Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Direct Immunofluorescence of IgG on Formalin-Fixed Paraffin-Embedded Tissue by Heat-Induced Antigen Retrieval as a Sensitive Method for the Diagnosis of Pemphigus
Authors Zhao W , Zhu H , Zhao X, Wu X, Sun F, Pan M, Zhou S
Received 1 March 2023
Accepted for publication 27 April 2023
Published 10 May 2023 Volume 2023:16 Pages 1233—1241
DOI https://doi.org/10.2147/CCID.S408613
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Wenzhe Zhao,1 Haiqin Zhu,1 Xiaoqing Zhao,1 Xinyi Wu,1 Fei Sun,1 Meng Pan,1,* Shengru Zhou2,*
1Department of Dermatology, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, 200025, People’s Republic of China; 2Department of Dermatology, Dushu Lake Hospital Affiliated to Soochow University (Medical Center of Soochow University, Suzhou Dushu Lake Hospital), Suzhou, 215123, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Shengru Zhou, Department of Dermatology, Dushu Lake Hospital Affiliated to Soochow University, (Medical Center of Soochow University, Suzhou Dushu Lake Hospital), No. 9, Chongwen Road, Suzhou, 215123, People’s Republic of China, Tel +86-17717453465, Fax +86-2164669691, Email [email protected]
Purpose: Direct immunofluorescence (DIF) on frozen sections (DIF-F) plays a key role in the identification and differential diagnosis of bullous dermatoses, which are a group of critical autoimmune diseases that include pemphigus, bullous pemphigoid (BP), and epidermolysis bullosa acquisita (EBA). However, this technique requires specialized laboratory equipment conditions, sample acquisition and sample preservation. In this study, the application value of DIF on paraffin-embedded tissue sections (DIF-P) detecting IgG using heat-induced antigen retrieval (HIAR) in the diagnosis of bullous dermatosis was explored.
Patients and Methods: Samples from 12 patients with pemphigus vulgaris (PV), 10 patients with pemphigus foliaceus (PF), 17 patients with BP, and 4 patients with EBA were retrospectively studied for DIF-P IgG detection. Formalin-fixed, paraffin-embedded tissue (FFPE) was used, and the antigen retrieval method used in the experiment was HIAR. All patients were diagnosed with the autoimmune bullous disease (AIBD) based on clinical presentation, histopathology, DIF-F, and enzyme-linked immunosorbent assay (ELISA).
Results: Intercellular staining for IgG in the epidermis was successful in paraffin-embedded tissue sections in 11 of 12 PV samples and in all 10 PF samples. IgG at the basement membrane zone (BMZ) was not detected by immunofluorescent staining in 17 BP samples and 4 EBA samples.
Conclusion: The detection of IgG by DIF-P using HIAR can be used for the diagnosis of pemphigus as an alternative method to DIF-F.
Keywords: pemphigus, bullous dermatoses, direct immunofluorescence, IgG, paraffin-embedded section, heat-induced antigen retrieval
Introduction
Autoimmune bullous diseases (AIBDs) are a group of life-threatening skin-specific autoimmune diseases. They can be divided into intraepidermal and subepidermal blistering diseases, depending on the location of the blisters caused by autoantibodies.1 Pemphigus is the most important intraepidermal blistering disease and mainly includes pemphigus vulgaris (PV) and pemphigus foliaceus (PF), while subepidermal blistering diseases mainly include bullous pemphigoid (BP), epidermolysis bullosa acquisita (EBA), and linear IgA bullous disease (LABD).2
Laboratory tests are crucial for the diagnosis of AIBD. Currently, direct immunofluorescence on frozen tissue sections (DIF-F), enzyme-linked immunosorbent assay (ELISA), indirect immunofluorescence (IIF) and histopathology are important tools for the diagnosis of AIBD.3 DIF-F has long been the gold standard technique for diagnosing AIBD.4 Positive DIF results in intraepidermal blistering diseases mainly show IgG and/or C3 deposits between epidermal (or epithelial) cells, while in subepidermal blistering diseases mainly show IgG and/or C3 linear deposits at the basement membrane zone (BMZ), and linear deposits of IgA along the BMZ can be seen in LABD.5,6 However, in some cases, it may not be possible to obtain fresh skin tissue for staining. In this situation, formalin-fixed, paraffin-embedded (FFPE) tissue, which is more readily available and can be preserved for long periods of time, has several advantages.
