some pathological changes of ocular tissue are part of systemic disease.21–23 Therefore, the results of target-based research and therapy may have similarities for ophthalmology and other disciplines. For example, aptamer AS1411 target nucleolin is located in nucleoli and expressed by various tissue types, including vascular and cancer tissue and could be used for therapy for ophthalmopathy despite its intended use as an anticancer drug.24,25 In contrast, the application of target-based research varies for ocular tissues, since the anterior and posterior segments of the eye differ in their anatomic structures and functions.26 Target-based research may, therefore, be applied to each segment, such as corneal and fundal staining techniques for diagnosis and administration techniques for therapy.27,28 When aptamers are designed for examination or treatment applications, the target anatomical structure and location, as well as the characteristics of tissues should be considered. For example, anti-VEGF transmission across the cornea is desirable to avoid the discomfort of retrobulbar injections, and carbon dot modification of anti-VEGF aptamer facilitates corneal administration.29 The blood-aqueous and blood-retinal barriers30,31 tightly restrict the passage of proteins between extra- and intraocular regions, presenting a challenge for the development of intraocular target-based drugs.
Aptamers Applied in Ophthalmology Research
Aptamer application in ophthalmology is based on the pathogenic mechanisms of eye disorders. The key molecules or cells in the pathogenesis of eye disorders may provide a target for aptamers, and a change in the expression or signal pathway in that target may have a diagnostic and therapeutic effect. To date, several types of aptamers have been developed to cure eye disorders, such as NX1838 for age-related macular degeneration (AMD), S58 for glaucoma, and AS1411 for corneal neovascularization.32–34 In addition to the development of aptamers for ophthalmology, those intended for non-ocular diseases have also been used in research on eye disease, as summarized in Table 1.
 |
Table 1 Aptamers Applied in Ophthalmology |
Aptamers Applied in Bacterial Keratitis
In addition to their use for targeting therapeutic binding sites in eye disease, aptamers have been used in the development of therapeutic drugs. Given the rapid clearance of eye drops from the ocular surface and the challenge of bioactive compounds reaching target tissues from the ocular surface, Jan Willem de Vries et al developed a DNA amphiphiles nanoparticle drug carrier system.55 This novel system was used to treat bacterial keratitis, and aptamers, in this case, targeted kanamycin or neomycin and were used as linkers between nanoparticles and antibiotics.
Aptamers Applied in Glaucoma
Glaucoma is a global optic neuropathy characterized by the degeneration of retinal ganglion cells.56 Aptamers are rarely used clinically for glaucoma therapy, and this application is still under development, but research in this field shows promise. As the most effective therapy for drug-refractory glaucoma, glaucoma filtration surgery, may not always be successful due to conjunctival fibrosis at the incision site promoted by TGF-β binding with TGF-β receptor II.57 Xie et al developed an aptamer S58 that targets TGF-β receptor II and inhibits the differentiation of tenon’s fibroblasts into myofibroblasts, effectively improving the outcome of glaucoma filtration surgery.37 In further research, S58 was combined with a chitosan thermo-sensitive gel or exosome, and the results showed that S58 reduced fibrosis in human fibroblasts as well as in a rat glaucoma filtration surgery model.58,59 In addition, recovery after glaucoma infiltration glaucoma is complicated, with several bioprocesses such as angiogenesis, which may affect the outcome of surgery. The anti-VEGF aptamer pegaptanib was the first to be approved by the FDA for use as an inhibitor of angiogenesis, inflammation, or collagen deposition and has been shown to reduce angiogenesis after glaucoma filtration surgery to improve its outcomes.38 An anti-VEGF aptamer was also used to treat neovascular glaucoma, characterized by massive neovascularization on the iris surface and atrial angle.39,60 Brain-derived neurotrophic factors are nerve growth factors with neuroprotective effects through Tropomyosin receptor kinase B and are significantly lower in aqueous humor, lacrimal fluid, and serum in glaucoma than in the normal eye and may be a biomarker for early glaucoma diagnosis.61 An aptamer targeting brain-derived neurotrophic factors has been developed to construct a biolayer interferometry-based 3D aptasensor41 in which aptamers have a detecting function and produce a signal for highly sensitive 3D matrix sensors, achieving low-abundance brain-derived neurotrophic factor detection for early diagnosis of glaucoma.41 Lipid DNA nanoparticles have been developed for drug delivery to overcome the disadvantages of low drug uptake on the ocular surface. In this system, the aptamer targets drugs and is a linker between drugs and DNA nanoparticles rather than disease-related targets. Schnichels et al used this system to enhance glaucoma drugs based on travoprost-target and brimonidine-target aptamers.62,63
Aptamer Application in AMD
