Back to Journals » OncoTargets and Therapy » Volume 15
Diffuse Large B-Cell Lymphoma (DLBCL): Early Patient Management and Emerging Treatment Options
Authors Vodicka P , Klener P, Trneny M
Received 30 August 2022
Accepted for publication 29 November 2022
Published 6 December 2022 Volume 2022:15 Pages 1481—1501
DOI https://doi.org/10.2147/OTT.S326632
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Sanjeev K. Srivastava
Prokop Vodicka, Pavel Klener, Marek Trneny
First Department of Medicine, First Faculty of Medicine, Charles University and General University Hospital, Prague, Czech Republic
Correspondence: Marek Trneny, First Department of Medicine, First Faculty of Medicine, Charles University and General University Hospital, U Nemocnice 499/2, Prague, 128 08, Czech Republic, Tel +420 224 96 25 27, Fax +420 224 96 35 56, Email [email protected]
Abstract: Diffuse large B-cell lymphoma (DLBCL) represents a curable disease with a 60– 70% chance of cure with current R-CHOP chemoimmunotherapy. However, 30– 40% of patients are refractory or relapsing. Many attempts failed to improve the outcome of DLBCL patients, including the intensification of R-CHOP regimen, consolidation, or maintenance therapy since the introduction of R-CHOP in 2000. Better understanding of both molecular biology of lymphoma cells and the tumor microenvironment raised the hope for future improvement of DLBCL patients’ survival. Novel molecular findings have initiated clinical trials exploring targeted therapy based on driver genetic alterations with an intent to improve survival of high-risk subsets of patients. But the preliminary results remain ambiguous. The approach “agnostic” to specific molecular alterations of lymphoma cell includes antibody-drug conjugates (especially polatuzumab vedotin), immunotherapy comprising different antibodies with immunomodulatory effect (tafasitamab, lenalidomide), and T-cell engaging therapy (bispecific antibodies, early use of CAR T-cell). This approach could increase the cure rates and change the current therapeutic paradigm. However, better prognostic stratification, smarter designs of clinical trials, modification of endpoints including the use of ctDNA are needed. This review covers the complexity of DLBCL management.
Keywords: diffuse large B-cell lymphoma, first-line therapy, R-CHOP, agnostic therapy, tailored therapy, polatuzumab vedotin
Introduction and Epidemiology
Diffuse large B-cell lymphoma (DLBCL) is the most common type of non-Hodgkin lymphomas (NHL) accounting for 30–40% of B-cell NHL (B-NHL). The incidence rises with age, reaching a median age at diagnosis in the 7th decade. However, DLBCL can occur at any age, with a slight predominance in males.1–3 It can arise de novo or as transformation from an underlying indolent lymphoma. Multiple risk factors are associated with development of the disease, including genetic features, infectious and environmental influences.4
Most patients present with generalized lymphadenopathy. Extranodal involvement can be found in approximately 30% of the patients, most commonly affecting gastrointestinal tract, bones, testes, spleen, central nervous system (CNS), and other sites.5
DLBCL is a potentially curable disease with an overall 60–70% chance of cure with the currently used front-line immunochemotherapy consisting of rituximab (R) in combination with cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP; Figure 1).6 Nevertheless, 30–40% of the patients are either refractory (ie, no response or primary progression after the treatment) to the first-line treatment or experience relapse (R/R).
Because of a large biological heterogeneity of the disease, this article mainly focuses on the DLBCL, not otherwise specified (NOS). The current first-line treatment regimen R-CHOP targets DLBCL tumor cells regardless of the molecular subtype of DLBCL. This therapeutical approach “agnostic” to the molecular biology of DLBCL also includes innovative drugs from the group of monoclonal antibodies, antibody-drug conjugates, and immune system engaging therapy. On the other hand, some clinical trials have started testing targeted therapy based on the knowledge of molecular pathogenesis of DLBCL. In this article, we review the first-line standards of care of DLBCL and discuss the emerging treatment options that might change the current paradigm of R-CHOP regimen superiority.
Taxonomy and Molecular Classification
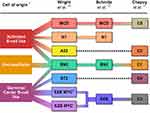
DLBCL is a highly heterogenous disease. The diagnosis is based on histopathological and molecular analysis of a tumor biopsy. In the era of gene expression profiling (GEP) the original morphological variants of DLBCL (ie, centroblastic, immunoblastic, anaplastic, and others) have lost their clinical significance. The novel classification systems based on molecular biology and genetics are being incorporated into a daily practice with an intent to identify higher-risk patient populations with inferior survival on the current standard of care R-CHOP.
Cell of Origin
A pioneer GEP in early 2000s led to discovery of two molecular subtypes of DLBCL: germinal center B-cell-like (GCB) DLBCL and activated B-cell-like (ABC) DLBCL.7 The molecular profile of the GCB subtype correlates with germinal center B cells including the expression of CD10, BCL-6, MYD88, and EZH2 molecules. Expression of the nuclear factor kappa B (NF-κB) and BCL-2 is typical for the ABC subtype, and it reflects the post-germinal origin of these lymphoma cells. Importantly, patients with the ABC subtype have inferior survival compared to the GCB subtype.8 Approximately 10–15% of cases remain unclassifiable. Since the GEP cannot be performed in all newly diagnosed DLBCL in a daily routine, surrogate immunohistochemical approaches, including so-called Hans algorithm, were developed to determine the cell of origin.9 However, the algorithms are considered only as an approximation of GEP dichotomizing DLBCL cases into GCB and non-GCB (ie, ABC and unclassifiable) subtypes.10
Molecular Classification
Currently, there are several proposed molecular classification systems of DLBCL (Figure 2). The next-generation sequencing of de novo DLBCL cases revealed five clusters (C1–C5) with different genetic aberrations and outcomes.11 The former ABC subgroup was divided into two clusters. A low-risk cluster C1 is associated with structural variants of BCL6 gene and mutations in the NOTCH2 and NF-κB pathways genetically related to marginal-zone B-cells. The cluster C5 exhibits 18q chromosomal gains with overexpression of BCL-2 protein and frequent mutations of CD79B and MYD88L265P genes, which might be associated with an extranodal involvement and poor prognosis. Another two clusters (C3, C4) are related to the GCB subtype. The C3 group carries BCL2 mutations and its structural variants, as well as frequent mutations of KMT2D, CREBBP, and EZH2 genes leading to a worse prognosis in comparison to the C4 group. The C4 group bears mutations in 4 linker and 4 core histone genes, as well as further molecular changes. The C3 and C4 groups presumably use different mechanisms to modify the intracellular signaling pathways including the PI3K pathway. The C2 group includes both ABC and GCB cases and is associated with inferior survival and biallelic inactivation of TP53 caused by various gene mutations as well as chromosomal 17p loss.
Another recently published genetic classification of DLBCL proposed four genetic subtypes called MCD, BN2, N1, and EZB.12 The MCD subtype exhibits frequent mutations of the MYD88L265P gene together with CD79B aberrations and thus closely correlates to the C5 cluster in the previous work.11 The N1 subtype harbors recurrent NOTCH1 mutations. MCD and N1 groups correspond to the ABC subtype and both correlate with inferior survival. The BN2 subtype is related to NOTCH2 mutations and BCL6 rearrangement, analogous to C1 cluster. The EZB subtype is characterized by EZH2 mutation and BCL2 translocation typical for GCB subtype and C3 cluster. Both BN2 and EZB are associated with a better prognosis in comparison to the other subtypes.
Based on the proposed molecular classification systems,11,12 subsequent analysis described seven genetic subtypes of DLBCL using an algorithm called “LymphGen” (MCD, N1, A53, BN2, ST2, EZB MYC+, and EZB MYC-).13 The specific subtypes have different gene expression profiles, microenvironmental features, and outcomes. The study revealed relationship between certain subtypes of DLBCL and extranodal lymphoma involvement or indolent lymphomas indicating a potential shared pathogenesis of these entities. Detection of genetic alterations is essential to recognize high-risk subsets of the disease moving towards tailored therapy for the patients. However, this approach does not have application in a daily clinical practice yet and is now being used only in some clinical trials. The detection of genetic alterations has certain limitations given mostly by a significant time prolongation of a diagnostic process. This fact may lead to a delayed treatment initiation, which could endanger especially those patients with high-risk disease. A potential compromise could be an early initiation of a non-targeted treatment (ie, one cycle of R-CHOP) with subsequent adjustment of the therapeutic schedule based on the results of genetic analysis.
Recurrent Genetic Changes
A MYC proto-oncogene rearrangement is detected in approximately 12% of DLBCL cases by fluorescence in situ hybridization. DLBCL with MYC rearrangement together with rearrangement of an anti-apoptotic gene BCL2 and/or transcription repressor gene BCL6 is classified by the revised 4th edition of the World Health Organization (WHO) classification as “High-grade B-cell lymphomas with MYC and BCL2 and/or BCL6 rearrangement”.14 These lymphomas are predominantly of GCB subtype.15,16 Patients with these so-called “double-” or “triple-hit” lymphomas (10% of all DLBCL cases) have inferior survival when treated with the standard R-CHOP. Although the patients might profit from an intensified first-line chemoimmunotherapy, prospective randomized trials are missing.17,18
Unlike double- and triple-hit neoplasms, an immunohistochemical overexpression of MYC protein is detected in approximately half of all newly diagnosed DLBCL. Almost one-third of all DLBCL cases present with overexpression of MYC and BCL-2; these lymphomas are called “double-” or “dual-expressors” and are considered to have a decreased survival.19 In contrast to double-hits, the double-expressors can be found among GCB as well as ABC subtypes of DLBCL.
Novelties in the Classification of Lymphoid Malignancies
The upcoming 5th version of the WHO classification brings some reorganizations and nomenclature modifications to the family of large B-cell lymphomas as a result of new insights into the molecular background of the lymphoid malignancies.20,21 DLBCL, NOS remains the most common type of the family of large B-cell lymphomas. It is a heterogenous group of lymphomas that does not harbor diagnostic criteria of specific large B-cell lymphoma entities. Among the group of DLBCL, NOS, the 5th WHO classification continues to recognize the GCB and non-GCB subtypes, although the clinical impact of the cell of origin remains limited. The recently published proposals for molecular classification using next-generation sequencing11–13 have not been included in the WHO classification, since no unifying principles of these classification systems were recognized; moreover, the clinical significance of these classifications remains to be established.
