Back to Journals » Infection and Drug Resistance » Volume 15
Differentiation Between Acinetobacter Baumannii Colonization and Infection and the Clinical Outcome Prediction by Infection in Lower Respiratory Tract
Authors Feng DY , Zhou JX, Li X, Wu WB , Zhou YQ, Zhang TT
Received 22 June 2022
Accepted for publication 7 September 2022
Published 12 September 2022 Volume 2022:15 Pages 5401—5409
DOI https://doi.org/10.2147/IDR.S377480
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Ding-Yun Feng,* Jian-Xia Zhou,* Xia Li, Wen-Bin Wu, Yu-Qi Zhou, Tian-Tuo Zhang
Department of Pulmonary and Critical Care Medicine, Third Affiliated Hospital of Sun Yat-Sen University, Institute of Respiratory Diseases of Sun Yat-Sen University, Guangzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Tian-Tuo Zhang, Department of Pulmonary and Critical Care Medicine, Third Affiliated Hospital of Sun Yat-sen University, Institute of Respiratory Diseases of Sun Yat-Sen University, 600 Tianhe Road, Guangzhou, 510630, People’s Republic of China, Tel +86-20-85252241, Fax +86-20-85253084, Email [email protected]
Purpose: Acinetobacter baumannii is the most common microorganism in sputum cultures from long-term hospitalized patients and is often the cause of hospital-acquired pneumonia (HAP), which is usually associated with poor prognosis and high mortality. It is sometimes difficult to distinguish between A. baumannii infection and colonization. This study aimed to evaluate factors that differentiate infection from colonization and predict mortality in patients with nosocomial pneumonia caused by A. baumannii.
Patients and Methods: The data used in this study were collected in our hospital between January 2018 and December 2020 from patients whose sputum cultures were positive for A. baumannii.
Results: A total of 714 patients were included, with 571 in the infection group and 143 in the colonization group. The in-hospital mortality rate in the infection group was 20.5%. Univariate and multivariate logistic regression analyses showed that age, total number of inpatient departments, absolute neutrophil count, and C-reactive protein (CRP) level helped distinguish between infection and colonization. The area under the receiver operating characteristic curve (ROC) of the identification model was 0.694. In the infection group, age, Charlson comorbidity score, neutrophil-to-lymphocyte ratio, blood urea nitrogen/albumin ratio, CRP level, presence of multidrug resistance, and clinical pulmonary infection score (≥ 6) ratio were associated with in-hospital mortality. The area under the ROC curve for the prediction model was 0.828. The top three drug resistance rates in the infection group were 100% (cefazolin), 98.77% (ceftriaxone), and 71.8% (cefuroxime).
Conclusion: The combination of common parameters helps identify A. baumannii respiratory tract infection or colonization. Several novel predictors can be used to predict the risk of death from A. baumannii pneumonia to reduce mortality. The drug resistance of A. baumannii remains high.
Keywords: Acinetobacter baumannii, infection, colonization, mortality, drug resistance
Introduction
Hospital-acquired infection (HAI) is an infection that happens within 48 hours after hospitalization or 3 days after discharge.1 This means that the longer the patient stays in the hospital, the higher the risk of developing HAI. Hospital-acquired pneumonia (HAP) is one of the most common types of nosocomial infections.2 Sputum culture examination is often required to identify the HAP pathogen and is widely used in hospitalized patients. Acinetobacter baumannii (A. baumannii) was the most prevalent organism, followed by Staphylococcus aureus (S. aureus), Pseudomonas aeruginosa (P. aeruginosa), and Escherichia coli (E. coli) from the sputum and endotracheal aspirates collected from each patient for bacterial isolation and detection.3 A. baumannii infection often results in poor prognosis and high mortality.4 In a multicenter retrospective cohort study, Ko RE found that A. baumannii was the most common multidrug-resistant (MDR) pathogen, leading to 28.2% of patients being admitted to the ICU for additional care and 28.1% dying.5
A. baumannii colonization and infection were usual in the lower respiratory tract. A. baumannii colonization means sputum culture from lower respiratory tract is positive without infection signs. And A. baumannii infection means sputum culture from lower respiratory tract is positive with infection signs. A. baumannii infection is a serious problem, and it is difficult to distinguish between A. baumannii infection and colonization. In clinical practice, it is very easy to culture A. baumannii from sputum culture specimens. Not every patient with HAP has evidence of typical clinical infection. This study aimed to evaluate factors that differentiate infection from colonization and predict mortality in patients with nosocomial pneumonia caused by A. baumannii.
