Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 18
Differential Response to 12 Weeks of Once-Daily Tiotropium/Olodaterol Fixed Dose Combination in Patients with COPD: A Multidimensional Response Profiling in the TORRACTO Study
Authors Posthuma R , Vanfleteren LEGW , Gaffron S , Vaes AW, Franssen FME , Spruit MA
Received 22 January 2023
Accepted for publication 13 May 2023
Published 8 June 2023 Volume 2023:18 Pages 1091—1102
DOI https://doi.org/10.2147/COPD.S405478
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Rein Posthuma,1– 3 Lowie EGW Vanfleteren,4,5 Swetlana Gaffron,6 Anouk W Vaes,1 Frits ME Franssen,1– 3 Martijn A Spruit1– 3
1Department of Research and Development, CIRO+, Center of Expertise for Chronic Organ Failure, Horn, the Netherlands; 2NUTRIM, School of Nutrition and Translational Research in Metabolism, Faculty of Health, Medicine and Life Sciences, Maastricht, the Netherlands; 3Department of Respiratory Medicine, Maastricht University Medical Center (MUMC+), Maastricht, the Netherlands; 4COPD Center, Department of Respiratory Medicine and Allergology, Sahlgrenska University Hospital, Gothenburg, Sweden; 5Department of Internal Medicine and Clinical Nutrition, Institute of Medicine, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden; 6Viscovery Software GmbH, Vienna, Austria
Correspondence: Rein Posthuma, CIRO+, Center of expertise for chronic organ failure, Hornerheide 1, Horn, 6085 NM, the Netherlands, Email [email protected]
Purpose: Long-acting bronchodilators (LABD), in general, reduce respiratory symptoms, improve exercise endurance time and pulmonary function in patients with chronic obstructive pulmonary disease (COPD). However, there might be heterogeneity in improvement for several outcomes on an individual level. Therefore, we aimed to profile the multidimensional response in patients receiving tiotropium/olodaterol (T/O) using self-organizing maps (SOM).
Materials and Methods: This is a secondary analysis of the TORRACTO study: a multicenter, multinational, randomized, double-blind, placebo-controlled, parallel-group trial to evaluate the effects of T/O (2.5/5 and 5/5 μg) compared with placebo after 6 and 12 weeks of treatment in patients with COPD. In the current study, we used endurance time, forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC), inspiratory capacity (IC) at rest and IC at isotime (ICiso) to identify clusters by means of SOM in patients treated with T/O.
Results: Six clusters with distinct response profiles were generated at week 12 in COPD patients receiving T/O (n = 268). Patients in cluster 1 improved significantly on all outcomes, whilst cluster 5 showed strong improvement in endurance time (357s); contrarily, FEV1, FVC, ICrest and ICiso decreased when compared to baseline.
Conclusion: Individual responses on endurance time and pulmonary function after 12 weeks of T/O are heterogeneous. This study identified clusters in COPD patients with markedly different multidimensional response on LABD.
Keywords: COPD, long-acting bronchodilators, outcomes
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by persistent airflow obstruction, exertional dyspnea, and exercise limitation, commonly related to lung hyperinflation.1 Inhalation therapy, primarily long-acting bronchodilators (LABD), is a central component in the management of stable COPD.2 Studies examining the efficacy of LABD in patients with COPD reported improvements in forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC), inspiratory capacity (IC) and exercise endurance time with dual bronchodilation providing greater efficacy than placebo or monotherapy.3–7 This was also confirmed in the TORRACTO study, a double-blind, placebo-controlled trial, which demonstrated that tiotropium/olodaterol 5/5 μg (T/O) improved endurance time during constant work-rate cycle ergometry (CWRCE) with 23% and 14% after, respectively, 6 and 12 weeks compared to placebo.6 In addition, the secondary endpoints, IC at rest (ICrest) and at isotime (ICiso), showed significant expansion confirming the strong association between reduction in hyperinflation and improvement in exercise tolerance. However, patients with COPD are complex and clinical outcomes are often not linearly associated. Although mean improvement on different outcomes can be seen, there might be heterogeneity in improvement for several outcomes on an individual level.8,9
To study and visualize this complexity, statistical analysis using self-organizing maps (SOM) has been shown to identify meaningful clusters and non-linear relationships in COPD research.10,11 Additionally, examining multiple outcomes simultaneously can give insight into the complex effects of LABDs. Moreover, assessing these multiple outcomes at different intervals (6 or 12 weeks) can contribute to understanding the stability of response clusters over time.