Since the 1990s, heat-induced antigen retrieval (HIAR) has gradually become an important modification in antigen retrieval of FFPE tissue sections.7 HIAR can be used in combination with detection methods both enzyme labeled-antibody method including horseradish peroxidase (HRP) and calf intestinal alkaline phosphatase (AP) in immunohistochemical staining and fluorescence-labeled antibody method.8 Several recent studies investigating the diagnostic methods of AIBD using immunohistochemistry to stain for IgG, IgA and complement have achieved favorable results.9–23 In some of these studies, HIAR was used as a reliable method for antigen retrieval of immunohistochemistry.11,16,18,20 And immunohistochemistry has been included in some guidelines for diagnosis of BP as a supplementary diagnostic tool.24,25
Now, HIAR has also been widely used in the immunofluorescence processing of samples prepared from FFPE tissue sections, for instance, in renal immunopathology.8 When we studied the function of skin infiltrating lymphocytes in pemphigus, we found that HIAR in combination with fluorescence could clearly display the IgG positive plasma cells, B cells and T cells in the dermis of pemphigus lesions.26–28 However, HIAR has not yet been used in the immunofluorescence analysis of IgG deposition of FFPE tissue from AIBD patients.
In this study, DIF on paraffin-embedded tissue sections (DIF-P) detecting IgG using HIAR was performed in pemphigus, BP and EBA patient samples to investigate the efficacy of this method for AIBD diagnosis.
Materials and Methods
Sample
Samples from 12 patients with PV, 10 patients with PF, 17 patients with BP, 4 patients with EBA and 10 normal control individuals were included in this retrospective study. The diagnosis of patients with PV, PF, and BP was confirmed from a combination of typical clinical manifestations and laboratory findings from histopathology, ELISA, IIF, and DIF experiments on frozen skin samples. The diagnosis of patients with EBA was confirmed by clinical manifestations and histopathology, ELISA and DIF on frozen skin samples. Normal skin was obtained from normal tissue surrounding the lesions collected during nevus surgery. All specimens were obtained with the approval of the Ethics Committee of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine.
Hematoxylin and Eosin Staining
The FFPE tissue was cut into thin slices and fixed on positively charged slides. The sections were placed in xylene 3 times for 5 minutes each, then in anhydrous ethanol, 95% ethanol, 85% ethanol, and 75% ethanol 3 times for 5 minutes each in turn, and then the sections were rinsed with distilled water. The sections were stained with hematoxylin and eosin (H&E) in turn and then observed by Olympus microscopy.
ELISA
Anti-desmoglein (Dsg)1, anti-Dsg3, and anti-type VII collagen IgG levels were measured by ELISA performed according to standard procedures (MBL, Nagoya, Japan). Anti-BP180 and anti-BP230 IgG levels were measured by ELISA performed according to standard procedures (EUROIMMUN, Lubeck, Germany).
IIF
IIF was performed according to standard protocols (EUROIMMUN, Lubeck, Germany).
DIF-F
Snap-frozen tissue was transported in Michel media and cut into 5-mm sections. Then, the sections were air-dried and washed 3 times with phosphate-buffered saline (PBS), pH 7.4. After that, the sections were incubated with rabbit anti-human IgG/FITC antibody (Dako, Santa Clara, CA, USA) for 30 minutes at temperature, washed 3 times with PBS at pH 7.4, and mounted with mounting media before viewing with an Olympus fluorescence microscope.
