Back to Journals » Journal of Pain Research » Volume 16
Digital Subtraction Angiography-Guided Percutaneous Kyphoplasty in Treatment of Multi-Segmental Osteoporotic Vertebral Compression Fracture: A retrospective single-Center study
Authors Tan B , Yang QY, Fan B, Li Q, Zhang XY
Received 30 August 2022
Accepted for publication 17 January 2023
Published 22 January 2023 Volume 2023:16 Pages 169—176
DOI https://doi.org/10.2147/JPR.S388068
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Krishnan Chakravarthy
Bing Tan, Qi-Yuan Yang, Bin Fan, Qin Li, Xiao-Yan Zhang
Department of Spine Surgery, The Third Hospital of MianYang, Sichuan Mental Health Center, Mianyang, Sichuan Province, 621000, People’s Republic of China
Correspondence: Qi-Yuan Yang, Department of Spine Surgery, The Third Hospital of MianYang, Sichuan Mental Health Center, Mianyang, Sichuan Province, 621000, People’s Republic of China, Tel +8615882889797, Email [email protected]
Purpose: This study aimed to explore the effectiveness and safety of digital subtractionangiography (DSA)-guided percutaneous kyphoplasty (PKP) in treating multi-segmental osteoporotic vertebral compression fracture (OVCF).
Methods: We retrospectively reviewed 68 patients with multi-segmental OVCF who had unilateral PKP surgeries using DSA and C arm guiding at our hospital between October 2016 and June 2020 and were followed for at least two years. All patients were divided into two groups: DSA guidance (n = 31) and C‐arm guidance (n=37). In addition, we collected the clinical and radiological evaluation results during postoperative and last follow-up periods.
Results: Our findings revealed that the DSA guidance group required lesser time for channel establishment and surgery than the C-arm guidance group at P < 0.05. The incidences of bone cement leakage, fluoroscopy times, and radiation dose of the DSA guidance group were significantly lesser than the C‐arm guidance group (P < 0.05). Compared to the C-arm guidance group, the deviation of puncture in the DSA guidance group was significantly lower, the puncture angle in the DSA guidance group was significantly larger, and better bone cement distribution was obtained (P < 0.05). Compared to preoperative data, the VAS score, median vertebral height, and Cobb angle were significantly improved one day after surgery and the final follow-up in both groups (P < 0.05). However, the VAS score, the median vertebral height, average length of stay, and Cobb angle were not significantly different between the two groups (P > 0.05).
Conclusion: DSA-guided PKP in treating multi-segmental OVCF can shorten the operation time, improve puncture accuracy, reduce the times and dose of fluoroscopy, reduce the leakage of bone cement, and achieve better cement distribution.
Keywords: digital subtraction angiography, percutaneous kyphoplasty, multi-segmental osteoporotic vertebral compression fracture
Introduction
With the advent of an aging society, the incidence of osteoporotic fractures is gradually increasing. OVCF is a common disease among the elderly, accounting for about 40% of all osteoporotic fractures.1 OVCF often causes significant pain, dysfunction, and many complications such as hypostatic pneumonia, spinal deformities, and venous thrombosis of lower limbs, which seriously affects the daily life of the elderly and even leads to death.2 Traditional open surgery carries a high risk of complications and is only suitable for patients with severe neurological dysfunction.3 Presently, percutaneous vertebroplasty (PVP) or PKP combined with anti-osteoporosis is the most effective treatment for OVCF, with less trauma, faster recovery, and fewer complications.4 Compared to PVP, PKP can restore vertebral height and correct kyphosis and obtain better long-term efficacy for vertebral fractures with compression of more than 1/3. However, PKP treatment still has problems such as pedicle cortical perforation, uneven distribution of bone cement, leakage of bone cement, and multiple fluoroscopy times.5–7
With the aging population in the contemporary world, the incidence of multi-segmental OVCF increases yearly, and about 20% of the patients previously diagnosed with compression fractures will develop into multi-segmental OVCF.8 The traditional C-arm-guided PKP has many disadvantages, such as long operation time, multiple fluoroscopy times, and high fluoroscopic dose.9 Multiple osteoporotic vertebral compression fractures frequently combine with a spinal deformity or thoracic fracture. The traditional C-arm fluoroscopy imaging is unclear, resulting in a high risk of surgical puncture, high bone cement leakage rate, and high incidence of complications.10 Therefore, one-stage or staged treatment for multi-vertebral fractures is still controversial. Because of the high risk and complications associated with traditional perspective-guided PKP or PVP treatment for multiple vertebral fractures, some researchers believe that conservative treatment for multiple vertebral fractures can also yield good clinical results.11 However, the number of included cases is small, and the results lack credibility. With the development of modern medical equipment and various new technologies, surgical methods and indications have also undergone tremendous changes by assisting various equipment. Using modern medical equipment, science, and technology to reduce the risk and complications of PKP surgery in treating multiple vertebral fractures is now a research hotspot.