The former group of “High-grade B-cell lymphomas with MYC and BCL2 and/or BCL6 rearrangement” was precise and renamed the “Diffuse large B-cell lymphoma/High-grade B-cell lymphoma with MYC and BCL2 rearrangements”. This novel more homogenous entity comprises only GCB subtypes with rearrangement of MYC and BCL2. Remaining cases with MYC and BCL6 rearrangement are considered to be more heterogenous and are classified as either “DLBCL, NOS”, or “High-grade B-cell lymphoma, NOS”. Due to its mutational spectrum being more similar to the GCB subtype of DLBCL than to Burkitt lymphoma, the entity “High-grade B-cell lymphoma with 11q aberration” was renamed from the previous “Burkitt-like lymphoma with 11q aberration”.
Beside the primary CNS lymphomas, a newly proposed group of “Large B-cell lymphomas of immune-privileged sites” includes also DLBCL of testis and vitreo-retina previously classified as DLBCL, NOS. Next, “Mediastinal gray zone lymphoma” is to replace the group “B-cell-lymphoma, unclassifiable with features intermediate between DLBCL and classic Hodgkin lymphoma”. Extra-mediastinal cases of these lymphomas will be classified as DLBCL, NOS.
Recently, another proposed classification system has been published, by the joint Clinical Advisory Committee of the Society for Hematopathology and European Association for Haematopathology.22 This classification system shares many similarities with the upcoming 5th version of the WHO classification, but there are some differences as well. Along with the WHO classification, the committee recommends maintaining the cell of origin designation of DLBCL, NOS, while the role of morphological and phenotypic variants has limited clinical impact. Although the novel molecular classification systems fail to classify all cases of DLBCL, this approach might be used in the near future, as well as in development of new clinical trials.11–13 Extranodal ABC subtypes of DLBCL share clinical and molecular features, especially those arising in the immune-privileged sites (ie, primary CNS lymphomas and DLBCL of testis). For this reason, there was a tendency to group these DLBCL cases. In contrast to the 5th version of the WHO classification (newly proposed group of “Large B-cell lymphomas of immune-privileged sites”), this subcategorization was concluded as premature.
The former entity “Burkitt-like lymphoma with 11q aberration” (“High-grade B-cell lymphoma with 11q aberration”) identified in the WHO classification was proposed to be renamed as “Large B-cell lymphoma with 11q aberration” since it shares more similarities with DLBCL than with Burkitt lymphomas. Along with the novel WHO classification, the committee recommends distinguishing the High-grade B-cell lymphoma with MYC and BCL-2 rearrangements as an aggressive lymphoma of GCB origin from the High-grade B-cell lymphoma with MYC and BCL-6 rearrangements. Other differences in the large B-cell lymphoproliferative disorders linked with different viral agents are beyond the content of this review.
Staging, Risk Stratification, and Circulating Tumor DNA
Staging
PET/CT Scan
Positron emission tomography/computed tomography (PET/CT) using 18F-fluorodeoxyglucose (FDG) has largely replaced a conventional CT scan and it represents a useful method to determine the clinical stage of a newly diagnosed DLBCL. PET/CT is routinely used also for the end-of-treatment evaluation of response to the therapy according to the Deauville score comparing the FDG uptake in the target lesion to the uptake of mediastinum and liver tissue.23 However, using PET/CT has some limitations including false positivity due to the uptake of FDG in other metabolic active sites (eg, inflammation), as well as false negative results in patients with minor residues of the tumor.24
Although interim PET/CT scan evaluation showed ambiguous results, it seems to be a useful tool to detect patients with no response to the first-line treatment, especially when combined with quantitative PET/CT methods.25 Recently published data demonstrated that the decrease of standard uptake volume (ΔSUV) of more than 66% on the interim PET/CT scans has an impact on the overall survival (OS) of patients with DLBCL.26 A PET/CT guided therapy was also investigated in a phase III trial randomizing the “negative” patients (with ΔSUV over 66% to 4 cycles R-CHOP ± 2 cycles of R) and the “positive” patients (to 6 cycles R-CHOP arm or Burkitt-like chemotherapy protocol), showing a predictive value of ΔSUV on the progression-free survival (PFS).27 However, the “positive” patients did not profit from dose-intensification treatment, which corresponds to the previously published data.28
The estimation of total metabolic tumor volume (TMTV) as a quantitative PET/CT method was shown to bear an independent prognostic value in the newly diagnosed DLBCL.29–32 The high TMTV was associated with inferior survival, but the cut-off value for high TMTV category varied among the studies. The value of pretreatment TMTV as a prognostic factor remains unclear when compared to the well-established prognostic indexes.29,32
Bone Marrow Examination
Bone marrow involvement is present in 10–25% of DLBCL cases, including up to 10% of cases with concordant DLBCL infiltration, while another 10% of cases are diagnosed with a discordant bone marrow infiltration by an indolent lymphoma.33 The DLBCL infiltration is associated with a poorer outcome, but the clinical significance of an indolent lymphoma infiltration remains largely unclear.33,34 Although PET/CT scan is less sensitive in detecting the bone marrow involvement than histological examination, the biopsy is no longer recommended for those patients who undergo PET/CT scan at the time of diagnosis. The bone marrow biopsy examination is still indicated in some PET/CT negative cases to identify discordant infiltration by an indolent lymphoma, especially in clinical trials, or if the information is relevant for further therapeutical management of the patient.
Risk Stratification
Staging systems according to the Ann Arbor clinical stage and Lugano criteria have remained generally accepted.35,36 The International Prognostic Index (IPI) as a predictor of outcomes of patients with newly diagnosed DLBCL has remained an essential stratification tool for decades.37 Moreover, the IPI serves as an important stratifier in clinical trials. Since the survival of the patients decreases with age, a new age-adjusted IPI (aaIPI) was defined as an age-independent prognostic index. Although a revised IPI (R-IPI) was validated in the rituximab era bringing a better predictive value of outcome,38 the proper IPI retained its clinical relevance.39 Another recently published prognostic factor, the National Comprehensive Cancer Network IPI (NCCN-IPI), represents a tool for further stratifying very high-risk subpopulations of patients.40,41
Circulating Tumor DNA
The circulating tumor DNA (ctDNA) is extensively investigated in patients with hematological malignancies, including DLBCL. The non-invasive assessment of ctDNA can be used as a marker of treatment efficacy, and as an indicator of DLBCL relapses. This was confirmed by a retrospective study of DLBCL cases analyzing clonal IGH-VDJ gene rearrangements with a high predictive value for relapse of the disease.42 The molecular signs of a relapse preceded the progression detectable by imaging methods by 3.5 months.42,43 The ctDNA level quantification at the time of diagnosis detected by Cancer Personalized Profiling by deep Sequencing (CAPP-Seq) independently predicted the outcome of DLBCL patients.44 The quantification of ctDNA levels might also be of help in distinguishing between the tumor flare/pseudo-progression and the relapsed/progressive disease as it was previously shown in patients with Hodgkin lymphomas treated by immune checkpoint inhibitors.45 The use of ctDNA is still investigational and has no application in daily clinical practice yet, but it might serve as a perspective predictor of treatment efficacy as well as an early marker of DLBCL relapses in the near future.
Standard of Care in the First Line
From CHOP to R-CHOP
A combination of four drugs cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) was developed for treatment of NHL in the 1970s.46 Multiple efforts were subsequently made to improve the outcome of aggressive NHL including DLBCL by intensifying the chemotherapeutical regimen or adding new drugs into the combination (Supplementary Table).47 However, the classical CHOP regimen remained unbeaten until early 2000s.
Based on clinical trials, a monoclonal anti-CD20 antibody IDEC-C2B8, later called rituximab, was first approved in 1997 by the US Food and Drug Administration to treat patients with R/R low-grade NHL.48 The efficacy of rituximab was further confirmed in the treatment of patients with DLBCL in monotherapy and in an R/R setting. A pivotal GELA trial showed the combination of 8 cycles of rituximab plus CHOP (ie, R-CHOP) with an interval of 21 days to be more effective in terms of complete remission (CR) rate and led to a superior event-free survival (EFS; 57% vs 38% at 2 years) and OS (OS; 70% vs 57% at 2 years) in patients between 60–80 years of age with DLBCL in comparison to the former CHOP regimen without any increase in the toxicity profile.49 The benefit of rituximab with CHOP was further confirmed by Habermann et al in DLBCL patients over 60 years of age,50 and by Pfreundschuh et al in the MInT trial in younger DLBCL patients (18–60 years of age; 2-year EFS 74% vs 56%).51 Subsequent studies showed no difference in survival of patients treated with six versus eight cycles of R-CHOP.52–54 As a consequence, 6 cycles of R-CHOP-21 has remained a standard of care for the following 2 decades.
Limited-Stage Disease
One-third of patients diagnosed with de novo DLBCL present with a limited-stage disease, ie, Ann Arbor stage I or II without bulky mass. The main objective in these patients is to maintain the efficacy of the treatment but avoid overtreatment. In the pre-rituximab era, the patients profited from an abbreviated schedule of 3 cycles of CHOP with subsequent radiotherapy (RT) in comparison to the original 8 cycles of CHOP (5-year OS 82% vs 72%).55 Nevertheless, late relapses did occur in both arms of this study with no difference in long-term survival.56 The implementation of rituximab into the abbreviated regimen (ie, 3 cycles of R-CHOP + RT) further improved survival of these patients (4-year PFS 88%, 4-year OS 92%).57 A phase III FLYER trial compared the efficacy of 4 cycles of R-CHOP + 2 cycles of R versus 6 cycles of R-CHOP in low-risk patients up to 60 years of age with Ann Arbor stages I–II, normal serum lactate dehydrogenase (LDH) concentration, performance status according to the Eastern Cooperative Oncology Group (PS ECOG) 0–1, and without bulky disease. This trial demonstrated no differences in survival between both subgroups (3-year PFS 96% vs 94%, 3-year OS 99% vs 98%).58 Thus, the 4 cycles R-CHOP + 2 cycles of R became a new standard of care.
The role of RT has been investigated by several studies. Patients between 18–75 years of age in CR after 4 cycles of R-CHOP were randomized to a consolidative RT vs observation. No significant differences in survival were shown (5-year EFS 92% vs 89%, 5-year OS 96% vs 92%) indicating that RT brings no benefit to these patients in CR.59 A recent phase II trial randomized the patients of all ages according to the residual disease on interim PET/CT scan after 3 cycles of R-CHOP to an additional one cycle of R-CHOP (if PET/CT negative) or RT in combination with radioimmunotherapy by ibritumomab tiuxetan (if PET/CT positive). The PET/CT negative patients achieved 5-year PFS of 89%, while the PET/CT positive patients 5-year PFS was 86%.60 Thus, the trial confirmed 4 cycles of R-CHOP as the new standard approach for most patients with limited-stage disease. However, more prospective trials are needed to establish the role of consolidative RT for those patients who do not reach CR on interim PET/CT.