DIF-P
Deparaffinization and rehydration were the same as those used for H&E staining. HIAR was performed using antigen retrieval buffer (Abcam, Cambridge, UK) at 98°C for approximately 20 minutes. The sections were incubated with Protein Block (Abcam, Cambridge, UK) for 1 hour at room temperature for tissue blocking. The sections were incubated overnight at 4°C with a rabbit anti-human IgG monoclonal antibody (Abcam, Cambridge, UK) at a ratio of 1:500, and then the sections were washed 3 times with PBS, followed by incubation with a goat anti-rabbit IgG H&L antibody (Alexa Fluor® 488) (Abcam, Cambridge, UK) at a ratio of 1:300 for 1 hour at room temperature. After washing, the sections were sealed and observed under an Olympus fluorescence microscope.
Statistical Analysis
Data were analyzed using GraphPad Prism version 8.3.0 (GraphPad Software, La Jolla, CA). Fisher’s exact test was used for enumeration data. Differences were considered statistically significant at P < 0.05.
Results
Intraepidermal Blistering Diseases
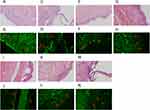
For pemphigus, we selected 12 PV patients and 10 PF patients for DIF-P. First, the paraffin tissue sections of these patients were subjected to fluorescence staining after HIAR to study the deposition of IgG fluorescence between the spinous cells. We found IgG deposits in the epidermis in 11 of the 12 PV samples included in this study (91.67%) and in all 10 PF samples (100%). Representative examples of H&E staining and DIF-P staining IgG results for pemphigus are presented in Figure 1A–D and Figure S1A and B, among which Figure 1A and B are at the perilesion area of pemphigus, while Figure 1C and D and Figure S1A and B are at the blister area. Basically, the fluorescence intensity of IgG of the perilesion (Figure 1B) site was stronger than that of the blister (Figure 1D and Figure S1B). In the 10 normal control samples, no IgG deposits were observed in the epidermis. Representative examples of H&E staining and IgG negative staining results by DIF-P for healthy controls are presented in Figure 1E and F. In comparison, DIF-F staining of IgG was performed in 11 of 12 PV patients, 10 of whom were positive, and DIF-F staining of IgG was positive in all 10 PF patients. General information, ELISA results, and DIF results for the 22 patients included are presented in Table 1. There was no significant difference between DIF-P by HIAR and DIF-F in the positive rates of pemphigus (P>0.9999) (Table 2). The sensitivity of DIF-P by HIAR in pemphigus is 95.45%, the specificity is 100%, and there is no significant difference between DIF-P by HIAR and DIF-F in the diagnosis of pemphigus. These results show that DIF-P using HIAR is effective in the diagnosis of pemphigus.
 |
Table 1 Patient Information and Laboratory Results of Pemphigus |
 |
Table 2 Fisher’s Exact Test of Pemphigus Samples |
Subepidermal Blistering Diseases
For subepidermal bullous disease, we selected 17 patients with BP and 4 patients with EBA. We studied the deposition of IgG fluorescence at the BMZ by DIF-P from these patients after HIAR. In 17 BP samples and 4 EBA samples, no IgG deposits were present at the BMZ by DIF-P. DIF-F IgG staining was performed in all 17 BP patients, 14 of whom were positive. DIF-F staining IgG was also performed in 3 of the 4 EBA patients, and 2 of 3 were positive. In the 10 normal control samples, no IgG deposits were observed at the BMZ by DIF-P. Representative examples of IgG negative staining by DIF-P and H&E staining results for BP are shown in Figure 1G–J, among which Figure 1G and H are at the perilesion area of BP, while Figure 1I and J are at the blister area. Representative examples of IgG negative staining by DIF-P on paraffin-embedded tissue sections and H&E staining results for EBA are shown in Figure 1K-N, among which Figure 1K and L are at the perilesion area of BP, while Figure 1M and N are at the blister area. Information on the 21 patients with subepidermal bullous diseases included and the results of their laboratory tests are listed in Table 3. These results suggest that DIF-P using HIAR has no diagnostic value for subepidermal bullous diseases compared to DIF on frozen sections (P<0.0001) (Table 4).