Materials and Methods
The data used in this study were collected from patients whose sputum cultures from lower respiratory tract were positive for A. baumannii in the third affiliated hospital of Sun Yat-sen University, Guangdong, China, between January 2018 and December 2020. This was a single-center, retrospective study. Each patient underwent chest radiography or computed tomography for diagnosis. The inclusion criteria were as follows: (1) the criterion for A. baumannii colonization in lower respiratory tract is with sputum culture from lower respiratory tract was positive for A. baumannii, but there were no respiratory tract symptoms or signs, and chest radiography or computed tomography had no pulmonary infiltrate. (2) the lower respiratory tract infection included in this study was nosocomial pneumonia. The criterion for a diagnosis of HAP6 is a new pulmonary infiltrate (occurring ≥48 h after admission) associated with at least one of the following: new or increased cough with or without purulent tracheobronchial secretion or new pathogenic bacteria isolated from sputum or tracheal aspirate culture with ≥104 colony-forming units/mL, fever (>37.8℃) or hypothermia (<35.6℃), leukocytosis, left shift, or leukopenia based on local normal values. (3) all included patients were at least 18 years of age. The exclusion criteria were as follows: (1) the patients who did not have pneumonia or have positive sputum cultures and one symptom ie pyrexia. (2) patients who were considered as community infection. (3) patients with acquired immunodeficiency syndrome and those with missing key data. Variables of interest included age, sex, comorbidities, in-hospital mortality and so on.
Pathogenic bacteria isolated from clinical specimens were further characterized by conventional biochemical tests to identify specific strains using standard microbiological methods.7 The collection time of sputum culture was when patients had sputum within 24 hours. Pathogenic organism susceptibility testing was conducted using the microdilution method (MicroScan System; Baxter Healthcare, West Sacramento, CA, USA), and the results were interpreted using the National Committee for Clinical Laboratory Standards guidelines published in 2020 (Clinical & Laboratory Standards Institute, 2020).8 MDR pathogens were defined as organisms resistant to at least one of three or more antimicrobial categories in susceptibility tests of isolates from patients with HAP.9 The Charlson score is a composite score for age and comorbid conditions.10 The Clinical Pulmonary Infection Score (CPIS), which determines temperature, blood leukocytes, tracheal secretions, PaO2/FiO2, and chest radiography, was used to assess the severity of HAP.11
The main endpoints of this study were the identification of infection and colonization and in-hospital survival. Infection was defined as meeting the criteria for HAP and a positive sputum culture with A. baumannii. Survival was defined as the time interval between HAP diagnosis and death or before discharge.
Statistical Analysis
Statistical analyses of parametric data are reported as frequency, percentage, mean value, and standard deviation. Nonparametric data were reported as frequencies and percentages. Simple logistic regression and multivariate logistic regression analyses were used, and each independent variable was analyzed together with the dependent variable HAP or death. This was reported as the P-value and odds ratio (OR). A receiver operating characteristic (ROC) curve was used to analyze the probability of A. baumannii infection or HAP-related mortality. The accepted level of significance was set at P< 0.05. Statistical analyses were performed using SPSS Statistics version 25 (IBM Corp., Armonk, NY, USA).