Recently, the application of DSA in surgery has gradually increased, and it is widely used in interventional surgery such as vascular surgery, neuro-surgery, and hepatobiliary surgery.12 Research depicts that DSA assisted surgery can achieve accurate positioning, three-dimensional scanning, and fast and clear imaging, to improve puncture accuracy, reduce the number of fluoroscopies, and shorten the operation time.13 However, to our knowledge, no studies on the safety and efficacy of DSA-assisted PKP in the treatment of multi-segment thoracolumbar OVCF have been reported to date.The aim of our study was to demonstrate that DSA - assisted PKP in the treatment of multi-segment thoracolumbar OVCF is safe and effective and can improve the effectiveness and safety of minimally invasive surgery for multi-segment thoracolumbar OVCF.Since 2016, our hospital has used DSA assisted PKP to treat patients with multi-segment thoracolumbar OVCF. The clinical data of patients were retrospectively analyzed and compared with patients undergoing traditional fluoroscopy. In addition, the safety and effectiveness of DSA-guided PKP were analyzed.
Materials and Methods
Study Population Selection
We performed a retrospective case-control study on 68 patients (including 23 men and 45 women; age range: 57–90 years; mean: 74.7±8.6 years) with multi-segment thoracolumbar OVCF at our hospital between October 2016 and June 2020. All patients received PKP surgery by unilateral puncture approach, with 37 cases receiving DSA (Siemens, US) guided PKP (DSA-guided group, DSA group) and 31 receiving traditional C-arm (Siemens, US) guided (Traditional fluoroscopy group, TRF group). The procedures were performed in the hybrid operating room. Table 1 summarizes the detailed general information of the patients in the two comparable groups (P > 0.05). All patients enrolled in this study met the following inclusion criteria: (1) T3 ~ L5 OVCF, 3 ~ 4 segments fractures; (2) Posterior margin of the vertebral body was intact without symptoms of the spinal cord and nerve root compression; (3) Dual-energy X-ray absorptiometry displayed decreased bone mass or osteoporosis. The patients who met the following exclusion criteria were excluded: (1) Other pathological fractures, such as metastatic tumors, myeloma, hemangioma, etc.; (2) Patients with incomplete clinical data or difficulty cooperating with surgery; (3) Patients suffering from severe coagulopathy. The function of cardiopulmonary was so weak that it could not tolerate surgery. This study was performed in conformity with the Declaration of Helsinki and approved by the ethics committee of the Third Hospital of Mianyang (2022, Reviewed, No.12). Due to the retrospective design of this work, there was no need to obtain informed consent from patients. All data were collected and analyzed anonymously.
 |
Table 1 Patient Demographics of the Two Groups |
Surgical Management
The same senior surgeon performed all the surgeries. The patients who received general anesthesia were laid in a prone position on the radiolucent operating table, and a radiometer was placed on their waist to record the radiation exposure. All cases involved unilateral transpedicular puncture, using the vertebral body shaper and balloon of Suzhou Aid-e Medical Technology Co., Ltd (China) and Heraeus Medical Company (Germany) bone cement.
Traditional fluoroscopy group: The responsible vertebral pedicle was marked using a C-arm X-ray machine. The puncture was performed under C-arm fluoroscopy. The inner core of the working sleeve was used to enter the responsible vertebrae from the outside and above the vertebral arch. The inner core was pulled out when entering about 1/3 of the vertebral body. The bone tunnel was prepared by bone drill, and the balloon was placed into the responsible vertebra. The contrast agent was used to dilate the balloon. After the vertebral height was recovered satisfactorily, the balloon was taken out. Next, a push rod injected the bone cement in the drawing stage into the responsible vertebral body. The amount of bone cement injected into each vertebral body was 2–6 mL, with an average of 3.5 mL. The operation ended after the bone cement hardening.
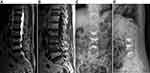
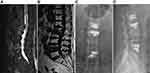
DSA-guided group: The surface marker of the responsible vertebral pedicle was guided by DSA. Multi-vertebral sequential puncture positioning was DSA-guided (Figure 1). After DSA scanning and imaging, the position of the puncture needle (Figure 1) was accurately adjusted. After the position and depth of the puncture needle were appropriate, the needle core was pulled out, the casing was fixed, and the working channel was established. Next, the fine drill was inserted through the working channel, the medulla was expanded, and the balloon was inserted. The balloon was slowly rotated and expanded. After the successful vertebroplasty, the balloon was removed, and bone cement was injected. The amount of bone cement injected into each vertebral body was 2–6 mL, with an average of 3.5 mL. The operation was ended after bone cement hardening (Figures 2 and 3).