The mechanism of AMD is complicated and could be classified into early and late stages, the latter characterized by neovascularization.64 During angiogenesis, cell factors such as VEGF, a mitogen for endothelial cells, enhance blood vessel formation.65 While there are several genes in the VEGF family, including VEGF-B, VEGF-C, and placental growth factor, VEGF-A is thought to play a major role in angiogenesis.66 Aptamers target VEGF develop rapidly since the first aptamer drug pegaptanib was proven to be effective in a clinical trial in 2004.40
Within the past decade, the applications of pegaptanib have been investigated through large-scale research and meta-analysis, and its therapeutic effects and those of other anti-VEGF drugs, such as monoclonal antibodies, have been tested. The results have shown that monoclonal antibodies such as bevacizumab and ranibizumab performed similarly based on best-corrected visual acuity, while indirect evidence showed that pegaptanib provided less improvement in visual acuity.67,68 Dalvin et al found that anti-VEGF therapy has no association with stroke or death,69 and a retrospective cohort study revealed that an injection of an anti-VEGF such as pegaptanib did not increase the risk of developing glaucoma.70
Platelet-derived growth factor (PDGF) regulates angiogenesis and interacts with VEGF,71,72 while PDGF inhibitors serve as a potential therapy for ocular neovascularization.73 Since aptamers ARC126 and ARC127 have been found to affect several retinal diseases through preclinical trials, anti-PDGF aptamers were developed for clinical usage.74 E10030 is an anti-platelet-derived growth factor aptamer that has been used in combination with ranibizumab for the treatment of AMD. Phase I and IIb clinical trials have been conducted, and the results have confirmed the efficacy of E10030 and permitted a phase III clinical trial.42,75
Fibroblast growth factor 2 (FGF2) participates in angiogenesis and fibrosis by promoting the proliferation of vascular endothelial cells and stimulating the secretion of VEGF.76 The aptamer target FGF2 was first developed and used for therapy for the bone disease known as APT-F2P.77 Since the bioprocess of angiogenesis is similar for eye and bone disease, Yusaku et al used APT-F2P (RBM-007) for retinal disease therapy.43 Through an angiogenesis mouse model, APT-F2P was found to reduce new vessel formation and subretinal fibrosis,43 based on which a phase I/IIa clinical study was conducted to further investigate the effect of APT-F2P in AMD.78
Complement component 5 (C5) mediates inflammation and was thought to participate in the local chronic inflammatory process in AMD.79 While research on single nucleotide polymorphisms of C5 did not support an association between C5 and AMD, immunolocalization has provided evidence of the C5 complement activation in AMD.80,81 The aptamer targeting C5 (avacincaptad pegol) has been used for AMD therapy,44 and the results have shown that avacincaptad pegol significantly reduces geographic atrophy in AMD.44
AS1411 is an aptamer targeting nucleolin with an anti-proliferation effect and acts as an anti-cancer drug, and a phase II clinical trial has shown that it has a therapeutic effect in metastatic renal cell carcinoma.82 AS1411 was introduced for AMD to suppress the function of endothelial cells and have a therapeutic effect on AMD.45 This aptamer has been found in animal models to reduce choroidal neovascularization and attenuate the infiltration of macrophages.45
In addition to fully developed aptamer therapeutics, some newly developed aptamers have a potential therapeutic effect on AMD. C promoter binding factor 1 is involved in Notch signaling and acts as an inhibitor of angiogenesis. VEGF promotes C promoter binding factor 1 proteasomal degradation and suppresses the activation of Notch signaling. An aptamer Apt-3 developed by Tezuka-Kagajo et al targeted C promoter binding factor 1 and active Notch signaling, thus inhibiting angiogenesis.47 Chandola et al developed an aptamer targeting CD44 (which is overexpressed in retinal pigment epithelium after oxidative stress) that could be transferred to lysosomes under oxidative stress.48 Since oxidative stress also occurs in AMD, CD44-aptamer could be used for lysosomal delivery of drugs to the retinal pigment epithelium and may have a therapeutic effect on AMD.48
In addition to therapy, aptamers have also been used to detect biomarkers in AMD, and various detection methods have been established to suit a range of clinical application scenarios. For example, an aptamer-based proteomic technology has been used to detect the biomarkers in the disease and show differential expression of vinculin and CD177 compared with controls.83 Lynch et al also found different protein expression assays in AMD using aptamer-based technology.84 An anti-VEGF aptamer has also been used to establish a target-induced dissociation assay using thermophoresis and microarrays, which can detect 0.1 nM of VEGF,49,85 and another has been used to develop an electrochemical aptasensor based on metallo nanoenzyme particles to amplify the VEGF signal to facilitate its detection.86 Gao et al constructed a biolayer interferometry-based enzyme-linked aptamer sorbent assay based on anti-PDGF-BB, allowing rapid, high-throughput processing and real-time monitoring.87 The generation of these detection methods indicates the new direction of aptamer application in AMD and the importance of biomarker detection for early diagnosis and therapy of the disease. In addition, current studies have conjugated aptamers with nanoparticles or other elements, further demonstrating the developing trends of application in AMD.