 |
Table 3 Patient Information and Laboratory Results of BP and EBA |
 |
Table 4 Fisher’s Exact Test of BP Samples |
Non-Autoimmune Blistering Diseases
In addition to the samples included as mentioned above, we also selected some non-autoimmune blistering diseases as controls, including 3 patients with erythema multiforme, 1 patient with epidermolysis bullosa, 2 patients with herpes simplex and 2 patients with drug eruption. In all these samples, no IgG deposits were observed in the epidermis and along the BMZ, at both lesion and perilesion sites. Representative examples of IgG negative staining by DIF-P and H&E staining results for them are shown in Figure S2.
Discussion
DIF-F is not always possible due to several limitations, such as lack of appropriate equipment or reagents, higher technical requirements for operators, frozen samples that cannot be obtained retrospectively because AIBD is not considered, or when perilesion skin is mistakenly placed into a 4% formaldehyde solution. Currently, FFPE tissues from routine histopathology are more easily available, have simpler preservation conditions and exhibit longer storage times, and can be used retrospectively to facilitate clinical auxiliary examination.
This study shows that DIF-P by the HIAR method can be used to confirm intercellular IgG deposition in pemphigus lesions. Out of 22 total pemphigus samples, 21 samples showed a disease-specific staining pattern, demonstrating that this method is a reliable means of diagnosis. In addition, DIF-P after antigen retrieval by enzyme digestion has long been studied in the field. S. L. MERA et al found that DIF-P after antigen retrieval by enzyme digestion in pemphigus, pemphigoid and lupus erythematosus presented disease-specific staining patterns similar to those of frozen sections.29 N. A. Firth et al found that DIF-P after antigen retrieval showed better specificity in the diagnosis of diseases involving the oral mucosa, such as mucous membrane pemphigoid and PV.30 Furthermore, when the frozen tissues were immersed in formalin, negative results would probably be obtained by DIF-F. According to the research by Joshua Arbesman et al, the amount of time the tissue is exposed to formalin determines whether immunofluorescence can be performed, and the longer the time, the less effective the DIF-F is.31
The negative results of the subepidermal blistering disease samples in this study may be related to the method of antigen retrieval. In the present study, we used HIAR, which is the first reported use of HIAR in this context, unlike the enzyme digestion method used in previous studies of bullous diseases.29 Both the HIAR and enzyme digestion methods are based on the principle of disrupting the cross-links between proteins that resulted from formalin fixation, thereby exposing the antigenic epitopes to be recognized by antibodies. The difference is that HIAR uses high temperatures instead of proteolysis to disrupt protein cross-links.32 The specific mechanism of antigen retrieval is not yet fully understood for both methods. Based on the present study and previous studies, we speculate that the heat-mediated method may not adequately expose IgG antigen epitopes in subepidermal blistering disease samples relative to enzyme digestion or may destroy conformational epitopes recognized by antibodies during antigen retrieval, resulting in negative findings.
In the present study, the reason for the failure of DIF-P to show IgG deposits at the BMZ in subepidermal blistering disease samples may also be related to the nature of the BMZ itself. The BMZ is a special structure between the epidermis and dermis of the skin with complex composition.33 We hypothesize that the IgG antigenic epitopes exposed to hyperthermia may interact with some component of the BMZ and are masked, resulting in a negative result due to the inability of the antibody to bind the corresponding antigenic epitopes.
In summary, when there are no fresh frozen samples but paraffin-embedded tissues are available, DIF-P could be chosen as a reliable diagnostic method. Both HIAR and enzyme digestion can be used in pemphigus samples, while only enzyme digestion is effective in pemphigoid.