Results
During the study period, 1044 inpatients had positive sputum culture results. Based on the inclusion and exclusion criteria, 714 patients were enrolled. According to the analysis process (Figure 1), 571 and 143 patients were in the infection and colonization groups, respectively. During hospitalization, 20.5% of the patients in the infection group died.
 |
Figure 1 The flow chart of this study. |
In the first analysis of the predictors of infection and colonization, univariate logistic regression analysis revealed that age, intensive care unit admission, total number of inpatient departments, white blood cell level(WBC), absolute value of neutrophils, blood urea nitrogen/albumin ratio (BUN/ALB ratio), and C-reactive protein (CRP) levels were related to A. baumannii infection in HAP. Multivariate logistic regression analysis revealed that age (odds ratio [OR]=1.011, 95% confidence interval (CI):1.000–1.022, P=0.047), total number of inpatient departments (OR=1.763, 95% CI: 1.053–2.950, P=0.031), absolute value of neutrophils (OR=1.068, 95% CI: 1.006–1.134, P=0.031), and CRP level (OR=1.007, 95% CI: 1.002–1.012, P=0.005) were still helpful in distinguishing between infection and colonization (Table 1). WBC, absolute values of neutrophils, and CRP levels are often used to distinguish between infection and colonization. To test whether the prediction model for distinguishing between infection and colonization established in this study was superior to these classical indicators, we compared their ROC values and found that this model was superior to the others. The area under the ROC curve of the identification model was 0.694 (Figure 2).
 |
Table 1 Significant Univariate and Multivariate Logistic Regression Analyses of Predictors Used to Distinguish Between Infection and Colonization |
 |
Figure 2 Comparison of different receiver-operating characteristics curves for predicting infection or colonization model. |
In the second step, this study found that hospital deaths occurred in 117 cases (20.5%). The infection group was analyzed to identify the risk factors for death. Univariate logistic regression analysis showed that age, ICU admission, total number of inpatient departments, Charlson score, absolute value of neutrophils, neutrophil-to-lymphocyte (NLR) ratio, BUN/ALB ratio, procalcitonin(PCT) level, CRP level, presence of MDR pathogens, and CPIS ≥6 ratio were associated with in-hospital mortality.
Multivariate logistic regression analysis revealed that age, Charlson score, NLR ratio, BUN/ALB ratio, CRP level, presence of MDR pathogens, and CPIS ≥6 ratio were associated with in-hospital mortality (Table 2). The area under the ROC curve for the prediction model was 0.828 (Figure 3). Analysis of drug resistance in the infected group revealed that the top three drug resistance rates were 100% (cefazolin), 98.77% (ceftriaxone), and 71.8% (cefuroxime) in the infection group (Table 3).
 |
Table 2 Significant Univariate and Multivariate Logistic Regression Analyses of Predictors for Hospital-Acquired Pneumonia in-Hospital Mortality |
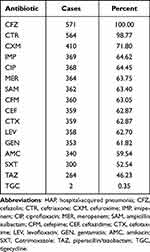 |
Table 3 The Drug Resistance of a.baumannii Infection Group of Hospital-Acquired Pneumonia |
 |
Figure 3 Analysis of receiver-operating characteristics curve for predicting mortality model. |
Discussion
Sputum and endotracheal aspirates are important for identifying the pathogen in patients with HAP. A. baumannii is one of the most common bacteria detected in sputum culture and endotracheal aspirate from long-term hospitalized patients, including those with HAP.3 A. baumannii is an opportunistic infectious agent that can cause lung infection and nosocomial infection transmission.12 It usually causes bacterial colonization, defined as the presence of bacteria on the body surface, including the airway, without causing clinical evidence of infection in the individual.13 As it is very easy to culture A. baumannii from sputum specimens in clinical practice, not every patient with HAP has evidence of a typical clinical infection. It is difficult to distinguish A. baumannii infections from colonization.14 In the current study, we found that age was associated with A. baumannii infection, with the risk of infection increasing with age. This may be due to the decline of immune organs and immune cell activity with age.15 Patients with long hospital stays, some of which may be complicated or critically ill, are often hospitalized in rotation among several departments. Therefore, this study focused on the patients’ total number of inpatient departments. Interestingly, this study found that the more departments a patient was hospitalized in, the more susceptible he was to infection. This may be related to the fact that patients with more inpatient departments are exposed to more A. baumannii in the environment. Absolute neutrophil and CRP levels are common predictors of bacterial infection. The current study also found that both predictors were related to A. baumannii infection, consistent with previous studies.16 This study compared the ROC of a single classical indicator and a synthetic model and found that this model was superior to other single classical indicators. Therefore, it is valuable to construct a model to predict colonization or infection. This model allows clinicians to identify infections more accurately than ever before.