Population with Advanced Age and/or Comorbidities
The R-CHOP regimen was confirmed to be effective for patients between 60 and 80 years of age who are feasible candidates for receiving full-dose anthracyclines.6,49 However, one-fourth of all patients with newly diagnosed DLBCL are not candidates for the full-dose R-CHOP and the therapeutical approach remains challenging for many reasons, including decreased fitness and frailty, as well as frequent underlying comorbidities and the advanced age itself. The attenuated anthracycline-based regimen R-miniCHOP with the 2-year PFS of 47% and OS of 59% is possibly the most widely used treatment regimen for these patients.61 Nevertheless, the attenuation of the anthracycline-based treatments for elderly patients and for those with cardiac comorbidities is subject to great variability in the real-life settings or the patients are often provided with different non-anthracycline-based chemotherapy regimens with reduced intensity.62,63 Although exceptions exist,64,65 very elderly patients are frequently excluded from clinical trials. Thus, the evidence-based data on treatment of these patients remains limited. A currently ongoing phase II/III SWOG 1918 study is investigating the efficacy of oral azacitidine in combination with R-miniCHOP versus R-miniCHOP alone in patients aged 75 years and older. Of note, this study incorporates an assessment of baseline frailty as well as comprehensive geriatric assessment.66 Previous studies reported the comprehensive geriatric assessment to be a useful tool to estimate the fitness and predict potential adverse events of the therapy in very elderly patients with DLBCL.67,68
Intensification of the R-CHOP Regimen
The Backbone
To improve the outcomes of high-risk DLBCL patients, many attempts were made to intensify the R-CHOP regimen by using two different strategies: either by shortening the intervals between the cycles from 21 to 14 days, or by dose intensification of the chemotherapeutical regimen. Although a significantly prolonged survival of patients aged ≥60 years was reported by Pfreundschuh et al when shortening the interval between the CHOP cycles to 14-day intervals,69 this was not confirmed in the general patient population with DLBCL treated by R-CHOP.70
The only dose-intensified regimen R-ACVBP led to a superior survival in younger patients with aaIPI ≤1 in comparison to the conventional R-CHOP regimen (3-year EFS 81% vs 67%).71 A subsequent GAINED trial comparing the efficacy of rituximab versus obinutuzumab with chemotherapy in patients with advanced-stage DLBCL and testing both R-CHOP and R-ACVBP regimens did not suggest a benefit of R-ACVBP over R-CHOP in the subgroup of patients treated by rituximab.72 Therefore, and due to an increased number of serious hematologic toxicities during the experimental treatment, this regimen was not implemented into a daily practice.
The addition of etoposide into the CHOP regimen (ie, CHOEP) did not significantly improve outcome in elderly DLBCL patients.69 Although there is no randomized phase III trial that has shown benefit of adding etoposide to R-CHOP in the first-line treatment, this regimen was associated with an improved survival in younger high-risk patients based on two retrospective studies73,74 and one prospective trial testing etoposide in both experimental and control arms with or without autologous stem cell transplantation (ASCT)75 when compared to previously published outcomes of R-CHOP regimen. The dose-adjusted EPOCH-R regimen containing etoposide showed no survival benefit in DLBCL patients when compared to R-CHOP and it was associated with increased toxicity.76 A phase II trial investigated efficacy of this regimen in DLBCL patients with MYC rearrangement including double-hit lymphomas.77 The treatment led to durable remissions, 4-year EFS of 55%, and tolerable toxicity profile. This study suggested that dose-adjusted EPOCH-R should be considered for the treatment of the aggressive B-NHL with MYC rearrangement.
Monoclonal Antibody
The use of rituximab as a part of standard R-CHOP regimen is well established by previously published trials. However, attempts to improve the outcome of patients with DLBCL by intensification of the rituximab dose failed,78,79 and the increased dose of rituximab led to multiple complications, mainly infections.80
Consolidation Treatment
RT as a consolidative treatment in patients with limited-stage disease who reach CR on the end-of-treatment PET/CT neither decreased the relapse rate nor improved their survival.59 Recently published data demonstrated the RT to have impact on the outcome of patients with limited-stage disease60 as well as advanced-stage DLBCL81 with PET-positive residual after the treatment termination, with a similar survival to those individuals who were PET-negative. This PET-guided approach could improve the outcome of RT-feasible patients in partial remission by avoiding the side effects of salvage chemoimmunotherapy and ASCT. Nevertheless, more data from randomized trials testing consolidative RT in advanced-stage DLBCL are essential to confirm these results.
ASCT is well established in the R/R DLBCL as a consolidation procedure after salvage chemotherapy improving the outcome of the patients.82 However, front-line consolidation by high-dose chemotherapy and subsequent ASCT did not improve survival of high-risk patients with DLBCL.83 Furthermore, a meta-analysis performed in the rituximab era in patients with DLBCL in the first complete remission demonstrated no beneficial effect of upfront ASCT consolidation in these patients.84 The role of consolidation of systemic DLBCL by ASCT retains its importance only in the second-line treatment following salvage chemotherapy.
Therapy “Agnostic” to Molecular Biology
The concept of “agnostic” treatment, ie, therapy based on targeting pan-B-cell markers of DLBCL cells, includes the group of naked monoclonal antibodies, antibody-drug conjugates, and immune system engaging therapy (T-cell engaging therapy and immunomodulatory drugs; Figure 3).
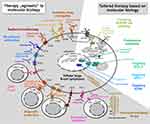 |
Figure 3 Therapeutic approaches in patients with newly diagnosed diffuse large B-cell lymphoma. Abbreviation: CAR, chimeric antigen receptor. |
Naked Antibodies
Several prospective trials have investigated a substitution of any of R-CHOP component with novel agents. The substitution of rituximab for another type II anti-CD20 monoclonal antibody obinutuzumab (G), ie, the G-CHOP regimen, was investigated in a large, randomized phase III GOYA study (Table 1).54 The patients received 8 cycles of either R or G plus 6 or 8 cycles of CHOP, with primary endpoint being the PFS. The G-CHOP chemoimmunotherapy did not show a PFS benefit over R-CHOP (5-years PFS 64% vs 63%), with serious adverse events being more frequently detected in the experimental arm of the study. Among the secondary endpoints of that study, patients with GCB subtype of DLBCL tended to benefit from the G-CHOP in comparison to the control arm.
 |
Table 1 Selected Clinical Trials in Patients with Newly Diagnosed Advanced-Stage Diffuse Large B-Cell Lymphoma |
Antibody-Drug Conjugates
A GO29365 study showed the combination of polatuzumab vedotin, an anti-CD79b antibody-drug conjugate carrying a microtubule inhibitor auristatin E, with bendamustine and rituximab to be an effective and safe treatment option for transplant-ineligible patients with R/R DLBCL,85 as well as bridging therapy to chimeric antigen receptor (CAR) T-cell treatment.86 The experimental results were subsequently confirmed by real-world data.87 The first-line substitution of vincristine by polatuzumab vedotin, ie, Pola-R-CHP vs R-CHOP regimen, was investigated in the POLARIX study and recently published.88 Patients between 18–80 years of age with IPI ≥2 and PS ECOG ≤2 entered the analysis. This study met its primary endpoint: the Pola-R-CHP arm had superior PFS in comparison to the control arm (2-year PFS 76.7% vs 70.2%). No differences in response rates and OS were reported. The safety profile did not significantly differ among the two arms, suggesting that the Pola-R-CHP combination could be considered as a novel first-line standard of care. Detailed molecular as well as clinical analysis is needed to assess whether there is a specific subgroup of patients who could profit from this treatment even more. Moreover, in the context of the effectivity of polatuzumab vedotin in the R/R settings, a right timing to use this drug (first line vs R/R) is now widely discussed in the expert community. The safety and tolerability of the combination of polatuzumab vedotin plus dose-adjusted EPOCH-R is currently being investigated in the NCT04231877 phase I trial for newly diagnosed aggressive B-NHL.
Loncastuximab tesirine is an antibody-drug conjugate comprising of an anti-CD19 monoclonal antibody conjugated to a pyrrolobenzodiazepine. This molecule was approved in monotherapy with an acceptable safety profile in the treatment of R/R DLBCL based on the phase II LOTIS-2 trial.89 The combination of loncastuximab tesirine with ibrutinib in the currently ongoing phase II LOTIS-3 trial showed high ORR (57%) and a manageable toxicity profile in patients with R/R DLBCL.90 This chemo-free regimen with encouraging anti-tumor activity might be an interesting approach also in patients with newly diagnosed DLBCL.
Zilovertamab vedotin, an anti-ROR1 antibody plus auristatin E antibody-drug conjugate was tested in R/R hematologic malignancies including DLBCL with promising efficacy and a tolerable toxicity profile.91 This molecule is now being investigated in a phase II/III trial in combination with a standard of care in patients with R/R DLBCL after at least one prior line of treatment (NCT05139017).
T-Cell Engaging Therapy and Immunomodulatory Drugs
T-cell engaging therapy is a quickly evolving field of hemato-oncology. The mechanism of action is based on eliciting T-cell mediated responses against the lymphoma and is thus in principle different from both the conventional chemotherapy and most targeted agents. The activation of patients´ autologous T-cells can be achieved by direct activation of T-cell with bispecific T-cell engagers (BiTEs), re-activation of tumor-suppressed T-cells by checkpoint inhibitors, or re-infusion of ex vivo genetically modified and expanded autologous T-cells (ie, chimeric antigen receptor, CAR T-cells).
Checkpoint Inhibitors
Programmed death 1 (PD-1) is an immune checkpoint surface antigen on B and T-cells which plays an important role in the downregulation of the immune system. Many tumors including DLBCL express PD-1 ligands PD-L1 and PD-L2, which enables an immune escape of the tumor cells.92
Expression of PD-L1 in DLBCL is associated with inferior survival. Pembrolizumab, an anti-PD-1 monoclonal antibody, was investigated in R/R DLBCL with ambiguous results.93 In the current first-line trial, patients with PS ECOG 0–1 entered the trial with 6 cycles of pembrolizumab and R-CHOP.94 The patients reached a 2-year PFS of 83% with acceptable toxicity profile. Not surprisingly, patients with tumors expressing PD-L1 tended to have an improved survival.
Another anti-PD-1 monoclonal antibody with antibody-dependent cell cytotoxicity and in vitro synergism with rituximab is avelumab. This antibody was tested in a phase II study in patients PS ECOG ≤2 and advanced stage disease.95 The patients received 2 cycles of avelumab as an induction treatment, followed by 6 cycles of R-CHOP, and a maintenance of 6 cycles of avelumab. The patients presented with a 1-year failure-free (FFS) survival of 76% and 1-year OS of 89%, surprisingly high overall response rate (ORR) of 60% after the first two cycles of avelumab, and manageable toxicity profile.