In this secondary analysis of the TORRACTO study we aimed to profile the multidimensional response in patients receiving tiotropium/olodaterol using endurance time, FEV1, FVC, ICrest, and ICiso. Furthermore, we wanted to explore the stability of these clusters over time between 6 and 12 weeks. We hypothesized that clusters of patients could be identified with significantly different response profiles on T/O and that the clusters remain relatively stable over time.
Materials and Methods
This is a secondary analysis from already published research data (Maltais et al, 2018 https://doi.org/10.1177/1753465818755091).6 Therefore, new ethics committee approval does not apply for our secondary analysis.
Overview of TORRACTO Study Methods
In summary, the TORRACTO® [ClinicalTrials.gov NCT01525615] was a multicenter, multinational, randomized, double-blind, placebo-controlled, parallel-group trial to evaluate the effects of once daily tiotropium/olodaterol (2.5/5 and 5/5 μg) compared with placebo after 12 weeks of treatment in patients with COPD. The primary outcome was endurance time during CWRCE.
The TORRACTO study protocol was reviewed and approved by the Institutional Review Boards, and all patients provided written, informed consent (co-ordinating investigator’s centre approval dated 15 March 2012, from Research Center, University Institute of Cardiology and Pneumology, Quebec, (IUCPQ Research Ethics Board), 2725 chemin Sainte-Foy, Quebec, Canada).
Patients with COPD, stable airway obstruction and between 40 and 75 years old were eligible when postbronchodilator FEV1/FVC was below 70% and postbronchodilator FEV1 <80% and ≥30% of predicted. Patients were former or current smokers with a minimum of 10 pack-years.
After inclusion but before randomization, ie run-in period, patients performed a protocolized incremental cycle ergometry to measure peak wattage (Wpeak) and subsequently 2 CWRCE tests at 75% of Wpeak.12,13 The CWRCEs were performed at least 4 days apart with the second test defined as the pretreatment baseline. During CWRCE, patients also performed an inspiratory capacity (IC) maneuver after each grading of breathing and leg discomfort using the modified Borg scale. Patients were stimulated to cycle to the point of symptom limitation. CWRCE at weeks 6 and 12 was performed 2 h post-trial medication.
Patients with known contraindications to exercise (unstable angina, uncontrolled heart failure, severe hypertension) or an exercise limitation other than leg fatigue or exertional dyspnea (eg arthritis in knee) or an endurance time >25 minutes were excluded from the study.
Included patients were randomized to receive one of the three treatments: T/O 2.5/5 μg, T/O 5/5 μg, or placebo, all delivered once daily via the Respimat® (Boehringer Ingelheim International GmbH, Ingelheim am Rhein, Germany) inhaler. Patients using long-acting muscarinic antagonist (LAMA) or long-acting β2-agonist (LABA) at baseline were switched to the trial medication. Inhaled corticosteroids were continued. Open-label salbutamol was provided as rescue medication throughout the study, and short-acting muscarinic antagonist was not permitted.
During the 12-week treatment period, trial medication was self-administered by the patient once daily between 7 a.m. and 10 a.m.; on test days, administration of trial medication was performed under research staff observation.
Pulmonary function testing and CWRCE were conducted at baseline, 6 and 12 weeks according to international standards.12–14 Isotime for each patient was outlined as the lowest exercise endurance time among baseline, week 6, and week 12 CWRCE tests. Isovalue was defined as the value of a particular variable at isotime, which could be an observed value or determined by interpolation.
Statistics
Data are presented as mean±SD or as frequency and percentage, as appropriate.
To analyze the multidimensional response of patients on T/O, the current study used self-organizing maps (SOM) using Viscovery Software SOMine 7.2 by Viscovery Software GmbH (www.viscovery.net), to create an ordered representation of the treated patients. We combined patients on T/O 2.5/5 μg and T/O 5/5 μg to one T/O group. Only patients using T/O were clustered, and the placebo group was excluded.
Patient data was ordered based on the similarity concerning selected attributes: the change in endurance time (measured with CWRCE), FEV1, FVC, ICrest, ICiso and overall response between baseline and week 12. The overall response was a composite attribute defined by the standardized differences between baseline and week 12 on CWRCE, FEV1, FVC, ICrest, and ICiso.