- baumannii was one of the most prevalent organism found in sputum and endotracheal aspirate collected from each patient with HAP for bacterial isolation and detection.17 A. baumannii infection often results in poor prognosis and high mortality.4 Shu et al18 reported that hospital deaths in patients who were diagnosed with HAP, caused by MDR-A. baumannii infections, occurred in 142 patients (38.80%). The current study also showed that A. baumannii HAP has a high mortality rate (20.5%). Multivariate logistic regression analysis revealed that age, Charlson score, NLR ratio, BUN/ALB ratio, CRP level, presence of MDR pathogens, and CPIS ≥6 ratio were associated with in-hospital mortality. As people age, their immune organs and cells deteriorate, making them susceptible to infection. This study also found that age was associated with in-hospital mortality. However, there is no clear positive correlation, which may be related to the selection bias of the enrolled population and the differences in population characteristics of the center. Because our hospital is characterized by rehabilitation medicine, many young patients who need rehabilitation will be hospitalized in our hospital, and this kind of patients are prone to hospital acquired infections because of their long hospitalization time. The current study found that the Charlson score was associated with in-hospital mortality, which is consistent with previous reports.19 This suggests that clinicians should pay special attention to infection management in patients with a high Charlson score to improve their prognosis. NLR has been widely used as a prognostic indicator of infectious diseases.20 However, data on A. baumannii HAP are lacking. This study identified NLR as a prognostic indicator for A. baumannii HAP. Zou et al showed that the BUN/ALB ratio was a simple but independent predictor of 30-day mortality and severity of E. coli bacteremia.21 Akahane et al found that a high BUN/ALB ratio was an independent prognostic predictor of mortality risk in patients with pneumocystis pneumonia.22 BUN/ALB ratio was first identified as an independent prognostic factor in patients with nosocomial pneumonia caused by A. baumannii in our study. This study provides a reference for future clinical diagnoses and treatments. CRP level and CPIS≥6 ratio are indicators of infection severity.23–25 It is well known that the more severe the infection, the higher the risk of death. Therefore, it is reasonable that these two indicators were associated with the risk of death in the current study. MDR-A. baumannii can lead to poorer prognosis and follow-up of patients with increased mortality.18,26 Therefore, MDR-A. baumannii was associated with mortality in the present study, with a model combining the above factors being reliable and having a high predictive value. However, resistance to A. baumannii remains high.18,26,27 A. baumannii HAP in an EICU was poorly susceptible to piperacillin-tazobactam, cefepime, amoxicillin+clavulonic acid, and ciprofloxacin in Tianjin, China.28 Our study also found that A. baumannii is poorly susceptible to cefazolin, ceftriaxone, and cefuroxime. Fortunately, the sensitivity to tigecycline remains good. Attention should be paid to controlling the spread of drug-resistant A. baumannii and striving to improve its prognosis.