Bispecific Antibodies
BiTEs are monoclonal antibody constructs simultaneously binding a tumor cell antigen and a surface T-cell antigen in order to activate the T-lymphocytes with subsequent lysis of the malignant cells. The tumor binding site of the BiTE targets molecules CD19 (blinatumomab) or CD20 (glofitamab, mosunetuzumab, epcoritamab, odronextamab), while the T-cell binding site targets CD3ε molecules.
Blinatumomab is a first-generation BiTE used in patients with acute lymphoblastic leukemia.96 The efficacy of this BiTE with a short half-life in the treatment of R/R DLBCL was quite low.97 In the first-line treatment, high-risk patients with DLBCL who reached CR after 6 cycles of R-CHOP, R-CHOEP, or dose-adjusted EPOCH-R, were subsequently treated by blinatumomab in a phase II study reaching ORR of 89% with a tolerable safety profile.98
Second-generation BiTE glofitamab with a larger molecule, and thus, longer half-life, was tested in the heavily pre-treated R/R DLBCL in combination with obinutuzumab, reaching a CR rate of 30% with tolerable incidence of cytokine release syndrome and neurological toxicities.99 Glofitamab is now being tested in 3 trials: a phase Ib study of patients with PS ECOG ≤3 in combination with rituximab or G-CHOP (NCT03467373), a phase Ib/II COALITION study of younger patients up to 65 years of age with high-risk DLBCL and PS ECOG 0–1 in combination with R-CHOP or Pola-R-CHP (NCT04914741), and a phase II study of patients with PS ECOG 0–2 and high-risk levels of ctDNA in combination with R-CHOP (NCT04980222).
Mosunetuzumab in monotherapy was tested among elderly patients ≥60 years of age who were not eligible to receive R-CHOP, with promising results in terms of efficacy and tolerability (ORR 68%, CR 42%).100 Another study investigated this BiTE in combination with 6 cycles of CHOP reaching ORR of 97% (CR rate 85%).101 Consolidation treatment by mosunetuzumab monotherapy or a combination with polatuzumab vedotin is evaluated in the NCT03677154 trial in patients after induction.
The EPCORE NHL-2 phase Ib/II trial evaluates the safety of multiple immunochemotherapeutic combinations with anti-CD3 x anti-CD20 BiTE epcoritamab (NCT04663347); and a phase I ELM-1 trial of BiTE odronextamab in monotherapy is now recruiting patients with B-NHL (NCT02290951).
The efficacy of BiTE has been confirmed in patients with R/R B-NHL, and those with chemo-resistant disease. However, the position of this treatment strategy versus the CAR T-cell therapy is still being discussed. The first-line therapeutical use of these products, especially in high-risk patient populations requires more data on effectivity from the current prospective trials.
CAR T-Cell Therapy
In highly pre-treated patients with R/R DLBCL after ≥2 lines of prior chemoimmunotherapy, three CAR T-cell products, axicabtagene ciloleucel (axi-cel), lisocabtagene maraleucel (liso-cel), and tisagenlecleucel (tisa-cel), were approved based on the registration studies.102–104 Subsequent phase III trials investigated the efficacy of CAR T-cell treatment in comparison to the standard of care salvage chemotherapy followed by ASCT in the second line in patients with early relapses ≤12 months. Two studies reported the superiority of CAR T-cell therapy to the standard of care in terms of EFS (ZUMA-7 trial for axi-cel; TRANSFORM for liso-cel), while in the third study the superiority was not observed (BELINDA trial for tisa-cel).
Patients with high-risk aggressive B-NHL, ie, those with IPI 3–5 and/or double-hit lymphomas with interim PET/CT positivity after 2 cycles of induction anthracycline-based chemoimmunotherapy entered the phase II ZUMA-12 trial investigating the CAR T-cell axi-cel product in the first line (conditioning cyclophosphamide and fludarabine).105 After leukapheresis, the patients were allowed to receive non-chemotherapeutical bridging therapy. The axi-cel was shown to be highly effective in the first-line treatment, with ORR of 89% (CR rate 78%) and a manageable toxicity profile including 22% of high-grade cytokine release syndrome incidence, and 12% of high-grade neurologic toxicity.
The effectivity of CAR T-cell therapy was confirmed in the R/R DLBCL, and preliminary data showed this approach to be effective as part of first-line treatment. Nevertheless, the optimal position of the CAR T-cell therapy is to be established similarly to the BiTEs, and the treatment modalities after CAR T-cell failure need to be investigated by subsequent prospective trials.
Immunomodulatory Drugs
Besides its immunomodulatory functions, lenalidomide binds to C3 ubiquitin ligase cereblon, which leads to proteasome-mediated degradation of Ikaros zinc finger (IKZF) transcription factors and transcriptional downregulation of IRF4 and MYC. In phase II studies in R/R DLBCL patients, lenalidomide demonstrated promising activity in combination with rituximab.106 A phase III ROBUST trial investigated efficacy and safety of the combination of lenalidomide and R-CHOP versus R-CHOP alone.107 Only patients with ABC subtype of DLBCL determined by GEP were included in the analysis. The experimental arm did not show a survival benefit in comparison to the R-CHOP (2-year PFS 67% vs 64%). Since lenalidomide was proven to downregulate the MYC proto-oncogene, a phase II HOVON trial was conducted for patients with MYC-rearranged aggressive B-NHL including double- and triple-hit lymphomas.108 The patients received 6 cycles of R-CHOP plus lenalidomide with an acceptable toxicity profile, a 2-year EFS of 63% and 2-year OS of 73%, which is comparable to results achieved by other dose-escalated regimens.
The combination of lenalidomide plus tafasitamab, an anti-CD19 antibody with enhanced antibody-dependent cellular cytotoxicity, and phagocytosis, was shown to be safe in patients with R/R DLBCL in a single-arm phase II L-MIND trial,109 and similarly in a phase Ib First-MIND trial in first-line combination with R-CHOP.110 Currently, a phase III Front-MIND study has been recruiting patients with de novo DLBCL, higher-risk IPI and PS ECOG ≤2.111
Lenalidomide plus ibrutinib and rituximab, a chemotherapy-free regimen, was tested in the pilot SMART Start study.112 Patients with non-GCB DLBCL who entered this trial were treated with 2 cycles of chemotherapy-free regimen followed by CHOP or EPOCH with 1-year PFS of 93%. This regimen was shown to be safe and effective, and opened up a new discussion about potential implementation of chemo-free regimens in daily practice.
Tailored Therapy Based on Molecular Biology
Targeting BCR/NF-kB Signaling Pathway
The ABC subtype of DLBCL is characterized by constitutive activation of BCR/NF-kB signaling.113 Patients with non-GCB (ABC) subtypes of DLBCL receiving R-CHOP regimen have inferior outcome compared to GCB subtype (5-year PFS 48% vs 73%, 5-year OS 56% vs 78%).8 It is thus not surprising that the non-GCB DLBCL patients are ideal candidates for the testing of innovative treatment targeting the aberrantly activated pathways.
The inhibitor of Bruton’s tyrosine kinase ibrutinib has been approved for the therapy of chronic lymphocytic leukemia and mantle cell lymphoma. In DLBCL, the combination of ibrutinib with R-CHOP versus R-CHOP alone was tested in a phase III PHOENIX trial.114 Only patients with the non-GCB subtype determined by the Hans algorithm were enrolled in the study. Ibrutinib did not improve EFS and slightly increased toxicity in the experimental arm of that study. However, a sub-analysis of patients younger than 60 years presented with significantly improved EFS, indicating that these patients might profit from the addition of ibrutinib to R-CHOP. Of note, a retrospective analysis of the DLBCL subtypes according to the newly published molecular-based classification revealed a survival benefit of the ibrutinib-R-CHOP combination in the sub-cohorts of patients with the MCD and N1 subtypes.12,115 The results suggest that targeting the activated BCR/NF-kB signaling pathway in MCD/N1 subtypes of DLBCL could improve outcome of the patients. However, this approach requires development of fast molecular typization of DLBCL in a clinical practice to avoid delays in treatment initiation. Alternatively, one cycle of an “agnostic” treatment regimen (ie, R-CHOP) can be administered to the patients as a “bridge” during the molecular analysis.
Second-generation Bruton’s tyrosine kinase inhibitor acalabrutinib with enhanced kinase selectivity is currently being tested in a phase III ESCALADE trial in combination with the R-CHOP regimen.116 Patient ≤65 years of age with non-GCB subtype, clinical stage II–IV, and R-IPI 2–5 can be enrolled into the study. Another Bruton’s tyrosine kinase inhibitor zanubrutinib was tested in a single-arm phase II study, together with R-CHOP (European Hematology Association 2022, abstract P1185).
Proteasome Inhibitors
Bortezomib is a proteasome inhibitor used in first-line treatment of patients with multiple myeloma. Although previous data reported promising outcomes of patients treated by bortezomib + R-CHOP (2-year PFS 64%, 2-year OS 70%), these results were not confirmed by a phase III REMoDL-B trial.117 Notably, molecular profiling was used for prospective stratification of the patients in this study.
Ixazomib, another proteasome inhibitor with the ability to down-regulate MYC expression in preclinical lymphoma models, was investigated in a phase I–II study in patients with aggressive B-NHL with MYC rearrangement, including double-hit lymphomas in combination with dose-adjusted EPOCH-R, and subsequent maintenance by ixazomib monotherapy.118,119 This regimen led to a 2-year PFS of 67% and 2-year OS of 79% with a tolerable toxicity profile. However, a prospective phase III trial is needed to confirm these promising results.
Targeting BCL-2
Venetoclax, a selective BCL-2 inhibitor approved for patients with chronic lymphocytic leukemia and acute myeloid leukemia was investigated in combination with R-CHOP in a single-arm phase II CAVALLI trial.120 Patients with newly diagnosed DLBCL with IPI 2–5 and PS ECOG ≤2 were enrolled. A control arm from the GOYA study was used. An increased rate of grade 3–4 hematologic toxicities was observed, with a trend toward improved PFS when compared to the GOYA study (2-year PFS 80% vs 67%). Thus, the addition of venetoclax in the first-line treatment might be effective.
Preliminary results from the phase II/III study of patients with double-hit lymphomas treated with the combination of venetoclax and dose-adjusted EPOCH-R reported increased treatment-related mortality.121 For this reason, the study was prematurely terminated.