Each attribute was given a weight, ie, priority, in driving the ordering process, CWRCE 1.5, FEV1 1.2, FVC 1.2, ICrest 1.0, ICiso 1.0, and overall response 0.5.
The software placed patients with a similar response profile close to each other on the map. Based on the ordering of the patients on the map, the hierarchical ward cluster algorithm was applied, to group the patients in outcome clusters. The values of all attributes included in the analysis were then retracted cluster by cluster and exported to the statistical tables.
Patients for whom all values of the prioritized attributes were missing could not be assigned to any node in the model and thus, were automatically excluded. If specific attributes were missing for a single patient, this patient was matched to the SOM based on all other attributes whose values were known.
To demonstrate the trajectory of patients between the clusters at week 6 and week 12 a Sankey diagram was made. Thus, the original week 12 model with the 6 attributes was correspondingly applied at week 6. Patients with all or part of the outcomes missing at week 6 or 12 were excluded from the Sankey analysis since no suitable cluster assignment was possible.
Results
A total of 404 patients were randomized and received trial medication. Six patients were excluded due to missing data or lost to follow-up. Thus, 398 patients were included in the current analyses of which 268 were allocated to T/O and 130 to placebo. Baseline characteristics are shown in Table 1. The T/O group had a mean age of 62 years and mainly consisted of Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage II (mean FEV1 of 59% of predicted).
 |
Table 1 Baseline Characteristics |
Mean Response Following 6 and 12 Weeks of T/O or Placebo
After 6 weeks, the endurance time was 620 seconds for the whole T/O group and 490 seconds for the placebo group. In turn, the T/O group had a 24% higher increase in exercise time compared to the placebo group. After 12 weeks, the endurance time was 630 seconds for the T/O group and 548 seconds for the placebo group. The T/O group had a 14% higher increase in endurance time compared to the placebo group. After 12 weeks ICrest and ICiso in the T/O group were, respectively, 2.60 L and 2.27 L compared to 2.43 L and 2.17 L in the placebo group.
Multidimensional Response Profiling of T/O Group
Six clusters with distinct response profiles, as shown in Figure 1, were generated using the 6 attributes at week 12 on the total T/O group (n = 268). The selected outcomes at 6 and 12 weeks are reported per cluster in Table 2.
 |
Table 2 Change from Baseline After 6- and 12-Weeks in Placebo and Total T/O Group |
Cluster 1 (n = 61) demonstrated a significant improvement on all attributes: endurance time, FEV1, FVC, ICrest, ICiso and overall outcome (Table 2) compared to the total T/O group after 12 weeks. Specifically, it showed the highest increase in FEV1, ICrest and ICiso of all clusters. Generally, this is the best performing cluster in the multidimensional response profiling.
Cluster 2 (n = 32) showed improvements in endurance time, FEV1, FVC, ICiso and overall outcome when compared to the entire T/O group. A small deterioration was detected on ICrest when compared to the total T/O group. From all the clusters, it had the highest improvement in endurance time, with mean improvement 378 ± 196 seconds (Table 2) when compared to baseline.
Cluster 3 (n = 36) showed a significant decrease in endurance time. However, it demonstrated improvements in FEV1, FVC and ICiso. No significant changes were detected on overall outcome and ICrest when compared to the whole T/O group (Table 2).
Cluster 4 (n = 41) showed a very small, but statistically significant, increase in endurance time and ICiso. A small, significant decrease was seen in ICrest and overall outcome. FEV1 and FVC showed no significant changes when compared to the whole T/O group
Cluster 5, the smallest cluster (n = 28), showed a significant considerable improvement in endurance time. Contrarily, it demonstrated a significant decrease in primarily FVC and to a lesser degree in FEV1, FVC, ICiso, ICrest and overall outcomes when compared to the whole T/O group.
Patients in cluster 6 (n = 70) demonstrated a significant decrease in endurance time, FEV1, FVC and overall outcome, whilst no significant changes existed in ICiso and ICrest when compared to the whole T/O group (Table 2). Overall, this cluster decreased the most when relating to the whole T/O group (Figure 1).