Aptamer Application in Diabetic Retinopathy (DR)
The development of anti-VEGF aptamer drugs in angiogenesis has led to their use in DR and assessment of their therapeutic effect and safety in this disease. A Phase 3 study of pegaptanib assessed its safety in diabetic macular edema (a complication in DR) and showed that side effects of the drug were mainly mild or moderate and were related to the injection.88 A network meta-analysis comparing the effects of various anti-VEGF drugs such as aflibercept, ranibizumab, bevacizumab, and pegaptanib showed that anti-VEGF drugs improved vision, but long-term effects remained unclear.89 Approximately 40% of the patients with diabetic macular edema who underwent anti-VEGF therapy switched to laser surgery.90
With the development of anti-VEGF drugs, other kinds of aptamer drugs were neglected. Advanced glycation end products were associated with the early phase of DR through VEGF.91 An aptamer targeting advanced glycation end products was developed and used in DR and was found to prevent abnormalities in electroretinograms and have an inhibitory effect on the early development of DR.50,92 Connective tissue growth factor serves as a biomarker for DR and may be used in early diagnosis.93 Shunxiang et al developed a BLI-based enzyme-linked aptamer sandwich assay based on connective tissue growth factor-targeting aptamer, which detected connective tissue growth factor at a level of 0.02 M, facilitating the early diagnosis of DR.51 Lipocalin 1 is another biomarker of DR that is related to its severity, and its detection in tears facilitates the early diagnosis of DR.94 Gao et al developed an aptamer targeting lipocalin 1 which folds into the B-DNA structure.52 After being assembled with G-rich DNA fragments and the Thioflavin T mediator, a lipocalin 1-targeting aptamer-based fluorescent aptasensor was developed, with potential application as a convenient detector of lipocalin 1 with high sensitivity and specificity.52
Aptamer Application in Ocular Tumors
As the most common intraocular malignant tumor in adults, uveal melanoma has been well studied, and several biomarkers, such as TRPM4, BAP1, and RBM15B, have been associated with its mechanism and therapy.95–97 These biomarkers provide a therapeutic binding target and a potential target for aptamers. CD71 is overexpressed in malignancy, and antibodies such as A24 may compete for receptor binding sites with CD71, inducing CD71 degeneration in adult T-cell leukemia cells.98 An aptamer XQ-2d has been developed to target CD71 and is used in uveal melanoma therapy.53 XQ-2d was designed to conjugate with Monomethyl Auristatin E, and it inhibits the progression of uveal melanoma in mouse models.53
Retinoblastoma is a childhood eye cancer with an incidence of 1:15,000–1:20,000 globally.99 Aptamers targeting retinoblastoma have been developed over the past 10 years, but their application is still in the experimental stages. Epithelial cell adhesion molecules are cancer stem cell biomarkers since they are overexpressed in most solid cancers.100 An aptamer targeting epithelial cell adhesion molecules was developed and used in retinoblastoma therapy due to the high quantity of epithelial cell adhesion molecules in retinoblastoma cells.100,101 The aptamer was developed with doxorubicin, which enhanced the targeting of drugs.101 Aptamers developed with siRNA silence the expression of epithelial cell adhesion molecules102 and have been used therapeutically. Since high mobility group A is elevated in both pancreatic cancer and retinoblastoma, the aptamer targeting high mobility group A developed via research on the former has been used in retinoblastoma.103 A nucleolin-targeting aptamer (AS1411) has also been used in retinoblastoma and found to reduce its proliferation.46 Based on the inhibitory effects of anti-nucleolin and high mobility group A on cancer, Kannan et al constructed two aptamer drugs: NCLap-HMGA2si and NCLAb-HMGAap. The nucleolin-targeting aptamer was developed with high-mobility group A siRNA, and the high-mobility group A targeting aptamer was developed with a nucleolin antibody, respectively, and both had therapeutic effects on retinoblastoma.54
Challenges and Opportunities
There are several reports of cases of aptamer use in research related to eye disorders. However, there are several challenges. On the one hand, only a few types of aptamers are used in ophthalmology. Aptamers for several targets important for the pathogeneses of eye diseases have not been reported. Several original aptamers are not developed for eye disorders, and the specific characteristics of eye disease make it difficult for adopting aptamers in clinical practice. On the other hand, the current aptamer-related drugs used in clinical practice have challenges, including the long period of drug development and their characteristics such as degeneration and affinity. To overcome these challenges, more types of aptamer need to be developed while existing aptamers may be improved to facilitate their application to eye disorders.
Conclusion
In this study, the development of aptamers in ophthalmology was systematically reviewed. Several types of aptamers were used for targeting in drug development or improvement of drug performance for conditions such as glaucoma, AMD, DR, uveal melanoma, and retinoblastoma. Besides, aptamer was also used for the detection of the pathogenesis of eye disorders. The development of aptamers in ophthalmology may lead to further clinical applications in the future.
Abbreviations
AMD, age-related macular degeneration; DR, diabetic retinopathy; SELEX, systematic evolution of ligands by exponential enrichment; VEGF, vascular endothelial growth factor; PDGF, platelet-derived growth factor; FGF2, fibroblast growth factors 2; C5, complement component 5.
Funding
This study was supported by the National Natural Science Foundation of China (82071006), the National Science Foundation for Young Scholars of China (82201185) and the Fundamental Research Funds for the Central Universities of Central South University (2023ZZTS0327).
Disclosure
The authors declare no potential conflicts of interest in this research.