Targeting EZH2
The EZH2 histone-methyl transferase was found to be mutated in 25% of patients with DLBCL, especially those in the C3 cluster and EZB subgroup.11,12 An inhibitor of the EZH2 transferase tazemetostat in combination with R-CHOP was investigated in a phase Ib study in patients between 60–80 years of age. This combination was safe,122 but more data on treatment efficacy is needed.
Targeting XPO-1
Selinexor, a selective inhibitor of exportin 1 (XPO-1), inhibits nuclear export of several oncoproteins. Selinexor was tested in multiple myeloma patients, and in R/R DLBCL after ≥2 lines of treatment in a phase II SADAL trial, inducing durable responses with low toxicity rates.123 Therefore, a phase Ib/II study examining the safety of selinexor in combination with R-CHOP in the first-line treatment is now recruiting patients.
Maintenance Treatment
Several studies tested the potential effectivity of maintenance therapy after the R-CHOP induction regimen in order to improve the long-term outcomes, as this was proven to be an effective strategy in low-grade lymphomas. The use of rituximab did not show any survival benefit in the maintenance therapy.50 Another monoclonal anti-PD-1 antibody avelumab was tested in a phase II study as an induction and maintenance therapy, with encouraging results in terms of ORR, but more data on survival of the patients is needed.95 Elderly patients between 60–80 years of age who reached CR or partial remission were treated by lenalidomide monotherapy maintenance vs placebo for 24 months, improving the PFS in experimental arm with no difference in the OS.124 The phase III PILLAR-2 trial showed that an mTOR inhibitor everolimus, and similarly a protein kinase C-β inhibitor enzastaurin in the phase III PRELUDE trial had no impact on survival of patients with DLBCL in the maintenance settings.125,126
Central Nervous System Prophylaxis
A total of 2–8% of DLBCL patients present with CNS relapse of the disease, resulting in an extremely poor outcome.127 The involvement of renal and/or adrenal extranodal sites together with other IPI factors are incorporated into the CNS-IPI risk stratification index.128 A combination of CNS-IPI with the molecular classification of DLBCL was recently shown to better predict patients at high risk of CNS relapse.129 Based on the current guidelines, CNS prophylaxis containing methotrexate is recommended for high-risk patients, according to CNS-IPI. The CNS prophylaxis can be administered either intrathecally or intravenously (intercalated or after the completion of induction chemoimmunotherapy). Recently published large retrospective analysis found no significant difference in CNS relapse rate between the intrathecal vs intravenous routes of administration,130 while other studies failed to show a risk reduction of CNS relapses in comparison to the historical data on CNS relapse incidence.131,132 Along with the published data, if the intravenous CNS prophylaxis is indicated by the treating physician, it is recommended to be postponed after the treatment completion.133,134
Conclusions and Future Directions
The R-CHOP chemotherapy regimen has remained a standard of the first-line treatment in an unchanged form for many years. Novel diagnostic tools including gene expression profiling have shown DLBCL to be a heterogenic disease with recurrent genetic background. This knowledge is being applied in the current clinical trials with an intent to use specific tailored therapy in order to improve survival of the high-risk subgroups of DLBCL patients. Since the molecular analysis cannot be made for every newly diagnostic DLBCL before treatment initiation in real life, the “agnostic” approach is not abandoned. This was confirmed by recently published data showing the Pola-R-CHP regimen to have survival benefit over R-CHOP, and thus that this regimen might beat the 20-year-old paradigm of the R-CHOP superiority. Moreover, T-cell engaging therapies including BiTEs show encouraging anti-tumor activity in the first-line setting. The results from the clinical trials should be constantly monitored and the treatment schedule adjusted accordingly.
Abbreviations
aaIPI, age-adjusted International Prognostic Index; ABC, activated B-cell-like; ASCT, autologous stem cell transplantation; axi-cel, axicabtagene ciloleucel; BiTE, bispecific T-cell engager; B-NHL, B-cell non-Hodgkin lymphoma; CAR, chimeric antigen receptor; CNS, central nervous system; CR, complete remission; ctDNA, circulating tumor DNA; EFS, event-free survival; FDG, fluorodeoxyglucose; FFS, failure-free survival; G, obinutuzumab; GCB, germinal center B-cell-like; GEP, gene expression profiling; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IPI, International Prognostic Index; LDH, lactate dehydrogenase; liso-cel, lisocabtagene maraleucel; NA, not applicable; NCCN-IPI, National Comprehensive Cancer Network International Prognostic Index; NF-κB, nuclear factor kappa B; NHL, non-Hodgkin lymphoma; NOS, not otherwise specified, NS, not significant; ORR, overall response rate; OS, overall survival; PD-1, programmed death 1; PD-L1, programmed death 1 ligand; PET/CT, positron emission tomography–computed tomography; PFS, progression-free survival; PS (ECOG), performance status according to the Eastern Cooperative Oncology Group; R, rituximab; R/R, relapsed or refractory; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone; R-IPI, revised International Prognostic Index; RT, radiotherapy; SUV, standard uptake volume; tisa-cel, tisagenlecleucel; TMTV, total metabolic tumor volume; WHO, World Health Organization; y., years; *, significant.
Author Contributions
All authors made a significant contribution in conception of the manuscript; revised the literature; took part in drafting, revising and critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Czech Health Research Council (Grant Number AZV NU21-03-00386), the project National Institute for Cancer Research (Programme EXCELES, ID Project No. LX22NPO5102; funded by the European Union – Next Generation EU), and by the Cooperation Program (research area “Oncology and Haematology”).
Disclosure
Dr Prokop Vodicka reports personal fees from Hoffmann-La Roche, outside the submitted work. Prof. Dr. Marek Trneny reports personal fees from Janssen, Gilead Sciences, BMS, Abbvie, Astra Zeneca, ROCHE Morphosys, Incyte, Novartis, and Takeda, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Howlader N, Noone A, Krapcho M, et al. SEER cancer statistics review, 1975–2016. Bethesda, MD: National Cancer Institute; 2019. Available from: https://seer.cancer.gov/csr/1975_2016/.
2. Morton LM, Wang SS, Devesa SS, Hartge P, Weisenburger DD, Linet MS. Lymphoma incidence patterns by WHO subtype in the United States, 1992–2001. Blood. 2006;107(1):265–276. doi:10.1182/blood-2005-06-2508
3. Trneny M, Campr V, Janikova A, et al. The improving outcome of non-Hodgkin lymphoma (NHL) within 15 years period-real world data of national-wide lymphoma project. Haematologica. 2016;2016(101):479–480.
4. Cerhan JR, Kricker A, Paltiel O, et al. Medical history, lifestyle, family history, and occupational risk factors for diffuse large B-cell lymphoma: the InterLymph Non-Hodgkin Lymphoma Subtypes Project. J Natl Cancer Inst Monogr. 2014;2014(48):15–25. doi:10.1093/jncimonographs/lgu010
5. Lopez-Guillermo A, Colomo L, Jimenez M, et al. Diffuse large B-cell lymphoma: clinical and biological characterization and outcome according to the nodal or extranodal primary origin. J Clin Oncol. 2005;23(12):2797–2804. doi:10.1200/JCO.2005.07.155
6. Coiffier B, Thieblemont C, Van Den Neste E, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d’Etudes des Lymphomes de l’Adulte. Blood. 2010;116(12):2040–2045. doi:10.1182/blood-2010-03-276246
7. Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403(6769):503–511. doi:10.1038/35000501
8. Scott DW, Mottok A, Ennishi D, et al. Prognostic significance of diffuse large B-cell lymphoma cell of origin determined by digital gene expression in formalin-fixed paraffin-embedded tissue biopsies. J Clin Oncol. 2015;33(26):2848–2856. doi:10.1200/JCO.2014.60.2383
9. Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103(1):275–282. doi:10.1182/blood-2003-05-1545
10. Meyer PN, Fu K, Greiner TC, et al. Immunohistochemical methods for predicting cell of origin and survival in patients with diffuse large B-cell lymphoma treated with rituximab. J Clin Oncol. 2011;29(2):200–207. doi:10.1200/JCO.2010.30.0368
11. Chapuy B, Stewart C, Dunford AJ, et al. Molecular subtypes of diffuse large B cell lymphoma are associated with distinct pathogenic mechanisms and outcomes. Nat Med. 2018;24(5):679–690. doi:10.1038/s41591-018-0016-8
12. Schmitz R, Wright GW, Huang DW, et al. Genetics and pathogenesis of diffuse large B-cell lymphoma. N Engl J Med. 2018;378(15):1396–1407. doi:10.1056/NEJMoa1801445
13. Wright GW, Huang DW, Phelan JD, et al. A probabilistic classification tool for genetic subtypes of diffuse large B cell lymphoma with therapeutic implications. Cancer Cell. 2020;37(4):551–568 e514. doi:10.1016/j.ccell.2020.03.015
14. Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–2390. doi:10.1182/blood-2016-01-643569
15. Rosenwald A, Bens S, Advani R, et al. Prognostic significance of MYC rearrangement and translocation partner in diffuse large B-cell lymphoma: a study by the Lunenburg lymphoma biomarker consortium. J Clin Oncol. 2019;37(35):3359–3368. doi:10.1200/JCO.19.00743
16. Scott DW, King RL, Staiger AM, et al. High-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements with diffuse large B-cell lymphoma morphology. Blood. 2018;131(18):2060–2064. doi:10.1182/blood-2017-12-820605
17. Johnson NA, Savage KJ, Ludkovski O, et al. Lymphomas with concurrent BCL2 and MYC translocations: the critical factors associated with survival. Blood. 2009;114(11):2273–2279. doi:10.1182/blood-2009-03-212191
18. Savage KJ, Johnson NA, Ben-Neriah S, et al. MYC gene rearrangements are associated with a poor prognosis in diffuse large B-cell lymphoma patients treated with R-CHOP chemotherapy. Blood. 2009;114(17):3533–3537. doi:10.1182/blood-2009-05-220095
19. Johnson NA, Slack GW, Savage KJ, et al. Concurrent expression of MYC and BCL2 in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol. 2012;30(28):3452–3459. doi:10.1200/JCO.2011.41.0985
20. Alaggio R, Amador C, Anagnostopoulos I, et al. The 5th edition of the World Health Organization classification of haematolymphoid tumours: lymphoid neoplasms. Leukemia. 2022;36(7):1720–1748. doi:10.1038/s41375-022-01620-2
21. Cree IA. The WHO classification of haematolymphoid tumours. Leukemia. 2022;36(7):1701–1702. doi:10.1038/s41375-022-01625-x
22. Campo E, Jaffe ES, Cook JR, et al. The international consensus classification of mature lymphoid neoplasms: a report from the Clinical Advisory Committee. Blood. 2022;140(11):1229–1253.