We also performed DIF-P of C3 by HIAR in AIBD samples, including PV, PF, BP and EBA. Unfortunately, the results in our study were all negative (data not shown). In the literature about AIBD and kidney diseases, the result of C3 staining was often negative or weak by DIF-P, including using enzyme digestion.34,35 Therefore, when we diagnose AIBD, lupus and other diseases by DIF-P, IgG staining is more recommended than C3.
At last, as mentioned above, immunohistochemistry could be used in the diagnosis of AIBD.9–23 However, immunofluorescence is easier to observe than immunohistochemistry due to the nature of the fluorescence signal. In addition, immunofluorescence eliminates the step of DAB coloration, thus further saving time. The use of DIF-P combines the advantages of both techniques.
Conclusion
In conclusion, this study found that DIF-P staining IgG using HIAR can be used as a diagnostic tool for pemphigus and has clinical significance as an alternative to DIF-F when it is not possible.
Abbreviations
DIF, Direct immunofluorescence; DIF-F, direct immunofluorescence on frozen tissue sections; DIF-P, direct immunofluorescence on paraffin-embedded tissue sections; Dsg, desmoglein; BP, bullous pemphigoid; EBA, epidermolysis bullosa acquisita; HIAR, heat-induced antigen retrieval; PV, pemphigus vulgaris; PF, pemphigus foliaceus; FFPE, formalin-fixed, paraffin-embedded tissue; AIBD, autoimmune bullous diseases; ELISA, enzyme-linked immunosorbent assay; LABD, linear IgA bullous disease; IIF, indirect immunofluorescence; BMZ, basement membrane zone; H&E, hematoxylin and eosin.
Ethics Approval and Informed Consent
The research involving human participants was reviewed and approved by the Ethics Committee of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine. The participants provided their written informed consent to participate in this study. The research was carried out following the Helsinki Declaration.
Acknowledgments
We would like to thank all patients who participated in this study.
Funding
This work was supported by grants from the National Natural Science Foundation of China (81903210, 82273514, 82173407, and 81730085), Jiangsu Innovative and Enterpreneurial Talent Programme (JSSCBS20211583) and Gusu Health Talent Program (GSWS2022123).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Egami S, Yamagami J, Amagai M. Autoimmune bullous skin diseases, pemphigus and pemphigoid. J Allergy Clin Immunol. 2020;145(4):1031–1047. doi:10.1016/j.jaci.2020.02.013
2. van Beek N, Zillikens D, Schmidt E. Diagnosis of autoimmune bullous diseases. J Dtsch Dermatol Ges. 2018;16(9):1077–1091. doi:10.1111/ddg.13637
3. Beek NV, Zillikens D, Schmidt E. Bullous autoimmune dermatoses–clinical features, diagnostic evaluation, and treatment options. Dtsch Arztebl Int. 2021;118(24):413–420. doi:10.3238/arztebl.m2021.0136
4. Witte M, Zillikens D, Schmidt E. Diagnosis of autoimmune blistering diseases. Front Med. 2018;5:296. doi:10.3389/fmed.2018.00296
5. Montagnon CM, Tolkachjov SN, Murrell DF, Camilleri MJ, Lehman JS. Intraepithelial autoimmune blistering dermatoses: clinical features and diagnosis. J Am Acad Dermatol. 2021;84(6):1507–1519. doi:10.1016/j.jaad.2020.11.075
6. Montagnon CM, Tolkachjov SN, Murrell DF, Camilleri MJ, Lehman JS. Subepithelial autoimmune blistering dermatoses: clinical features and diagnosis. J Am Acad Dermatol. 2021;85(1):1–14. doi:10.1016/j.jaad.2020.11.076