References
1. Dehghani S, Nosrati R, Yousefi M, et al. Aptamer-based biosensors and nanosensors for the detection of vascular endothelial growth factor (VEGF): a review. Biosens Bioelectron. 2018;110:23–37. doi:10.1016/j.bios.2018.03.037
2. Zhu G, Chen X. Aptamer-based targeted therapy. Adv Drug Deliv Rev. 2018;134:65–78. doi:10.1016/j.addr.2018.08.005
3. Tuerk C, Gold L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science. 1990;249(4968):505–510. doi:10.1126/science.2200121
4. Wang Y, Mistry BA, Chou T. Discrete stochastic models of SELEX: aptamer capture probabilities and protocol optimization. J Chem Phys. 2022;156(24):244103. doi:10.1063/5.0094307
5. Bock LC, Griffin LC, Latham JA, et al. Selection of single-stranded DNA molecules that bind and inhibit human thrombin. Nature. 1992;355(6360):564–566.
6. McKeague M, McConnell EM, Cruz-Toledo J, et al. Analysis of in vitro aptamer selection parameters. J Mol Evol. 2015;81(5–6):150–161. doi:10.1007/s00239-015-9708-6
7. Feng T, Liu J, Chen G, et al. The fluorescent aptasensor based on CRISPR-Cas12a combined with TdT for highly sensitive detection of cocaine. Anal Bioanal Chem. 2022;414(24):7291–7297. doi:10.1007/s00216-022-04280-4
8. Khoshbin Z, Shakour N, Iranshahi M, et al. Aptamer-based Biosensors: promising Sensing Technology for Diabetes Diagnosis in Biological Fluids. Curr Med Chem. 2022.
9. Zhang Y, Zhang H, Chan D, et al. Strategies for developing long-lasting therapeutic nucleic acid aptamer targeting circulating protein: the present and the future. Front Cell Dev Biol. 2022;10:1048148. doi:10.3389/fcell.2022.1048148
10. Nimjee SM, White RR, Becker RC, et al. Aptamers as Therapeutics. Annu Rev Pharmacol Toxicol. 2017;57:61–79. doi:10.1146/annurev-pharmtox-010716-104558
11. Healy JM, Lewis SD, Kurz M, et al. Pharmacokinetics and biodistribution of novel aptamer compositions. Pharm Res. 2004;21(12):2234–2246. doi:10.1007/s11095-004-7676-4
12. Maio G, Enweronye O, Zumrut HE, et al. Systematic optimization and modification of a DNA aptamer with 2’-O-methyl RNA analogues. ChemistrySelect. 2017;2(7):2335–2340. doi:10.1002/slct.201700359
13. Yang C, Zhao H, Sun Y, et al. Programmable manipulation of oligonucleotide-albumin interaction for elongated circulation time. Nucleic Acids Res. 2022;50(6):3083–3095. doi:10.1093/nar/gkac156
14. Sun Y, Geng X, Ma Y, et al. Artificial Base-Directed In Vivo Formulation of Aptamer-Drug Conjugates with Albumin for Long Circulation and Targeted Delivery. Pharmaceutics. 2022;14(12):2781. doi:10.3390/pharmaceutics14122781
15. Costello AM, Elizondo-Riojas MA, Li X, et al. Selection and Characterization of Vimentin-Binding Aptamer Motifs for Ovarian Cancer. Molecules. 2021;26(21):6525. doi:10.3390/molecules26216525
16. Farrel CM, Marli BT, Ribeiro DB, et al. Selection and Identification of a DNA Aptamer for Multidrug-Resistant Acinetobacter baumannii Using an In-House Cell-SELEX Methodology. Front Cell Infect Microbiol. 2022;12:818737. doi:10.3389/fcimb.2022.818737
17. Li L, Wan J, Wen X, et al. Identification of a New DNA Aptamer by Tissue-SELEX for Cancer Recognition and Imaging. Anal Chem. 2021;93(19):7369–7377. doi:10.1021/acs.analchem.1c01445
18. Zhang Y, Lai B, Juhas M. Recent Advances in Aptamer Discovery and Applications. Molecules. 2019;24(5):941. doi:10.3390/molecules24050941
19. Lin N, Wu L, Xu X, et al. Aptamer Generated by Cell-SELEX for Specific Targeting of Human Glioma Cells. ACS Appl Mater Interfaces. 2021;13(8):9306–9315. doi:10.1021/acsami.0c11878
20. Kels BD, Grzybowski A, Grant-Kels JM. Human ocular anatomy. Clin Dermatol. 2015;33(2):140–146. doi:10.1016/j.clindermatol.2014.10.006
21. Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376(9735):124–136. doi:10.1016/S0140-6736(09)62124-3
22. Bjordal O, Norheim KB, Rodahl E, et al. Primary Sjogren’s syndrome and the eye. Surv Ophthalmol. 2020;65(2):119–132. doi:10.1016/j.survophthal.2019.10.004
23. Rosenberg C, Finger PT. Cutaneous malignant melanoma metastatic to the eye, lids, and orbit. Surv Ophthalmol. 2008;53(3):187–202. doi:10.1016/j.survophthal.2008.02.003
24. Girvan AC, Teng Y, Casson LK, et al. AGRO100 inhibits activation of nuclear factor-kappaB (NF-kappaB) by forming a complex with NF-kappaB essential modulator (NEMO) and nucleolin. Mol Cancer Ther. 2006;5(7):1790–1799. doi:10.1158/1535-7163.MCT-05-0361
25. Vindigni G, Raniolo S, Iacovelli F, et al. AS1411 Aptamer Linked to DNA Nanostructures Diverts Its Traffic Inside Cancer Cells and Improves Its Therapeutic Efficacy. Pharmaceutics. 2021;13(10):1671. doi:10.3390/pharmaceutics13101671