Evaluation Criteria
Three independent authors collected clinical data for this study, including clinical and radiological evaluation results before the operation, on postoperative day 1, and at the last follow-up. All patients were followed up for more than one year after surgery.
Clinical evaluation index: (1) Channel establishment time: the establishment time of injured vertebra passage was started when the skin was cut through the puncture point and ended when the working sleeve reached an appropriate position. (2)Intraoperative fluoroscopy times and fluoroscopy dose were recorded in the two groups (fluoroscopy dose was directly recorded by X-ray dose detector); (3) The VAS score was used to evaluate the degree of pain before the operation, one day after the operation, and the last follow-up. We also recorded the operation time and length of stay in the hospital.
Imaging evaluation indicators: (1) Degree of puncture deviation: CT was reviewed within one day after the operation. According to the Gertzbein-Robbins classification standard,13 the degree of puncture deviation was divided into four grades: grade A without deviation, grade B deviation <2 mm, grade C deviation 2–4 mm, and grade D deviation ≥4 mm. (2) Bone cement distribution was based on the bone cement dispersion standard proposed by Lin et al.9 Within one day of the operation, the maximum cross-section of bone cement was selected for analysis of CT images. The distribution of bone cement was divided into four levels: A: bone cement does not cross the middle line of the vertebral body and is not filled unilateral distribution. B: the main body of bone cement reaches the middle line of the vertebral body but does not exceed the maximum cross-sectional width of 10% filling unilateral distribution. C: bone cement beyond the maximum cross-sectional width of 10%, but the filling area does not exceed the maximum cross-sectional area of 3/5 of the non-filling bilateral distribution. D: Bone cement across the midline of the vertebral body and filling area reached the maximum cross-sectional area of 3/5 filling bilateral distribution. (3) Leakage of bone cement: CT imaging was used to determine whether bone cement had leaked into the surrounding tissue within one day of the operation. (4) Puncture angle: The angle between the midline of the vertebral body on the cross-section and the puncture route was observed by three-dimensional reconstruction of CT one day after the operation. (5) The midline height and Cobb angle of the vertebral body were measured on lateral X-ray films before the operation, one day after the operation, and during the last follow-up.
Statistical Analysis
All statistical data analyses were executed using SPSS software, version 23.0 (SPSS Inc, Chicago, IL, USA). Data are expressed as mean±SD. In order to analyze the treatment outcomes, repeated measures multivariate analysis of variances (MANOVA) will be used. Furthermore, an independent two-sample t-test was used to determine whether there was a significant difference between the two groups, whereas the chi-squared test (Fisher’s exact test for small samples) was used to analyze the categorical data in the two groups. P < 0.05 was considered statistically significant for all analyses.
Results
Overall, 68 patients were enrolled in this study, comprising 23 men and 45 women (age range: 57–90 years; mean: 74.7±8.6 years). All patients were followed up for 12–25 months, averaging 15.8±3.6 months. We observed no significant differences in age, sex, BMI, BMD, duration of disease, operative levels, the average length of stay, underlying diseases, follow-up, and average length of stay between DSA-guided and traditional fluoroscopy groups (P > 0.05, Tables 1 and 2). However, the channel establishment time of the DSA-guided group (3.8±0.7 min) was significantly shorter than that of the traditional fluoroscopy group (10±1.4 min) at P < 0.05 (Table 2). In addition, the operation time of the DSA-guided group (59.2±6.6 min) was significantly shorter than that of the traditional fluoroscopy group (95.9±8.0 min) at P < 0.05 (Table 2). Besides, the dose of fluoroscopy (192.7±38.7 cGycm2) and times of fluoroscopy frequency (13.9±2.8 times) of the DSA-guided group was significantly lower than that of the traditional fluoroscopy group (607±51.3 cGycm2, 32.1±5.9 times), respectively (P < 0.05, Table 2). In the DSA-guided group, asymptomatic bone cement leakage occurred in 12 segments (10.1%, 12/119), leakage in the disc space in 8 segments, and paravertebral leakage in the other six segments with no intraspinal and venous plexus leakage (Table 2). In the traditional fluoroscopy group, asymptomatic bone cement leakage occurred in 23 segments (23.2%, 23/99), leakage in the disc space in 12 segments, paravertebral leakage in 8 segments, venous plexus leakage in 1 segment, and intraspinal leakage in 2 segments (Table 2). Statistically significant differences regarding cement penetration were observed between the groups (P < 0.05, Table 2).