Cluster Characteristics
Baseline characteristics of the 6 clusters treated with T/O are presented in Table 3. Patients in cluster 2 had a lower endurance time and contained more COPD GOLD III patients with a significantly lower mean FEV1 of 51% compared to 59% in the total T/O group. Patients in cluster 4 contained significantly more women and current smokers when compared to the whole treated group. Importantly, no significant differences between the allocation of the two doses of T/O were detected when compared to the entire T/O group.
 |
Table 3 Baseline Characteristics in Placebo and T/O Group |
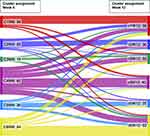
Stability of Clusters Between Week 6 and 12
In Figure 2 the flow of patients between the clusters is presented after 6 and 12 weeks of T/O treatment. In total, 250 patients had complete data at week 6 and week 12 for adequate cluster assignment. In general, there was substantial movement of patients in all clusters, with 48% of patients having the same cluster assignment at week 6 and week 12. The greatest change, percentage wise, was seen in cluster 4 in which 66% was assigned to a different cluster from week 6 to week 12.
Discussion
This study identified clusters with different multidimensional outcome profiles after 12 weeks of treatment with T/O in moderate-to-severe COPD patients. We demonstrated that response to LABD is heterogeneous and non-linear. Additionally, the study showed that treatment effects of T/O may differ over time.
The current GOLD report advocates initial therapy with LABD in all COPD patient groups with subsequent reassessment with a potential step-up approach based on dyspnea and exacerbations.1 An important mechanism of LABD is pharmacological lung volume reduction. By improving airflow and reducing airflow resistance, LABD leads to better lung emptying and consequently improves IC.15 However, response on LABD may differ between patients.8,9 The current analysis of 268 mild-to-moderate COPD patients treated with T/O verified these findings.
The present analysis classified, using non-parametric regression techniques, a cluster with a very good multidimensional response to T/O (cluster 1) and a cluster who seemed to be non-responding and partially showed deterioration in the selected outcomes (cluster 6). Interestingly, outcomes can also have completely different directions; cluster 3 showed an increase in FEV1 of 220mL but a reduction in endurance time of almost 2 minutes in contrast to cluster 5 which showed a negative change of −150mL in FEV1 but an improvement in endurance time of approximately 6 minutes. With the minimal clinically important difference of 100 s in CWRCE,16 cluster 5 showed, despite the deterioration in FEV1, an enormous improvement in endurance time. Thus, if this patient was assessed solely on one outcome, FEV1, it was regarded as a non-responder, however, the endurance time in daily life could have significantly improved. Therefore, changes in FEV1 with therapy should not be viewed as a surrogate for changes in exercise endurance and clearly underlines the need for looking at several outcomes concurrently. This is in line with existing literature demonstrating that changes in spirometric measurements like FEV1 are a poor predictor of improvement in endurance time in response to bronchodilators and improvements in static hyperinflation are largely independent of changes in expiratory flow limitation.17,18
Studying a set of different effects simultaneously in the pharmacological management of COPD has also been encouraged in several reviews and statements since the relationship between spirometry and symptoms appears to be modest.19–21 Moreover, recently, a multicomponent measure for assessing disease worsening in COPD has been studied in the IMPACT trial.22 Han et al demonstrated that prevention of clinically important deterioration (defined as any of the following on-treatment events: moderate/severe exacerbation, deterioration in lung function or deterioration in health status) is likely to reduce future risks of exacerbations requiring hospitalization and all-cause mortality. This analysis contributes to multidimensional response profiling and shows that SOM can be a helpful instrument to achieve this goal.
The GOLD report advises reassessment of the inhalation therapy if treatment goals like reducing respiratory symptoms are being met sufficiently.23 However, this is poorly defined and no time period is given when to reassess. The current study demonstrated that when looking simultaneously at different outcomes, the timing of assessment, 6 or 12 weeks, can have a large influence identifying the patient as a responder or non-responder. Indeed, patients at 6 weeks could flow from cluster 1 to cluster 6 or vice versa. For clinicians, this is important to be aware of and keep in mind when reviewing the symptoms after initiation of pharmacotherapy in COPD patients.
Several limitations of this study must be taken into account. Firstly, only Gold II and Gold III COPD patients were included which limits the extrapolation to GOLD I and the very severe GOLD IV patients. Secondly, key baseline values were not available, like modified Medical Research Scale of Dyspnea, exacerbation frequency and disease-specific health-related quality of life. Also, exacerbation frequency during the study per treatment arm was not reported. Potentially, the placebo group could have experienced more exacerbations during the study, when patients were withdrawn from LAMA or LABA, possibly affecting outcomes. A broader set of baseline characteristics could have allowed for identifying other clusters and possibly do more justice to the complexity of COPD management. Thirdly, the sample size is limited, leading to clusters with relatively small numbers. Preferably larger studies are needed for assessing multidimensional outcomes in more detail. Finally, no data was available if initiation of an exercise program during the treatment period was allowed which potentially could influence outcomes.