26. Kaplan HJ. Anatomy and function of the eye. Chem Immunol Allergy. 2007;92:4–10.
27. Wilson G, Ren H, Laurent J. Corneal epithelial fluorescein staining. J Am Optom Assoc. 1995;66(7):435–441.
28. Sun G, Liu X, Yu X. Multi-path cascaded U-net for vessel segmentation from fundus fluorescein angiography sequential images. Comput Methods Programs Biomed. 2021;211:106422. doi:10.1016/j.cmpb.2021.106422
29. Carrasquillo KG, Ricker JA, Rigas IK, et al. Controlled delivery of the anti-VEGF aptamer EYE001 with poly(lactic-co-glycolic)acid microspheres. Invest Ophthalmol Vis Sci. 2003;44(1):290–299. doi:10.1167/iovs.01-1156
30. Coca-Prados M. The blood-aqueous barrier in health and disease. J Glaucoma. 2014;23(8 Suppl 1):S36–S38. doi:10.1097/IJG.0000000000000107
31. Cunha-Vaz J, Bernardes R, Lobo C. Blood-retinal barrier. Eur J Ophthalmol. 2011;21(Suppl 6):S3–S9. doi:10.5301/EJO.2010.6049
32. Vivanco-Rojas O, Garcia-Bermudez MY, Iturriaga-Goyon E, et al. Corneal neovascularization is inhibited with nucleolin-binding aptamer, AS1411. Exp Eye Res. 2020;193:107977. doi:10.1016/j.exer.2020.107977
33. Li X, Leng Y, Li X, et al. The TbetaR II-targeted aptamer S58 prevents fibrosis after glaucoma filtration surgery. Aging. 2020;12(10):8837–8857. doi:10.18632/aging.102997
34. Drolet DW, Nelson J, Tucker CE, et al. Pharmacokinetics and safety of an anti-vascular endothelial growth factor aptamer (NX1838) following injection into the vitreous humor of rhesus monkeys. Pharm Res. 2000;17(12):1503–1510. doi:10.1023/A:1007657109012
35. Song KM, Cho M, Jo H, et al. Gold nanoparticle-based colorimetric detection of kanamycin using a DNA aptamer. Anal Biochem. 2011;415(2):175–181. doi:10.1016/j.ab.2011.04.007
36. Wallis MG, von Ahsen U, Schroeder R, et al. A novel RNA motif for neomycin recognition. Chem Biol. 1995;2(8):543–552. doi:10.1016/1074-5521(95)90188-4
37. Zhu X, Li L, Zou L, et al. A novel aptamer targeting TGF-beta receptor II inhibits transdifferentiation of human tenon’s fibroblasts into myofibroblast. Invest Ophthalmol Vis Sci. 2012;53(11):6897–6903. doi:10.1167/iovs.12-10198
38. Van Bergen T, Vandewalle E, Van de Veire S, et al. The role of different VEGF isoforms in scar formation after glaucoma filtration surgery. Exp Eye Res. 2011;93(5):689–699. doi:10.1016/j.exer.2011.08.016
39. Jia X. Duan X.[Application of anti-VEGF agents in treatment of neovascular glaucoma and anti-scarring in filtering surgery]. Zhonghua Yan Ke Za Zhi. 2015;51(4):314–318.