7. Shi SR, Key ME, Kalra KL. Antigen retrieval in formalin-fixed, paraffin-embedded tissues: an enhancement method for immunohistochemical staining based on microwave oven heating of tissue sections. J Histochem Cytochem. 1991;39(6):741–748. doi:10.1177/39.6.1709656
8. Yamashita S. Heat-induced antigen retrieval: mechanisms and application to histochemistry. Prog Histochem Cytochem. 2007;41(3):141–200. doi:10.1016/j.proghi.2006.09.001
9. Turbitt ML, Mackie RM, Young H, Campbell I. The use of paraffin-processed tissue and the immunoperoxidase technique in the diagnosis of bullous diseases, lupus erythematosus and vasculitis. Br J Dermatol. 1982;106(4):411–417. doi:10.1111/j.1365-2133.1982.tb04533.x
10. Zaenglein AL, Hafer L, Helm KF. Diagnosis of dermatitis herpetiformis by an avidin-biotin-peroxidase method. Arch Dermatol. 1995;131(5):571–573. doi:10.1001/archderm.1995.01690170073010
11. Magro CM, Dyrsen ME. The use of C3d and C4d immunohistochemistry on formalin-fixed tissue as a diagnostic adjunct in the assessment of inflammatory skin disease. J Am Acad Dermatol. 2008;59(5):822–833. doi:10.1016/j.jaad.2008.06.022
12. Chandler W, Zone J, Florell S. C4d immunohistochemical stain is a sensitive method to confirm immunoreactant deposition in formalin-fixed paraffin-embedded tissue in bullous pemphigoid. J Cutan Pathol. 2009;36(6):655–659. doi:10.1111/j.1600-0560.2008.01129.x
13. Pfaltz K, Mertz K, Rose C, Scheidegger P, Pfaltz M, Kempf W. C3d immunohistochemistry on formalin-fixed tissue is a valuable tool in the diagnosis of bullous pemphigoid of the skin. J Cutan Pathol. 2010;37(6):654–658. doi:10.1111/j.1600-0560.2009.01450.x
14. Zhang X, Hyjek E, Soltani K, Petronic-Rosic V, Shea CR. Immunohistochemistry for immunoglobulin G4 on paraffin sections for the diagnosis of pemphigus. Arch Pathol Lab Med. 2012;136(11):1402–1407. doi:10.5858/arpa.2011-0425-OA
15. Kwon EJ, Ntiamoah P, Shulman KJ. The utility of C4d immunohistochemistry on formalin-fixed paraffin-embedded tissue in the distinction of polymorphic eruption of pregnancy from pemphigoid gestationis. Am J Dermatopathol. 2013;35(8):787–791. doi:10.1097/DAD.0b013e3182a6b6cc
16. Abé T, Maruyama S, Babkair H, Yamazaki M, Cheng J, Saku T. Simultaneous immunolocalization of desmoglein 3 and IgG4 in oral pemphigus vulgaris: igG4 predominant autoantibodies in its pathogenesis. J Oral Pathol Med. 2015;44(10):850–856. doi:10.1111/jop.12290
17. Villani AP, Chouvet B, Kanitakis J. Application of C4d immunohistochemistry on routinely processed tissue sections for the diagnosis of autoimmune bullous dermatoses. Am J Dermatopathol. 2016;38(3):186–188. doi:10.1097/DAD.0000000000000333
18. Glauser S, Rutz M, Cazzaniga S, Hegyi I, Borradori L, Beltraminelli H. Diagnostic value of immunohistochemistry on formalin-fixed, paraffin-embedded skin biopsy specimens for bullous pemphigoid. Br J Dermatol. 2016;175(5):988–993. doi:10.1111/bjd.14686
19. Al-Shenawy HA. Can immunohistochemistry replace immunofluorescence in diagnosis of skin bullous diseases? APMIS. 2017;125(2):114–121. doi:10.1111/apm.12643
20. Shimanovich I, Nitz JM, Witte M, Zillikens D, Rose C. Immunohistochemical diagnosis of mucous membrane pemphigoid. J Oral Pathol Med. 2018;47(6):613–619. doi:10.1111/jop.12732