PCT is an important indicator in the evaluation of bacterial infection. In bacterial septic conditions, PCT levels are always elevated, and serum concentrations are correlated with severity of microbial invasion.29 This study also included PCT to analyze, found it was not sensitive to distinguish between A. baumannii infection and colonization. In the infection group, the level of PCT was higher in patients who died than in those who survived, although the difference was not statistically significant. The value and clinical application of PCT in distinguishing between A. baumannii infection and colonization were inferior to CRP and other indicators.30 It was similar for predicting the prognosis of A. baumannii infection in low respiratory tract.31 This may be related to the fact that the level of PCT is more correlated with the severity of systemic inflammation caused by infection.29 PCT levels are associated with severe trauma and renal insufficiency.32,33
This study has some limitations. First, although we had very high admission criteria, there may still be an information bias in individual cases, which is a common shortcoming of retrospective studies. Second, the accuracy of the differential model between infection and colonization constructed in this study needs to be improved. Further research involving data from a larger sample is required. Third, as a retrospective study, this study did not include information for the appropriateness of antibiotic treatment and data for the severity of respiratory failure, and only included the Clinical Pulmonary Infection Score (CPIS), this is a limitation. In the future, we will carry out a future prospective study to further explore related issues.
Conclusion
The combination of common parameters helps identify A. baumannii respiratory tract infection or colonization. Several novel predictors can be used to predict the risk of death from A. baumannii pneumonia to reduce mortality. The drug resistance of A. baumannii remains high. PCT has no significant advantage in differentiating lower respiratory tract infection from colonization or predicting the risk of death.
Statement of Ethics
This study was approved by the institutional review board of the hospital and the ethics committee of the Third Affiliated Hospital of Sun Yat-sen University (No. [2021]02-13). This study was conducted and designed in accordance with the Declaration of Helsinki. The need for written informed consent was waived because of the non-interventional design. Patient information was kept confidential.
Funding
National Natural Science Foundation of China (No. 82170014).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Khammarnia M, Ansari-Moghaddam A, Barfar E, et al. Systematic review and meta-analysis of hospital acquired infections rate in a Middle East country (1995–2020). Med J Islam Repub Iran. 2021;35:102. PMID: 34956948; PMCID: PMC8683797. doi:10.47176/mjiri.35.102
2. Vo-Pham-Minh T, Duong-Thi-Thanh V, Nguyen T, et al. The impact of risk factors on treatment outcomes of nosocomial pneumonia due to gram-negative bacteria in the intensive care unit. Pulm Ther. 2021; (2):563–574. PMID: 34652610; PMCID: PMC8517295. doi:10.1007/s41030-021-00175-4
3. Mazloomirad F, Hasanzadeh S, Sharifi A, Nikbakht G, Roustaei N, Khoramrooz SS. Identification and detection of pathogenic bacteria from patients with hospital-acquired pneumonia in southwestern Iran; evaluation of biofilm production and molecular typing of bacterial isolates. BMC Pulm Med. 2021;21(1):408. PMID: 34886838; PMCID: PMC8662843. doi:10.1186/s12890-021-01773-3
4. Feng DY, Zhou YQ, Zou XL, et al. Differences in microbial etiology between hospital-acquired pneumonia and ventilator-associated pneumonia: a single-center retrospective study in Guang Zhou. Infect Drug Resist. 2019;12:993–1000. doi:10.2147/IDR.S204671
5. Ko RE, Min KH, Hong SB, et al. Korean HAP/VAP study group. characteristics, management, and clinical outcomes of patients with hospital-acquired and ventilator-associated pneumonia: a multicenter cohort study in Korea. Tuberc Respir Dis. 2021;84:317–325. PMID: 34134465; PMCID: PMC8497766. doi:10.4046/trd.2021.0018
6. Shi Y, Huang Y, Zhang TT, et al. Chinese guidelines for the diagnosis and treatment of hospital-acquired pneumonia and ventilator-associated pneumonia in adults (2018 Edition). J Thorac Dis. 2019;11(6):2581–2616. PMID: 31372297; PMCID: PMC6626807. doi:10.21037/jtd.2019.06.09
7. Jorgensen JH, Pfaller MA, Carroll KC, et al. Manual of Clinical Microbiology.
8. CLSI. Performance standards for antimicrobial susceptibility testing. 30sted. CLSI supplement M100. Wayne, PA: Clinical and Laboratory Standards Institute; 2020.
9. Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–281. doi:10.1111/j.1469-0691.2011.03570.x
10. Borja AJ, Connolly J, Kvint S, et al. Charlson Comorbidity Index score predicts adverse post-operative outcomes after far lateral lumbar discectomy. Clin Neurol Neurosurg. 2021;206:106697. PMID: 34030078. doi:10.1016/j.clineuro.2021.106697
11. Fujitani S, Yu VL. Diagnosis of ventilator-associated pneumonia: focus on nonbronchoscopic techniques (nonbronchoscopic bronchoalveolar lavage, including Mini-BAL, blinded protected specimen brush, and blinded bronchial sampling) and endotracheal aspirates. J Intensive Care Med. 2006;21:17–21. doi:10.1177/0885066605283094
12. da Fonseca AS, Mencalha AL, de Paoli F. Antimicrobial photodynamic therapy against Acinetobacter baumannii. Photodiagnosis Photodyn Ther. 2021;35:102430. PMID: 34233224. doi:10.1016/j.pdpdt.2021.102430
13. Gordon NC, Wareham DW. Evaluation of CHROMagar Acinetobacter for detection of enteric carriage of multidrug-resistant Acinetobacter baumannii in samples from critically ill patients. J Clin Microbiol. 2009;47:2249–2251. doi:10.1128/JCM.00634-09
14. Casadevall A, Pirofski LA. Host-pathogen interactions: basic concepts of microbial commensalism, colonization, infection, and disease. Infect Immun. 2000;68:6511–6518. doi:10.1128/IAI.68.12.6511-6518.2000
15. Carrasco E, Gómez de Las Heras MM, Gabandé-Rodríguez E, Desdín-Micó G, Aranda JF, Mittelbrunn M. The role of T cells in age-related diseases. Nat Rev Immunol. 2022;22(2):97–111. PMID: 34099898. doi:10.1038/s41577-021-00557-4
16. Zarkesh M, Sedaghat F, Heidarzadeh A, Tabrizi M, Bolooki-Moghadam K, Ghesmati S. Diagnostic value of IL-6, CRP, WBC, and absolute neutrophil count to predict serious bacterial infection in febrile infants. Acta Med Iran. 2015;53(7):408–411. PMID: 26520627.
17. Jean SS, Chang YC, Lin WC, Lee WS, Hsueh PR, Hsu CW. Epidemiology, treatment, and prevention of nosocomial bacterial pneumonia. J Clin Med. 2020;9(1):275. PMID: 31963877; PMCID: PMC7019939. doi:10.3390/jcm9010275
18. Shu H, Li L, Wang Y, et al. Prediction of the risk of hospital deaths in patients with hospital-acquired pneumonia caused by multidrug-resistant Acinetobacter baumannii infection: a multi-center study. Infect Drug Resist. 2020;13:4147–4154. PMID: 33244244; PMCID: PMC7683351. doi:10.2147/IDR.S265195
19. Jiao J, Li Z, Wu X, et al. Risk factors for 3-month mortality in bedridden patients with hospital-acquired pneumonia: a multicentre prospective study. PLoS One. 2021;16(3):e0249198. PMID: 33784317; PMCID: PMC8009424. doi:10.1371/journal.pone.0249198
20. Liu Y, Du X, Chen J, et al. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J Infect. 2020;81(1):e6–e12. PMID: 32283162; PMCID: PMC7195072. doi:10.1016/j.jinf.2020.04.002
21. Zou XL, Feng DY, Wu WB, Yang HL, Zhang TT. Blood urea nitrogen to serum albumin ratio independently predicts 30-day mortality and severity in patients with Escherichia coli bacteraemia. Med Clin (Barc). 2021. 157(5):219–225. English, Spanish. PMID: 33059940. doi:10.1016/j.medcli.2020.06.060