40. Gragoudas ES, Adamis AP, Cunningham EJ, et al. Pegaptanib for neovascular age-related macular degeneration. N Engl J Med. 2004;351(27):2805–2816. doi:10.1056/NEJMoa042760
41. Gao S, Li Q, Zhang S, et al. One-step high-throughput detection of low-abundance biomarker BDNF using a biolayer interferometry-based 3D aptasensor. Biosens Bioelectron. 2022;215:114566. doi:10.1016/j.bios.2022.114566
42. Jaffe GJ, Eliott D, Wells JA, et al. A Phase 1 Study of Intravitreous E10030 in Combination with Ranibizumab in Neovascular Age-Related Macular Degeneration. Ophthalmology. 2016;123(1):78–85. doi:10.1016/j.ophtha.2015.09.004
43. Matsuda Y, Nonaka Y, Futakawa S, et al. Anti-Angiogenic and Anti-Scarring Dual Action of an Anti-Fibroblast Growth Factor 2 Aptamer in Animal Models of Retinal Disease. Mol Ther Nucleic Acids. 2019;17:819–828. doi:10.1016/j.omtn.2019.07.018
44. Jaffe GJ, Westby K, Csaky KG, et al. C5 Inhibitor Avacincaptad Pegol for Geographic Atrophy Due to Age-Related Macular Degeneration: a Randomized Pivotal Phase 2/3 Trial. Ophthalmology. 2021;128(4):576–586. doi:10.1016/j.ophtha.2020.08.027
45. Leaderer D, Cashman SM, Kumar-Singh R. Topical application of a G-Quartet aptamer targeting nucleolin attenuates choroidal neovascularization in a model of age-related macular degeneration. Exp Eye Res. 2015;140:171–178. doi:10.1016/j.exer.2015.09.005
46. Subramanian N, Srimany A, Kanwar JR, et al. Nucleolin-aptamer therapy in retinoblastoma: molecular changes and mass spectrometry-based imaging. Mol Ther Nucleic Acids. 2016;5(8):e358. doi:10.1038/mtna.2016.70
47. Tezuka-Kagajo M, Maekawa M, Ogawa A, et al. Development of Human CBF1-Targeting Single-Stranded DNA Aptamers with Antiangiogenic Activity In Vitro. Nucleic Acid Ther. 2020;30(6):365–378. doi:10.1089/nat.2020.0875
48. Chandola C, Casteleijn MG, Chandola UM, et al. CD44 aptamer mediated cargo delivery to lysosomes of retinal pigment epithelial cells to prevent age-related macular degeneration. Biochem Biophys Rep. 2019;18:100642. doi:10.1016/j.bbrep.2019.100642
49. Kurth T, Witt S, Bolten S, et al. Development of Aptamer-Based TID Assays Using Thermophoresis and Microarrays. Biosensors. 2019;9(4). doi:10.3390/bios9040124.
50. Maeda S, Matsui T, Ojima A, et al. DNA Aptamer Raised against Advanced Glycation End Products Prevents Abnormalities in Electroretinograms of Experimental Diabetic Retinopathy. Ophthalmic Res. 2015;54(4):175–180. doi:10.1159/000440768
51. Gao S, Hu W, Zheng X, et al. Functionalized aptamer with an antiparallel G-quadruplex: structural remodeling, recognition mechanism, and diagnostic applications targeting CTGF. Biosens Bioelectron. 2019;142:111475. doi:10.1016/j.bios.2019.111475
52. Gao S, Zhang S, Sun X, et al. Fluorescent aptasensor based on G-quadruplex-assisted structural transformation for the detection of biomarker lipocalin 1. Biosens Bioelectron. 2020;169:112607. doi:10.1016/j.bios.2020.112607
53. Zhang H, Jin C, Zhang L, et al. CD71-Specific Aptamer Conjugated with Monomethyl Auristatin E for the Treatment of Uveal Melanoma. ACS Appl Mater Interfaces. 2022;14(1):32–40. doi:10.1021/acsami.1c13980
54. Balachandran A, Zambre A, Kainth JS, et al. Targeting HMGA protein inhibits retinoblastoma cell proliferation. RSC Adv. 2018;8(55):31510–31514. doi:10.1039/C8RA06026F
55. Willem DVJ, Schnichels S, Hurst J, et al. DNA nanoparticles for ophthalmic drug delivery. Biomaterials. 2018;157:98–106. doi:10.1016/j.biomaterials.2017.11.046
56. Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA. 2014;311(18):1901–1911. doi:10.1001/jama.2014.3192
57. Zhu X, Xu D, Zhu X, et al. Evaluation of Chitosan/Aptamer Targeting TGF-beta Receptor II Thermo-Sensitive Gel for Scarring in Rat Glaucoma Filtration Surgery. Invest Ophthalmol Vis Sci. 2015;56(9):5465–5476. doi:10.1167/iovs.15-16683
58. Chen X, Zhu X, Li L, et al. Investigation on novel chitosan nanoparticle-aptamer complexes targeting TGF-beta receptor II. Int J Pharm. 2013;456(2):499–507. doi:10.1016/j.ijpharm.2013.08.028
59. Lin QY, Li XJ, Leng Y, et al. Exosome-mediated aptamer S58 reduces fibrosis in a rat glaucoma filtration surgery model. Int J Ophthalmol. 2022;15(5):690–700. doi:10.18240/ijo.2022.05.02
60. Hayreh SS. Neovascular glaucoma. Prog Retin Eye Res. 2007;26(5):470–485. doi:10.1016/j.preteyeres.2007.06.001
61. Lambuk L, Mohd LM, Ahmad S, et al. Brain-Derived Neurotrophic Factor-Mediated Neuroprotection in Glaucoma: a Review of Current State of the Art. Front Pharmacol. 2022;13:875662. doi:10.3389/fphar.2022.875662
62. Schnichels S, Hurst J, de Vries JW, et al. Improved Treatment Options for Glaucoma with Brimonidine-Loaded Lipid DNA Nanoparticles. ACS Appl Mater Interfaces. 2021;13(8):9445–9456. doi:10.1021/acsami.0c18626
63. Schnichels S, Hurst J, de Vries JW, et al. Self-assembled DNA nanoparticles loaded with travoprost for glaucoma-treatment. Nanomedicine. 2020;29:102260. doi:10.1016/j.nano.2020.102260
64. Mitchell P, Liew G, Gopinath B, et al. Age-related macular degeneration. Lancet. 2018;392(10153):1147–1159. doi:10.1016/S0140-6736(18)31550-2
65. Hoeben A, Landuyt B, Highley MS, et al. Vascular endothelial growth factor and angiogenesis. Pharmacol Rev. 2004;56(4):549–580. doi:10.1124/pr.56.4.3
66. Apte RS, Chen DS, Ferrara N. VEGF in Signaling and Disease: beyond Discovery and Development. Cell. 2019;176(6):1248–1264. doi:10.1016/j.cell.2019.01.021
67. Solomon SD, Lindsley K, Vedula SS, et al. Anti-vascular endothelial growth factor for neovascular age-related macular degeneration. Cochrane Database Syst Rev. 2019;3:D5139.