 |
Table 2 Comparison of Perspective Indicators, Operation Time, Hospitalization Time and Complications Between the Two Groups |
Compared to the traditional fluoroscopy group, the degree of puncture deviation decreased markedly, the cement distribution was better, and the puncture angle was significantly increased in the DSA-guided group. All the differences were statistically significant (P < 0.05, Table 3).
 |
Table 3 Comparison of Imaging Indexes Between Two Groups |
Compared to preoperative data, the values of the VAS score, Cobb angle, and Mid- height of vertebrae fractured were significantly improved one day after surgery and during the last follow-up in the two groups (P < 0.05, Table 4). Moreover, there was no statistical difference between DSA guided and traditional fluoroscopy groups (P > 0.05, Table 4).
 |
Table 4 Comparison of Surgical Results Between Two Groups |
Discussion
The incidence of osteoporosis is increasing rapidly with the aging population.
Severe osteoporosis often leads to multiple vertebral compression fractures.14 It has been reported that simultaneous puncture PKP is effective in treating multi-vertebral OVCF. Accurate puncture is the key to the success of PKP surgery.15 Research demonstrates that PKP surgery assisted by computer navigation and an orthopedic robot system can shorten the operation time, improve the puncture accuracy, reduce the times and dose of fluoroscopy, reduce bone cement leakage, and achieve better cement distribution.9,16 However, computer navigation and orthopedic robot systems are expensive, making it difficult to popularize hospital technology at all levels. DSA-guided PKP can reduce the risk of puncture, promote the uniform dispersion of bone cement, reduce the operation time and radiation exposure, and improve operational efficiency. Since DSA can be used to assist surgery in different departments, the prevalence of equipment is high.12 However, PKP treatment of multi-vertebral OVCF has not been reported for the DSA-guided unilateral lateral pedicle approach. Study Suggests unilateral approach percutaneous vertebroplasty is an effective treatment for multiple osteoporotic vertebral compression fractures.10 However, This procedure is time-consuming, high-risk and has high surgical complications, etc.Therefore, our study used multi-vertebral sequence puncture and DSA scanning reconstruction after precise adjustment of puncture needle position for PKP treatment of multi-vertebral OVCF. The results revealed that the puncture deviation of the DSA-guided group was significantly better than the traditional fluoroscopy group. The DSA-guided PKP can significantly improve puncture accuracy and reduce the risk of puncture deviation.
In the traditional fluoroscopy-guided puncture, the puncture point and angle are often difficult to control, which easily leads to the end of the working sleeve not reaching the ideal position, resulting in uneven bone cement distribution.17 When designing the puncture path before the operation, the puncture angle can be appropriately increased, and the puncture needle can be guided to penetrate the vertebral body one time by DSA three-dimensional scanning during operation so that the end of the working channel is as close as possible to the middle line of the vertebral body.18 The results demonstrated that the puncture angle of the DSA-guided group was significantly larger than that of the traditional fluoroscopy group, which may be one of the reasons for the more uniform distribution of bone cement in DSA guided group. Related studies have demonstrated that DSA-guided surgery can reduce intraoperative fluoroscopy times and radiation injury.12,18 Furthermore, a recent study demonstrated that PKP guided by DSA could reduce the times and doses of fluoroscopy.19 However, there is no report on the fluoroscopy dose of PKP guided by DSA in treating multi-vertebral osteoporotic fractures. Therefore, this study observed that the times and doses of fluoroscopy in the DSA-guided group were significantly lower than those in the traditional fluoroscopy group. This further indicated that DSA-guided PKP could significantly reduce the times and doses of fluoroscopy in treating multi-segment OVCF.
Conclusion
In summary, this study reveals that the DSA-guided PKP in the treatment of multi-segmental OVCF can shorten the operation time, improve puncture accuracy, reduce the times and doses of fluoroscopy, reduce bone cement leakage, achieve better cement distribution, and has good clinical application prospects. However, this study was only a retrospective study with limited data because of the small sample size. Therefore, a large, multicenter study is required to confirm these promising results.
Acknowledgments
The study was not supported by any financial funding. No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript.
Author Contributions
All authors significantly contributed to the work reported, whether in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; provided final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no competing interests in this work.
References
1. Wang L, Yu W, Yin X, et al. Prevalence of osteoporosis and fracture in China: the China osteoporosis prevalence study. JAMA Netw Open. 2021;4(8):e2121106. doi:10.1001/jamanetwor-kopen.2021.21106
2. Ning L, Zhu J, Tian S, et al. Analysis between basic diseases and subsequent vertebral fractures after percutaneous kyphoplasty (PKP) for osteoporotic vertebral compression fractures. Pain Physician. 2021;24(6):E803–E810.