22. Akahane J, Ushiki A, Kosaka M, et al. Blood urea nitrogen-to-serum albumin ratio and A-DROP are useful in assessing the severity of Pneumocystis pneumonia in patients without human immunodeficiency virus infection. J Infect Chemother. 2021;27(5):707–714. PMID: 33376033. doi:10.1016/j.jiac.2020.12.017
23. von Dach E, Albrich WC, Brunel AS, et al. Effect of C-reactive protein-guided antibiotic treatment duration, 7-day treatment, or 14-day treatment on 30-day clinical failure rate in patients with uncomplicated gram-negative bacteremia: a randomized clinical trial. JAMA. 2020;323(21):2160–2169. PMID: 32484534; PMCID: PMC7267846. doi:10.1001/jama.2020.6348
24. Wang Y, Zhang S, Li L, Xie J. The usefulness of serum procalcitonin, C-reactive protein, soluble triggering receptor expressed on myeloid cells 1 and Clinical Pulmonary Infection Score for evaluation of severity and prognosis of community-acquired pneumonia in elderly patients. Arch Gerontol Geriatr. 2019;80:53–57. PMID: 30366226. doi:10.1016/j.archger.2018.10.005
25. Monajati M, Ala S, Aliyali M, et al. Clinical effectiveness of a high dose versus the standard dose of meropenem in ventilator-associated pneumonia caused by multidrug resistant bacteria: a randomized, single-blind clinical trial. Infect Disord Drug Targets. 2021;21(2):274–283. PMID: 32106807. doi:10.2174/1871526520666200227102013
26. Ren J, Li X, Wang L, Liu M, Zheng K, Wang Y. Risk factors and drug resistance of the MDR Acinetobacter baumannii in pneumonia patients in ICU. Open Med. 2019;14:772–777. PMID: 31667355; PMCID: PMC6814959. doi:10.1515/med-2019-0090
27. Butler DA, Biagi M, Tan X, Qasmieh S, Bulman ZP, Wenzler E. Multidrug resistant Acinetobacter baumannii: resistance by any other name would still be hard to treat. Curr Infect Dis Rep. 2019;21(12):46. PMID: 31734740. doi:10.1007/s11908-019-0706-5
28. Zhang Y, Shou S. Pathogens and drug-resistance of hospital-acquired pneumonia in an EICU in Tianjin, China. Int J Biochem Mol Biol. 2021;12(2):49–54. PMID: 34084593; PMCID: PMC8166653.
29. Hamade B, Huang DT. Procalcitonin: where are we now? Crit Care Clin. 2020;36(1):23–40. PMID: 31733680; PMCID: PMC6866676. doi:10.1016/j.ccc.2019.08.003
30. Wussler D, Kozhuharov N, Tavares Oliveira M, et al. Clinical utility of procalcitonin in the diagnosis of pneumonia. Clin Chem. 2019;65(12):1532–1542. PMID: 31615771. doi:10.1373/clinchem.2019.306787
31. Kamat IS, Ramachandran V, Eswaran H, Guffey D, Musher DM. Procalcitonin to distinguish viral from bacterial pneumonia: a systematic review and meta-analysis. Clin Infect Dis. 2020;70(3):538–542. doi:10.1093/cid/ciz545
32. Lakshmi VS, Cherian A, Adole P. Procalcitonin assay has no role in the routine assessment of severe trauma patients at admission to the emergency department. Cureus. 2021;13(7):e16228. PMID: 34268060; PMCID: PMC8262111. doi:10.7759/cureus.16228
33. Bowman C, Covington EW. Determination of the optimal procalcitonin threshold for infection in patients with impaired renal function at a community hospital. J Pharm Technol. 2020;36(4):157–163. PMID: 34752523; PMCID: PMC7359663. doi:10.1177/8755122520924803
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