68. Nguyen CL, Oh LJ, Wong E, et al. Anti-vascular endothelial growth factor for neovascular age-related macular degeneration: a meta-analysis of randomized controlled trials. BMC Ophthalmol. 2018;18(1):130. doi:10.1186/s12886-018-0785-3
69. Dalvin LA, Starr MR, AbouChehade JE, et al. Association of Intravitreal Anti-Vascular Endothelial Growth Factor Therapy With Risk of Stroke, Myocardial Infarction, and Death in Patients With Exudative Age-Related Macular Degeneration. JAMA Ophthalmol. 2019;137(5):483–490. doi:10.1001/jamaophthalmol.2018.6891
70. Shah SM, Boopathiraj N, Starr MR, et al. Risk, Prevalence, and Progression of Glaucoma in Eyes With Age-Related Macular Degeneration Treated With Intravitreal Anti-Vascular Endothelial Growth Factor Injections. Am J Ophthalmol. 2022;243:98–108. doi:10.1016/j.ajo.2022.07.025
71. Gianni-Barrera R, Butschkau A, Uccelli A, et al. PDGF-BB regulates splitting angiogenesis in skeletal muscle by limiting VEGF-induced endothelial proliferation. Angiogenesis. 2018;21(4):883–900. doi:10.1007/s10456-018-9634-5
72. Xie H, Cui Z, Wang L, et al. PDGF-BB secreted by preosteoclasts induces angiogenesis during coupling with osteogenesis. Nat Med. 2014;20(11):1270–1278. doi:10.1038/nm.3668
73. Sadiq MA, Hanout M, Sarwar S, et al. Platelet derived growth factor inhibitors: a potential therapeutic approach for ocular neovascularization. Saudi J Ophthalmol. 2015;29(4):287–291. doi:10.1016/j.sjopt.2015.05.005
74. Akiyama H, Kachi S, Silva RL, et al. Intraocular injection of an aptamer that binds PDGF-B: a potential treatment for proliferative retinopathies. J Cell Physiol. 2006;207(2):407–412. doi:10.1002/jcp.20583
75. Jaffe GJ, Ciulla TA, Ciardella AP, et al. Dual Antagonism of PDGF and VEGF in Neovascular Age-Related Macular Degeneration: a Phase IIb, Multicenter, Randomized Controlled Trial. Ophthalmology. 2017;124(2):224–234. doi:10.1016/j.ophtha.2016.10.010
76. Belgore F, Lip GY, Blann AD. Basic fibroblast growth factor induces the secretion of vascular endothelial growth factor by human aortic smooth muscle cells but not by endothelial cells. Eur J Clin Invest. 2003;33(10):833–839. doi:10.1046/j.1365-2362.2003.01223.x
77. Jin L, Nonaka Y, Miyakawa S, et al. Dual Therapeutic Action of a Neutralizing Anti-FGF2 Aptamer in Bone Disease and Bone Cancer Pain. Mol Ther. 2016;24(11):1974–1986. doi:10.1038/mt.2016.158
78. Nakamura Y. Multiple Therapeutic Applications of RBM-007, an Anti-FGF2 Aptamer. Cells. 2021;10(7):1617. doi:10.3390/cells10071617
79. Scholl HP, Charbel IP, Walier M, et al. Systemic complement activation in age-related macular degeneration. PLoS One. 2008;3(7):e2593. doi:10.1371/journal.pone.0002593
80. Baas DC, Ho L, Ennis S, et al. The complement component 5 gene and age-related macular degeneration. Ophthalmology. 2010;117(3):500–511. doi:10.1016/j.ophtha.2009.08.032
81. Nozaki M, Raisler BJ, Sakurai E, et al. Drusen complement components C3a and C5a promote choroidal neovascularization. Proc Natl Acad Sci U S A. 2006;103(7):2328–2333. doi:10.1073/pnas.0408835103
82. Rosenberg JE, Bambury RM, Van Allen EM, et al. A Phase II trial of AS1411 (a novel nucleolin-targeted DNA aptamer) in metastatic renal cell carcinoma. Invest New Drugs. 2014;32(1):178–187. doi:10.1007/s10637-013-0045-6
83. Lynch AM, Wagner BD, Weiss SJ, et al. Proteomic Profiles in Advanced Age-Related Macular Degeneration Using an Aptamer-Based Proteomic Technology. Transl Vis Sci Technol. 2019;8(1):14. doi:10.1167/tvst.8.1.14
84. Lynch AM, Wagner BD, Palestine AG, et al. Plasma Biomarkers of Reticular Pseudodrusen and the Risk of Progression to Advanced Age-Related Macular Degeneration. Transl Vis Sci Technol. 2020;9(10):12. doi:10.1167/tvst.9.10.12
85. Nonaka Y, Sode K, Ikebukuro K. Screening and improvement of an anti-VEGF DNA aptamer. Molecules. 2010;15(1):215–225. doi:10.3390/molecules15010215
86. Mei C, Zhang Y, Pan L, et al. A One-Step Electrochemical Aptasensor Based on Signal Amplification of Metallo Nanoenzyme Particles for Vascular Endothelial Growth Factor. Front Bioeng Biotechnol. 2022;10:850412. doi:10.3389/fbioe.2022.850412
87. Gao S, Zheng X, Wu J. A biolayer interferometry-based enzyme-linked aptamer sorbent assay for real-time and highly sensitive detection of PDGF-BB. Biosens Bioelectron. 2018;102:57–62. doi:10.1016/j.bios.2017.11.017
88. Ishibashi T, Yuzawa M, Yoshimura N, et al. Japan phase 3 study of pegaptanib sodium in patients with diabetic macular edema. Nippon Ganka Gakkai Zasshi. 2014;118(9):773–782.