23. Meignan M, Barrington S, Itti E, Gallamini A, Haioun C, Polliack A. Report on the 4th international workshop on positron emission tomography in lymphoma held in Menton, France, 3–5 October 2012. Leuk Lymphoma. 2014;55(1):31–37. doi:10.3109/10428194.2013.802784
24. Mamot C, Klingbiel D, Hitz F, et al. Final results of a prospective evaluation of the predictive value of interim positron emission tomography in patients with diffuse large B-cell lymphoma treated with R-CHOP-14 (SAKK 38/07). J Clin Oncol. 2015;33(23):2523–2529. doi:10.1200/JCO.2014.58.9846
25. Casasnovas RO, Meignan M, Berriolo-Riedinger A, et al. SUVmax reduction improves early prognosis value of interim positron emission tomography scans in diffuse large B-cell lymphoma. Blood. 2011;118(1):37–43. doi:10.1182/blood-2010-12-327767
26. Schoder H, Polley MC, Knopp MV, et al. Prognostic value of interim FDG-PET in diffuse large cell lymphoma: results from the CALGB 50303 Clinical Trial. Blood. 2020;135(25):2224–2234. doi:10.1182/blood.2019003277
27. Duhrsen U, Muller S, Hertenstein B, et al. Positron emission tomography-guided therapy of aggressive non-Hodgkin lymphomas (PETAL): a multicenter, randomized phase III trial. J Clin Oncol. 2018;36(20):2024–2034. doi:10.1200/JCO.2017.76.8093
28. Zijlstra JM, Burggraaff CN, Kersten MJ, Barrington SF. FDG-PET as a biomarker for early response in diffuse large B-cell lymphoma as well as in Hodgkin lymphoma? Ready for implementation in clinical practice? Haematologica. 2016;101(11):1279–1283. doi:10.3324/haematol.2016.142752
29. Vercellino L, Cottereau AS, Casasnovas O, et al. High total metabolic tumor volume at baseline predicts survival independent of response to therapy. Blood. 2020;135(16):1396–1405. doi:10.1182/blood.2019003526
30. Esfahani SA, Heidari P, Halpern EF, Hochberg EP, Palmer EL, Mahmood U. Baseline total lesion glycolysis measured with (18) F-FDGPET/CT as a predictor of progression-free survival in diffuse large B-cell lymphoma: a pilot study. Am J Nucl Med Mol Imaging. 2013;3(3):272–281.
31. Gallicchio R, Mansueto G, Simeon V, et al. F-18 FDG PET/CT quantization parameters as predictors of outcome in patients with diffuse large B-cell lymphoma. Eur J Haematol. 2014;92(5):382–389. doi:10.1111/ejh.12268
32. Adams HJ, de Klerk JM, Fijnheer R, et al. Prognostic superiority of the National Comprehensive Cancer Network International Prognostic Index over pretreatment whole-body volumetric-metabolic FDG-PET/CT metrics in diffuse large B-cell lymphoma. Eur J Haematol. 2015;94(6):532–539. doi:10.1111/ejh.12467
33. Sehn LH, Scott DW, Chhanabhai M, et al. Impact of concordant and discordant bone marrow involvement on outcome in diffuse large B-cell lymphoma treated with R-CHOP. J Clin Oncol. 2011;29(11):1452–1457. doi:10.1200/JCO.2010.33.3419
34. Chung R, Lai R, Wei P, et al. Concordant but not discordant bone marrow involvement in diffuse large B-cell lymphoma predicts a poor clinical outcome independent of the International Prognostic Index. Blood. 2007;110(4):1278–1282. doi:10.1182/blood-2007-01-070300
35. Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059–3068. doi:10.1200/JCO.2013.54.8800
36. Barrington SF, Mikhaeel NG, Kostakoglu L, et al. Role of imaging in the staging and response assessment of lymphoma: consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J Clin Oncol. 2014;32(27):3048–3058. doi:10.1200/JCO.2013.53.5229
37. International Non-Hodgkin’s Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin’s lymphoma. N Engl J Med. 1993;329(14):987–994. doi:10.1056/NEJM199309303291402
38. Sehn LH, Berry B, Chhanabhai M, et al. The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood. 2007;109(5):1857–1861. doi:10.1182/blood-2006-08-038257
39. Ziepert M, Hasenclever D, Kuhnt E, et al. Standard International prognostic index remains a valid predictor of outcome for patients with aggressive CD20+ B-cell lymphoma in the rituximab era. J Clin Oncol. 2010;28(14):2373–2380. doi:10.1200/JCO.2009.26.2493
40. Zhou Z, Sehn LH, Rademaker AW, et al. An enhanced International Prognostic Index (NCCN-IPI) for patients with diffuse large B-cell lymphoma treated in the rituximab era. Blood. 2014;123(6):837–842. doi:10.1182/blood-2013-09-524108
41. Ruppert AS, Dixon JG, Salles G, et al. International prognostic indices in diffuse large B-cell lymphoma: a comparison of IPI, R-IPI, and NCCN-IPI. Blood. 2020;135(23):2041–2048. doi:10.1182/blood.2019002729
42. Roschewski M, Dunleavy K, Pittaluga S, et al. Circulating tumour DNA and CT monitoring in patients with untreated diffuse large B-cell lymphoma: a correlative biomarker study. Lancet Oncol. 2015;16(5):541–549. doi:10.1016/S1470-2045(15)70106-3
43. Kurtz DM, Scherer F, Jin MC, et al. Circulating tumor DNA measurements as early outcome predictors in diffuse large B-cell lymphoma. J Clin Oncol. 2018;36(28):2845–2853. doi:10.1200/JCO.2018.78.5246
44. Scherer F, Kurtz DM, Newman AM, et al. Distinct biological subtypes and patterns of genome evolution in lymphoma revealed by circulating tumor DNA. Sci Transl Med. 2016;8(364):364ra155. doi:10.1126/scitranslmed.aai8545
45. Spina V, Bruscaggin A, Cuccaro A, et al. Circulating tumor DNA reveals genetics, clonal evolution, and residual disease in classical Hodgkin lymphoma. Blood. 2018;131(22):2413–2425. doi:10.1182/blood-2017-11-812073
46. McKelvey EM, Gottlieb JA, Wilson HE, et al. Hydroxyldaunomycin (Adriamycin) combination chemotherapy in malignant lymphoma. Cancer. 1976;38(4):1484–1493. doi:10.1002/1097-0142(197610)38:4<1484::AID-CNCR2820380407>3.0.CO;2-I
47. Fisher RI, Gaynor ER, Dahlberg S, et al. Comparison of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non-Hodgkin’s lymphoma. N Engl J Med. 1993;328(14):1002–1006. doi:10.1056/NEJM199304083281404
48. Maloney DG, Grillo-López AJ, White CA, et al. IDEC-C2B8 (Rituximab) anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin’s lymphoma. Blood. 1997;90(6):2188–2195. doi:10.1182/blood.V90.6.2188
49. Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346(4):235–242. doi:10.1056/NEJMoa011795
50. Habermann TM, Weller EA, Morrison VA, et al. Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J Clin Oncol. 2006;24(19):3121–3127. doi:10.1200/JCO.2005.05.1003
51. Pfreundschuh M, Kuhnt E, Trumper L, et al. CHOP-like chemotherapy with or without rituximab in young patients with good-prognosis diffuse large-B-cell lymphoma: 6-year results of an open-label randomised study of the MabThera International Trial (MInT) Group. Lancet Oncol. 2011;12(11):1013–1022. doi:10.1016/S1470-2045(11)70235-2
52. Pfreundschuh M, Schubert J, Ziepert M, et al. Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell lymphomas: a randomised controlled trial (RICOVER-60). Lancet Oncol. 2008;9(2):105–116. doi:10.1016/S1470-2045(08)70002-0
53. Wasterlid T, Biccler JL, Brown PN, et al. Six cycles of R-CHOP-21 are not inferior to eight cycles for treatment of diffuse large B-cell lymphoma: a Nordic lymphoma group population-based study. Ann Oncol. 2018;29(8):1882–1883. doi:10.1093/annonc/mdy184
54. Sehn LH, Martelli M, Trneny M, et al. A randomized, open-label, Phase III study of obinutuzumab or rituximab plus CHOP in patients with previously untreated diffuse large B-cell lymphoma: final analysis of GOYA. J Hematol Oncol. 2020;13(1):71. doi:10.1186/s13045-020-00900-7
55. Miller TP, Dahlberg S, Cassady JR, et al. Chemotherapy alone compared with chemotherapy plus radiotherapy for localized intermediate- and high-grade non-Hodgkin’s lymphoma. N Engl J Med. 1998;339(1):21–26. doi:10.1056/NEJM199807023390104
56. Stephens DM, Li H, LeBlanc ML, et al. Continued risk of relapse independent of treatment modality in limited-stage diffuse large B-cell lymphoma: final and long-term analysis of Southwest Oncology Group Study S8736. J Clin Oncol. 2016;34(25):2997–3004. doi:10.1200/JCO.2015.65.4582
57. Persky DO, Unger JM, Spier CM, et al. Phase II study of rituximab plus three cycles of CHOP and involved-field radiotherapy for patients with limited-stage aggressive B-cell lymphoma: Southwest Oncology Group study 0014. J Clin Oncol. 2008;26(14):2258–2263. doi:10.1200/JCO.2007.13.6929
58. Poeschel V, Held G, Ziepert M, et al. Four versus six cycles of CHOP chemotherapy in combination with six applications of rituximab in patients with aggressive B-cell lymphoma with favourable prognosis (FLYER): a randomised, Phase 3, non-inferiority trial. Lancet. 2019;394(10216):2271–2281. doi:10.1016/S0140-6736(19)33008-9
59. Lamy T, Damaj G, Soubeyran P, et al. R-CHOP 14 with or without radiotherapy in nonbulky limited-stage diffuse large B-cell lymphoma. Blood. 2018;131(2):174–181. doi:10.1182/blood-2017-07-793984
60. Persky DO, Li H, Stephens DM, et al. Positron emission tomography-directed therapy for patients with limited-stage diffuse large B-cell lymphoma: results of Intergroup National Clinical Trials Network Study S1001. J Clin Oncol. 2020;38(26):3003–3011. doi:10.1200/JCO.20.00999
61. Peyrade F, Jardin F, Thieblemont C, et al. Attenuated immunochemotherapy regimen (R-miniCHOP) in elderly patients older than 80 years with diffuse large B-cell lymphoma: a multicentre, single-arm, Phase 2 trial. Lancet Oncol. 2011;12(5):460–468. doi:10.1016/S1470-2045(11)70069-9