3. Hou Y, Zhou B, Amuti A, et al. Rapid efficacy of percutaneous kyphoplasty (PKP) in treating thoracolumbar fractures in elderly patients. Am J Transl Res. 2021;13(4):2662–2669.
4. Wang H, Sribastav SS, Ye F, et al. Comparison of percutaneous vertebroplasty and balloon kyphoplasty for the treatment of single level vertebral compression fractures: a meta-analysis of the literature. Pain Physician. 2015;18(3):209–222.
5. Wei H, Dong C, Zhu Y, et al. Analysis of two minimally invasive procedures for osteoporotic vertebral compression fractures with intravertebral cleft: a systematic review and meta-analysis. J Orthop Surg Res. 2020;15(1):401. doi:10.1186/s13018-020-01938-6
6. Ma XL, Xing D, Ma JX, et al. Balloon kyphoplasty versus percutaneous vertebroplasty in treating osteoporotic vertebral compression fracture: grading the evidence through a systematic review and meta-analysis. Eur Spine J. 2012;21(9):1844–1859. doi:10.1007/s00586-012-2441-6
7. Hu KZ, Chen SC, Xu L. Comparison of percutaneous balloon dilation kyphoplasty and percutaneous vertebroplasty in treatment for thoracolumbar vertebral compression fractures. Eur Rev Med Pharmacol Sci. 2018;22(1 Suppl):96–102. doi:10.26355/eurrev_201807_15370
8. Tseng YY, Yang TC, Tu PH, et al. Repeated and multiple new vertebral compression fractures after percutaneous transpedicular vertebroplasty. Spine. 2009;34(18):1917–1922. doi:10.1097/BRS.0b013e318
9. Lin S, Hu J, Wan L, et al. Robot-guided percutaneous kyphoplasty in treatment of multi-segmental osteoporotic vertebral compression fracture. ChinJ Reparat Reconstruct Surg. 2020;34(9):1136–1141. doi:10.7507/1002-1892.202002131
10. Saracen A, Kotwica Z. Treatment of multiple osteoporotic vertebral compression fractures by percutaneous cement augmentation. Int Orthop. 2014;38(11):2309–2312. doi:10.1007/s00264-014-2470-3
11. Buchbinder R, Osborne RH, Ebeling PR, et al. randomized trial of vertebroplasty for painful osteoporotic vertebral fractures. N Engl J Med. 2009;361(6):557–568. doi:10.1056/NEJMoa0900429
12. Tan B, Yang QY, Fan B, et al. Is it necessary to approach the severe osteoporotic vertebral biconcave-shaped fracture bilaterally during the process of PKP? J Pain Res. 2021;14. doi:10.2147/JPR.S293528
13. Gertzbein SD, Robbins SE. Accuracy of pedicular screw placement in vivo. Spine. 1990;15(1):11–14. doi:10.1097/00007632-199001000-00004
14. Ballane G, Cauley JA, Luckey MM, et al. Worldwide prevalence and incidence of osteoporotic vertebral fractures. Osteoporos Int. 2017;28(5):1531–1542. doi:10.1007/s00198-017-3909-3
15. Fang SY, Dai JL, Min JK, et al. Analysis of risk factors related to the re-fracture of adjacent vertebral body after PKP. Eur J Med Res. 2021;26(1):127. doi:10.1186/s40001-021-00592-w
16. Laudato PA, Pierzchala K, Schizas C. Pedicle screw insertion accuracy using O-arm, robotic guidance, or freehand technique: a comparative study. Spine. 2018;43(6):E373–E378. doi:10.1097/BRS.00.00000000002449
17. Barake M, El Eid R, Ajjour S, et al. Osteoporotic Hip and vertebral fractures in the Arab region: a systematic review. Osteoporos Int. 2021;32(8):1499–1515. doi:10.1007/s00198-021-05937-z
18. Ban J, Peng L, Li P, et al. Performance of Double-Arm Digital Subtraction Angiography (DSA)-Guided and C-Arm-Guided Percutaneous Kyphoplasty (PKP) to treat senile osteoporotic vertebral compression fractures. Med Sci Monit. 2020;26:e923619. doi:10.12659/MSM.923619
19. Chen Z, Lou C, Yu W, et al. Comparison of intravertebral clefts between kümmell disease and acute osteoporotic vertebral compression fracture: a radiological study. Orthop Surg. 2021;13(7):1979–1986. doi:10.1111/os.13025
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.