89. Virgili G, Parravano M, Evans JR, et al. Anti-vascular endothelial growth factor for diabetic macular oedema: a network meta-analysis. Cochrane Database Syst Rev. 2018;10:D7419.
90. Jiang S, Barner JC, Park C, et al. Treatment Patterns of Anti-Vascular Endothelial Growth Factor and Laser Therapy Among Patients with Diabetic Macular Edema. J Manag Care Spec Pharm. 2015;21(9):735–741. doi:10.18553/jmcp.2015.21.9.735
91. Segawa Y, Shirao Y, Yamagishi S, et al. Upregulation of retinal vascular endothelial growth factor mRNAs in spontaneously diabetic rats without ophthalmoscopic retinopathy. A possible participation of advanced glycation end products in the development of the early phase of diabetic retinopathy. Ophthalmic Res. 1998;30(6):333–339. doi:10.1159/000055493
92. Higashimoto Y, Matsui T, Nishino Y, et al. Blockade by phosphorothioate aptamers of advanced glycation end products-induced damage in cultured pericytes and endothelial cells. Microvasc Res. 2013;90:64–70. doi:10.1016/j.mvr.2013.08.010
93. Klaassen I, van Geest RJ, Kuiper EJ, et al. The role of CTGF in diabetic retinopathy. Exp Eye Res. 2015;133:37–48. doi:10.1016/j.exer.2014.10.016
94. Csosz S, Lapeva-Gjonova A, Marko B. New data on the geographical distribution and host utilization of the entomopathogenic fungus Myrmicinosporidium durum. J Insect Sci. 2012;12:129. doi:10.1673/031.012.12901
95. Wang J, Qiao S, Liang S, et al. TRPM4 and TRPV2 are two novel prognostic biomarkers and promising targeted therapy in UVM. Front Mol Biosci. 2022;9:985434. doi:10.3389/fmolb.2022.985434
96. Cole YC, Zhang YZ, Gallo B, et al. Correlation between BAP1 Localization, Driver Mutations, and Patient Survival in Uveal Melanoma. Cancers. 2022;14(17):56. doi:10.3390/cancers14174105
97. Wang T, Bai J, Zhang Y, et al. N(6)-Methyladenosine regulator RBM15B acts as an independent prognostic biomarker and its clinical significance in uveal melanoma. Front Immunol. 2022;13:918522. doi:10.3389/fimmu.2022.918522
98. Luria-Perez R, Helguera G, Rodriguez JA. Antibody-mediated targeting of the transferrin receptor in cancer cells. Bol Med Hosp Infant Mex. 2016;73(6):372–379. doi:10.1016/j.bmhimx.2016.11.004
99. Fabian ID, Onadim Z, Karaa E, et al. The management of retinoblastoma. Oncogene. 2018;37(12):1551–1560. doi:10.1038/s41388-017-0050-x
100. Shigdar S, Lin J, Yu Y, et al. RNA aptamer against a cancer stem cell marker epithelial cell adhesion molecule. Cancer Sci. 2011;102(5):991–998. doi:10.1111/j.1349-7006.2011.01897.x
101. Subramanian N, Raghunathan V, Kanwar JR, et al. Target-specific delivery of doxorubicin to retinoblastoma using epithelial cell adhesion molecule aptamer. Mol Vis. 2012;18:2783–2795.
102. Subramanian N, Kanwar JR, Kanwar RK, et al. EpCAM Aptamer-siRNA Chimera Targets and Regress Epithelial Cancer. PLoS One. 2015;10(7):e132407.
103. Nalini V, Deepa PR, Raguraman R, et al. Targeting HMGA2 in Retinoblastoma Cells in vitro Using the Aptamer Strategy. Ocul Oncol Pathol. 2016;2(4):262–269. doi:10.1159/000447300
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