62. Lyman GH, Dale DC, Friedberg J, Crawford J, Fisher RI. Incidence and predictors of low chemotherapy dose-intensity in aggressive non-Hodgkin’s lymphoma: a nationwide study. J Clin Oncol. 2004;22(21):4302–4311. doi:10.1200/JCO.2004.03.213
63. Tien YY, Link BK, Brooks JM, Wright K, Chrischilles E. Treatment of diffuse large B-cell lymphoma in the elderly: regimens without anthracyclines are common and not futile. Leuk Lymphoma. 2015;56(1):65–71. doi:10.3109/10428194.2014.903589
64. Peyrade F, Bologna S, Delwail V, et al. Combination of ofatumumab and reduced-dose CHOP for diffuse large B-cell lymphomas in patients aged 80 years or older: an open-label, multicentre, single-arm, phase 2 trial from the LYSA group. Lancet Haematol. 2017;4(1):e46–e55. doi:10.1016/S2352-3026(16)30171-5
65. Sehn LH, Herrera AF, Flowers CR, et al. Polatuzumab vedotin in relapsed or refractory diffuse large B-cell lymphoma. J Clin Oncol. 2020;38(2):155–165. doi:10.1200/JCO.19.00172
66. Brem EA, Li H, Beaven AW, et al. SWOG 1918: a phase II/III randomized study of R-miniCHOP with or without oral azacitidine (CC-486) in participants age 75 years or older with newly diagnosed aggressive non-Hodgkin lymphomas - aiming to improve therapy, outcomes, and validate a prospective frailty tool. J Geriatr Oncol. 2022;13(2):258–264. doi:10.1016/j.jgo.2021.10.003
67. Tanaka T, Sakai R, Choi I, et al. Comprehensive geriatric assessment as a useful tool in predicting adverse events in elderly patients with diffuse large B-cell lymphoma. Sci Rep. 2022;12(1):3124. doi:10.1038/s41598-022-07164-w
68. Merli F, Luminari S, Rossi G, et al. Outcome of elderly frail patients with Diffuse Large B-Cell Lymphoma (DLBCL) prospectively identified by Comprehensive Geriatric Assessment (CGA). Results from a study of the Intergruppo Italiano Linfomi (IIL). Blood. 2010;116(21):1771. doi:10.1182/blood.V116.21.1771.1771
69. Pfreundschuh M, Trümper L, Kloess M, et al. Two-weekly or 3-weekly CHOP chemotherapy with or without etoposide for the treatment of elderly patients with aggressive lymphomas: results of the NHL-B2 trial of the DSHNHL. Blood. 2004;104(3):634–641. doi:10.1182/blood-2003-06-2095
70. Cunningham D, Hawkes EA, Jack A, et al. Rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone in patients with newly diagnosed diffuse large B-cell non-Hodgkin lymphoma: a phase 3 comparison of dose intensification with 14-day versus 21-day cycles. Lancet. 2013;381(9880):1817–1826. doi:10.1016/S0140-6736(13)60313-X
71. Recher C, Coiffier B, Haioun C, et al. Intensified chemotherapy with ACVBP plus rituximab versus standard CHOP plus rituximab for the treatment of diffuse large B-cell lymphoma (LNH03-2B): an open-label randomised phase 3 trial. Lancet. 2011;378(9806):1858–1867. doi:10.1016/S0140-6736(11)61040-4
72. Le Gouill S, Ghesquieres H, Oberic L, et al. Obinutuzumab vs rituximab for advanced DLBCL: a PET-guided and randomized phase 3 study by LYSA. Blood. 2021;137(17):2307–2320. doi:10.1182/blood.2020008750
73. Gang AO, Strom C, Pedersen M, et al. R-CHOEP-14 improves overall survival in young high-risk patients with diffuse large B-cell lymphoma compared with R-CHOP-14. A population-based investigation from the Danish Lymphoma Group. Ann Oncol. 2012;23(1):147–153. doi:10.1093/annonc/mdr058
74. Pedersen MO, Gang AO, Brown P, et al. Real world data on young patients with high-risk diffuse large B-cell lymphoma treated with R-CHOP or R-CHOEP - MYC, BCL2 and BCL6 as prognostic biomarkers. PLoS One. 2017;12(10):e0186983. doi:10.1371/journal.pone.0186983
75. Schmitz N, Nickelsen M, Ziepert M, et al. Conventional chemotherapy (CHOEP-14) with rituximab or high-dose chemotherapy (MegaCHOEP) with rituximab for young, high-risk patients with aggressive B-cell lymphoma: an open-label, randomised, phase 3 trial (DSHNHL 2002-1). Lancet Oncol. 2012;13(12):1250–1259. doi:10.1016/S1470-2045(12)70481-3
76. Bartlett NL, Wilson WH, Jung SH, et al. Dose-adjusted EPOCH-R compared with R-CHOP as frontline therapy for diffuse large B-cell lymphoma: clinical outcomes of the Phase III intergroup trial alliance/CALGB 50303. J Clin Oncol. 2019;37(21):1790–1799. doi:10.1200/JCO.18.01994
77. Dunleavy K, Fanale MA, Abramson JS, et al. Dose-adjusted EPOCH-R (etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab) in untreated aggressive diffuse large B-cell lymphoma with MYC rearrangement: a prospective, multicentre, single-arm phase 2 study. Lancet Haematol. 2018;5(12):e609–e617. doi:10.1016/S2352-3026(18)30177-7
78. Ohmachi K, Kinoshita T, Tobinai K, et al. A randomized phase 2/3 study of R-CHOP vs CHOP combined with dose-dense rituximab for DLBCL: the JCOG0601 trial. Blood Adv. 2021;5(4):984–993. doi:10.1182/bloodadvances.2020002567
79. Friedrichs B, Nickelsen M, Ziepert M, et al. Doubling rituximab in high-risk patients with aggressive B-cell lymphoma -results of the DENSE-R-MegaCHOEP trial. Br J Haematol. 2019;184(5):760–768. doi:10.1111/bjh.15710
80. Lugtenburg PJ, de Nully Brown P, van der Holt B, et al. Rituximab-CHOP with early rituximab intensification for diffuse large B-cell lymphoma: a randomized phase III trial of the HOVON and the Nordic lymphoma group (HOVON-84). J Clin Oncol. 2020;38(29):3377–3387. doi:10.1200/JCO.19.03418
81. Freeman CL, Savage KJ, Villa DR, et al. Long-term results of PET-guided radiation in patients with advanced-stage diffuse large B-cell lymphoma treated with R-CHOP. Blood. 2021;137(7):929–938. doi:10.1182/blood.2020005846
82. Philip T, Guglielmi C, Hagenbeek A, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin’s lymphoma. N Engl J Med. 1995;333(23):1540–1545. doi:10.1056/NEJM199512073332305
83. Cortelazzo S, Tarella C, Gianni AM, et al. Randomized trial comparing R-CHOP versus high-dose sequential chemotherapy in high-risk patients with diffuse large B-cell lymphomas. J Clin Oncol. 2016;34(33):4015–4022. doi:10.1200/JCO.2016.67.2980
84. Epperla N, Hamadani M, Reljic T, et al. Upfront autologous hematopoietic stem cell transplantation consolidation for patients with aggressive B-cell lymphomas in first remission in the rituximab era: a systematic review and meta-analysis. Cancer. 2019;125(24):4417–4425. doi:10.1002/cncr.32464
85. Sehn LH, Hertzberg M, Opat S, et al. Polatuzumab vedotin plus bendamustine and rituximab in relapsed/refractory diffuse large B-cell lymphoma: updated results of a phase Ib/II randomized study and preliminary results of a single-arm extension. Blood. 2020;136(Supplement 1):17–19. doi:10.1182/blood-2020-137078
86. Liebers N, Duell J, Fitzgerald D, et al. Polatuzumab vedotin as a salvage and bridging treatment in relapsed or refractory large B-cell lymphomas. Blood Adv. 2021;5(13):2707–2716. doi:10.1182/bloodadvances.2020004155
87. Vodicka P, Benesova K, Janikova A, et al. Polatuzumab vedotin plus bendamustine and rituximab in patients with relapsed/refractory diffuse large B-cell lymphoma in the real world. Eur J Haematol. 2022;109:162–165. doi:10.1111/ejh.13784
88. Tilly H, Morschhauser F, Sehn LH, et al. Polatuzumab vedotin in previously untreated diffuse large B-cell lymphoma. N Engl J Med. 2022;386(4):351–363. doi:10.1056/NEJMoa2115304
89. Caimi PF, Ai W, Alderuccio JP, et al. Loncastuximab tesirine in relapsed or refractory diffuse large B-cell lymphoma (LOTIS-2): a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2021;22(6):790–800. doi:10.1016/S1470-2045(21)00139-X
90. Carlo-Stella C, Zinzani PLL, Janakiram M, et al. Planned interim analysis of a phase 2 study of loncastuximab tesirine plus ibrutinib in patients with advanced diffuse large B-cell lymphoma (LOTIS-3). Blood. 2021;138:54. doi:10.1182/blood-2021-147765
91. Wang ML, Barrientos JC, Furman RR, et al. Zilovertamab vedotin targeting of ROR1 as therapy for lymphoid cancers. NEJM Evid. 2022;1(1):EVIDoa2100001. doi:10.1056/EVIDoa2100001
92. Cha JH, Chan LC, Li CW, Hsu JL, Hung MC. Mechanisms controlling PD-L1 expression in cancer. Mol Cell. 2019;76(3):359–370. doi:10.1016/j.molcel.2019.09.030
93. Frigault MJ, Armand P, Redd RA, et al. PD-1 blockade for diffuse large B-cell lymphoma after autologous stem cell transplantation. Blood Adv. 2020;4(1):122–126. doi:10.1182/bloodadvances.2019000784
94. Smith SD, Till BG, Shadman MS, et al. Pembrolizumab with R-CHOP in previously untreated diffuse large B-cell lymphoma: potential for biomarker driven therapy. Br J Haematol. 2020;189(6):1119–1126. doi:10.1111/bjh.16494
95. Hawkes EA, Chong G, Smith C, et al. Safety and efficacy of induction and maintenance avelumab plus R-CHOP in patients with diffuse large B-cell lymphoma (DLBCL): analysis of the Phase II Avr-CHOP study. Blood. 2020;136(Supplement 1):43–44. doi:10.1182/blood-2020-136024
96. Kantarjian H, Stein A, Gokbuget N, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. 2017;376(9):836–847. doi:10.1056/NEJMoa1609783
97. Viardot A, Goebeler ME, Hess G, et al. Phase 2 study of the bispecific T-cell engager (BiTE) antibody blinatumomab in relapsed/refractory diffuse large B-cell lymphoma. Blood. 2016;127(11):1410–1416. doi:10.1182/blood-2015-06-651380