21. Kamyab K, Abdolreza M, Ghanadan A, et al. C4d immunohistochemical stain of formalin-fixed paraffin-embedded tissue as a sensitive method in the diagnosis of bullous pemphigoid. J Cutan Pathol. 2019;46(10):723–728. doi:10.1111/cup.13490
22. Wang LL, Moshiri AS, Novoa R, et al. Comparison of C3d immunohistochemical staining to enzyme-linked immunosorbent assay and immunofluorescence for diagnosis of bullous pemphigoid. J Am Acad Dermatol. 2020;83(1):172–178. doi:10.1016/j.jaad.2020.02.020
23. Kasperkiewicz M, Lai O, Kim G, et al. Immunoglobulin and complement immunohistochemistry on paraffin sections in autoimmune bullous diseases: a systematic review and meta-analysis. Am J Dermatopathol. 2021;43(10):689–699. doi:10.1097/DAD.0000000000001817
24. Feliciani C, Joly P, Jonkman MF, et al. Management of bullous pemphigoid: the European Dermatology Forum consensus in collaboration with the European Academy of Dermatology and Venereology. Br J Dermatol. 2015;172(4):867–877. doi:10.1111/bjd.13717
25. Borradori L, Van Beek N, Feliciani C, et al. Updated S2 K guidelines for the management of bullous pemphigoid initiated by the European Academy of Dermatology and Venereology (EADV). J Eur Acad Dermatol Venereol. 2022;36(10):1689–1704. doi:10.1111/jdv.18220
26. Zou Y, Yuan H, Zhou S, et al. The Pathogenic Role of CD4(+) tissue-resident memory T Cells Bearing T follicular helper-like phenotype in pemphigus lesions. J Invest Dermatol. 2021;141(9):2141–2150. doi:10.1016/j.jid.2021.01.030.
27. Yuan H, Zhou S, Liu Z, et al. Pivotal role of lesional and perilesional T/B lymphocytes in pemphigus pathogenesis. J Invest Dermatol. 2017;137(11):2362–2370. doi:10.1016/j.jid.2017.05.032.
28. Zhou S, Liu Z, Yuan H, et al. Autoreactive B Cell differentiation in diffuse ectopic lymphoid-like structures of inflamed pemphigus lesions. J Invest Dermatol. 2020;140(2):309–18.e8. doi:10.1016/j.jid.2019.07.717
29. Mera SL, Young EW, Bradfield JW. Direct immunofluorescence of skin using formalin-fixed paraffin-embedded sections. J Clin Pathol. 1980;33(4):365–369. doi:10.1136/jcp.33.4.365
30. Firth NA, Rich AM, Radden BG, Reade PC. Direct immunofluorescence of oral mucosal biopsies: a comparison of fresh-frozen tissue and formalin-fixed, paraffin-embedded tissue. J Oral Pathol Med. 1992;21(8):358–363. doi:10.1111/j.1600-0714.1992.tb01365.x
31. Arbesman J, Grover R, Helm TN, Beutner EH. Can direct immunofluorescence testing still be accurate if performed on biopsy specimens after brief inadvertent immersion in formalin? J Am Acad Dermatol. 2011;65(1):106–111. doi:10.1016/j.jaad.2010.06.019
32. Yamashita S, Katsumata O. Heat-induced antigen retrieval in immunohistochemistry: mechanisms and applications. Methods Mol Biol. 2017;1560:147–161. doi:10.1007/978-1-4939-6788-9_10
33. McMillan JR, Akiyama M, Shimizu H. Epidermal basement membrane zone components: ultrastructural distribution and molecular interactions. J Dermatol Sci. 2003;31(3):169–177. doi:10.1016/s0923-1811(03)00045-8
34. Nasr SH, Galgano SJ, Markowitz GS, et al. Immunofluorescence on pronase-digested paraffin sections: a valuable salvage technique for renal biopsies. Kidney Int. 2006;70(12):2148–2151. doi:10.1038/sj.ki.5001990
35. Messias NC, Walker PD, Larsen CP. Paraffin immunofluorescence in the renal pathology laboratory: more than a salvage technique. Mod Pathol. 2015;28:854–860. doi:10.1038/modpathol.2015.1
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.