98. Katz DA, Chu MP, David KA, et al. Open-label, phase 2 study of blinatumomab after first-line rituximab-chemotherapy in adults with newly diagnosed, high-risk diffuse large B-cell lymphoma. Blood. 2019;134:4077. doi:10.1182/blood-2019-121708
99. Hutchings M, Morschhauser F, Iacoboni G, et al. Glofitamab, a novel, bivalent CD20-targeting T-cell-engaging bispecific antibody, induces durable complete remissions in relapsed or refractory B-cell lymphoma: a phase I trial. J Clin Oncol. 2021;39(18):1959–1970. doi:10.1200/JCO.20.03175
100. Olszewski AJ, Avigdor A, Babu S, et al. Mosunetuzumab monotherapy in elderly/unfit pts with first-line diffuse large B-cell lymphoma (DLBCL): safety and efficacy remain promising with durable complete responses. Hematol Oncol. 2021;39(S2). doi:10.1002/hon.152_2880
101. Phillips TJ, Olszewski AJ, Munoz J, et al. Mosunetuzumab, a novel CD20/CD3 bispecific antibody, in combination with CHOP confers high response rates in patients with diffuse large B-cell lymphoma. Blood. 2020;136:37–38. doi:10.1182/blood-2020-136295
102. Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380(1):45–56. doi:10.1056/NEJMoa1804980
103. Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396(10254):839–852. doi:10.1016/S0140-6736(20)31366-0
104. Locke FL, Ghobadi A, Jacobson CA, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, Phase 1–2 trial. Lancet Oncol. 2019;20(1):31–42. doi:10.1016/S1470-2045(18)30864-7
105. Neelapu SS, Dickinson M, Munoz J, et al. Axicabtagene ciloleucel as first-line therapy in high-risk large B-cell lymphoma: the phase 2 ZUMA-12 trial. Nat Med. 2022;28(4):735–742. doi:10.1038/s41591-022-01731-4
106. Wang M, Fowler N, Wagner-Bartak N, et al. Oral lenalidomide with rituximab in relapsed or refractory diffuse large cell, follicular and transformed lymphoma: a phase II clinical trial. Leukemia. 2013;27(9):1902–1909. doi:10.1038/leu.2013.95
107. Nowakowski GS, Chiappella A, Gascoyne RD, et al. ROBUST: a phase III study of lenalidomide plus R-CHOP versus placebo plus R-CHOP in previously untreated patients with ABC-type diffuse large B-cell lymphoma. J Clin Oncol. 2021;39(12):1317–1328. doi:10.1200/JCO.20.01366
108. Chamuleau MED, Burggraaff CN, Nijland M, et al. Treatment of patients with MYC rearrangement positive large B-cell lymphoma with R-CHOP plus lenalidomide: results of a multicenter HOVON phase II trial. Haematologica. 2020;105(12):2805–2812. doi:10.3324/haematol.2019.238162
109. Salles G, Duell J, Gonzalez Barca E, et al. Tafasitamab plus lenalidomide in relapsed or refractory diffuse large B-cell lymphoma (L-MIND): a multicentre, prospective, single-arm, phase 2 study. Lancet Oncol. 2020;21(7):978–988. doi:10.1016/S1470-2045(20)30225-4
110. Belada D, Kopeckova K, Bergua JM, et al. First-MIND: a phase Ib, open-label, randomized study to assess safety of tafasitamab (tafa) or tafa + lenalidomide (LEN) in addition to R-CHOP in patients with newly diagnosed DLBCL. J Clin Oncol. 2021;39(15_suppl):7540. doi:10.1200/JCO.2021.39.15_suppl.7540
111. Vitolo U, Nowakowski GS, Burke JM, et al. ABCL-021: FRONT-MIND: a phase III, randomized, double-blind, placebo-controlled study comparing efficacy and safety of tafasitamab + lenalidomide + R-CHOP vs R-CHOP alone for newly-diagnosed high-intermediate and high-risk diffuse large B-cell lymphoma (DLBCL). Clin Lymphoma Myeloma Leuk. 2021;21:S376–S377.
112. Westin J, Davis RE, Feng L, et al. Smart start: rituximab, lenalidomide, and ibrutinib in patients with newly diagnosed large B-cell lymphoma. J Clin Oncol. 2022;Jco2200597. doi:10.1200/JCO.22.00597
113. Buchner M, Muschen M. Targeting the B-cell receptor signaling pathway in B lymphoid malignancies. Curr Opin Hematol. 2014;21(4):341–349. doi:10.1097/MOH.0000000000000048
114. Younes A, Sehn LH, Johnson P, et al. Randomized phase III trial of ibrutinib and rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone in non-germinal center B-cell diffuse large B-cell lymphoma. J Clin Oncol. 2019;37(15):1285–1295. doi:10.1200/JCO.18.02403
115. Wilson WH, Wright GW, Huang DW, et al. Effect of ibrutinib with R-CHOP chemotherapy in genetic subtypes of DLBCL. Cancer Cell. 2021;39(12):1643–1653.e1643. doi:10.1016/j.ccell.2021.10.006
116. Sehn LH, Kahl BS, Matasar MJ, et al. ESCALADE: a phase 3 study of acalabrutinib in combination with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) for patients ≤65y with untreated non-germinal center B-cell–like (non-GCB) diffuse large B-cell lymphoma (DLBCL). J Clin Oncol. 2021;39(15_suppl):TPS7572–TPS7572. doi:10.1200/JCO.2021.39.15_suppl.TPS7572
117. Davies A, Cummin TE, Barrans S, et al. Gene-expression profiling of bortezomib added to standard chemoimmunotherapy for diffuse large B-cell lymphoma (REMoDL-B): an open-label, randomised, phase 3 trial. Lancet Oncol. 2019;20(5):649–662. doi:10.1016/S1470-2045(18)30935-5
118. Ravi D, Beheshti A, Abermil N, et al. Proteasomal inhibition by ixazomib induces CHK1 and MYC-dependent cell death in T-cell and Hodgkin lymphoma. Cancer Res. 2016;76(11):3319–3331. doi:10.1158/0008-5472.CAN-15-2477
119. Galvez C, Karmali R, Hamadani M, et al. A phase I-II trial of DA-EPOCH-R plus ixazomib as frontline therapy for patients with MYC-aberrant lymphoid malignancies: the Daciphor regimen. Blood. 2020;136:44–45. doi:10.1182/blood-2020-138532
120. Morschhauser F, Feugier P, Flinn IW, et al. A phase 2 study of venetoclax plus R-CHOP as first-line treatment for patients with diffuse large B-cell lymphoma. Blood. 2021;137(5):600–609. doi:10.1182/blood.2020006578
121. Abramson JS, Ruppert AS, Giri S, et al. Randomized phase II/III study of DA-EPOCH-R ± venetoclax in previously untreated double hit lymphoma: initial results from alliance A051701. Blood. 2021;138(Supplement 1):523. doi:10.1182/blood-2021-151266
122. Sarkozy C, Morschhauser F, Dubois S, et al. A LYSA phase Ib study of tazemetostat (EPZ-6438) plus R-CHOP in patients with newly diagnosed diffuse large B-cell lymphoma (DLBCL) with poor prognosis features. Clin Cancer Res. 2020;26(13):3145–3153. doi:10.1158/1078-0432.CCR-19-3741
123. Kalakonda N, Maerevoet M, Cavallo F, et al. Selinexor in patients with relapsed or refractory diffuse large B-cell lymphoma (SADAL): a single-arm, multinational, multicentre, open-label, phase 2 trial. Lancet Haematol. 2020;7(7):e511–e522. doi:10.1016/S2352-3026(20)30120-4
124. Thieblemont C, Tilly H, Gomes da Silva M, et al. Lenalidomide maintenance compared with placebo in responding elderly patients with diffuse large B-cell lymphoma treated with first-line rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol. 2017;35(22):2473–2481. doi:10.1200/JCO.2017.72.6984
125. Witzig TE, Tobinai K, Rigacci L, et al. Adjuvant everolimus in high-risk diffuse large B-cell lymphoma: final results from the PILLAR-2 randomized phase III trial. Ann Oncol. 2018;29(3):707–714. doi:10.1093/annonc/mdx764
126. Crump M, Leppa S, Fayad L, et al. Randomized, double-blind, phase III trial of enzastaurin versus placebo in patients achieving remission after first-line therapy for high-risk diffuse large B-cell lymphoma. J Clin Oncol. 2016;34(21):2484–2492. doi:10.1200/JCO.2015.65.7171
127. Eyre TA, Djebbari F, Kirkwood AA, Collins GP. Efficacy of central nervous system prophylaxis with stand-alone intrathecal chemotherapy in diffuse large B-cell lymphoma patients treated with anthracycline-based chemotherapy in the rituximab era: a systematic review. Haematologica. 2020;105(7):1914–1924. doi:10.3324/haematol.2019.229948
128. Schmitz N, Zeynalova S, Nickelsen M, et al. CNS international prognostic index: a risk model for CNS relapse in patients with diffuse large B-cell lymphoma treated with R-CHOP. J Clin Oncol. 2016;34(26):3150–3156. doi:10.1200/JCO.2015.65.6520
129. Klanova M, Sehn LH, Bence-Bruckler I, et al. Integration of cell of origin into the clinical CNS International Prognostic Index improves CNS relapse prediction in DLBCL. Blood. 2019;133(9):919–926. doi:10.1182/blood-2018-07-862862
130. Orellana-Noia VM, Reed DR, McCook AA, et al. Single-route CNS prophylaxis for aggressive non-Hodgkin lymphomas: real-world outcomes from 21 US academic institutions. Blood. 2022;139(3):413–423. doi:10.1182/blood.2021012888
131. Bobillo S, Joffe E, Sermer D, et al. Prophylaxis with intrathecal or high-dose methotrexate in diffuse large B-cell lymphoma and high risk of CNS relapse. Blood Cancer J. 2021;11(6):113. doi:10.1038/s41408-021-00506-3
132. Jeong H, Cho H, Kim H, et al. Efficacy and safety of prophylactic high-dose MTX in high-risk DLBCL: a treatment intent–based analysis. Blood Adv. 2021;5(8):2142–2152. doi:10.1182/bloodadvances.2020003947
133. Portell CA. CNS prophylaxis in DLBCL: first do no harm. Blood. 2022;139(16):2420–2421. doi:10.1182/blood.2022015432
134. Wilson MR, Eyre TA, Kirkwood AA, et al. Timing of high-dose methotrexate CNS prophylaxis in DLBCL: a multicenter international analysis of 1384 patients. Blood. 2022;139(16):2499–2511. doi:10.1182/blood.2021014506
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.