Conclusion
This study identified clusters with markedly different multidimensional response after 12 weeks of T/O. Self-organizing maps allowed to simultaneously assess different outcomes and demonstrated that outcomes can behave differentially. This is essential to appreciate the healthcare workers and medicine agencies who review and approve pharmacotherapies. Future COPD research should incorporate these statistical techniques to allow for better and more detailed response analysis as opposed to general means for individual outcomes only.
Acknowledgments
This publication is based on research using data from Boehringer Ingelheim. Boehringer Ingelheim had no role in the design, analysis or interpretation of the results of this research/study. Boehringer Ingelheim was given the opportunity to review the publication for medical and scientific accuracy, supplementary scientific information, as well as intellectual property considerations.
Disclosures
Dr Lowie EGW Vanfleteren reports personal fees from AstraZeneca, GSK, Boehringer, Novartis, Chiesi, Pulmonx, outside the submitted work. Prof. Dr. Frits ME Franssen reports grants, personal fees from AstraZeneca, personal fees, non-financial support from Chiesi, personal fees from GlaxoSmithKline and MSD, outside the submitted work. Prof. Dr. Martijn A Spruit reports grants from Boehringer Ingelheim, during the conduct of the study; grants from Netherlands Lung Foundation, Stichting Astma Bestrijding, Chiesi, AstraZeneca, TEVA and Boehringer Ingelheim, outside the submitted work. The authors report no other conflicts of interest in this study.
Funding
There is no funding to report.
References
1. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for prevention, diagnosis and management of COPD: 2023 report; 2023.
2. Nici L, Mammen MJ, Charbek E, et al. Pharmacologic management of chronic obstructive pulmonary disease. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;201(9):e56–e69. doi:10.1164/rccm.202003-0625ST
3. Kew KM, Mavergames C, Walters JA. Long-acting beta2-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013;10:CD010177.
4. Oba Y, Sarva ST, Dias S. Efficacy and safety of long-acting beta-agonist/long-acting muscarinic antagonist combinations in COPD: a network meta-analysis. Thorax. 2016;71(1):15–25. doi:10.1136/thoraxjnl-2014-206732
5. Oba Y, Keeney E, Ghatehorde N, Dias S. Dual combination therapy versus long-acting bronchodilators alone for chronic obstructive pulmonary disease (COPD): a systematic review and network meta-analysis. Cochrane Database Syst Rev. 2018;12:CD012620. doi:10.1002/14651858.CD012620.pub2
6. Maltais F, O’Donnell D, Galdiz Iturri JB, et al. Effect of 12 weeks of once-daily tiotropium/olodaterol on exercise endurance during constant work-rate cycling and endurance shuttle walking in chronic obstructive pulmonary disease. Ther Adv Respir Dis. 2018;12:1753465818755091. doi:10.1177/1753465818755091
7. Miravitlles M, Garcia-Rivero JL, Ribera X, et al. Exercise capacity and physical activity in COPD patients treated with a LAMA/LABA combination: a systematic review and meta-analysis. Respir Res. 2022;23(1):347. doi:10.1186/s12931-022-02268-3
8. Koopman M, Franssen FME, Gaffron S, et al. Differential outcomes following 4 weeks of aclidinium/formoterol in patients with COPD: a reanalysis of the ACTIVATE Study. Int J Chron Obstruct Pulmon Dis. 2022;17:517–533. doi:10.2147/COPD.S308600
9. Lee JH, Lee YK, Kim EK, et al. Responses to inhaled long-acting beta-agonist and corticosteroid according to COPD subtype. Respir Med. 2010;104(4):542–549. doi:10.1016/j.rmed.2009.10.024
10. Spruit MA, Augustin IM, Vanfleteren LE, et al. Differential response to pulmonary rehabilitation in COPD: multidimensional profiling. Eur Respir J. 2015;46(6):1625–1635. doi:10.1183/13993003.00350-2015
11. Vanfleteren LE, Spruit MA, Groenen M, et al. Clusters of comorbidities based on validated objective measurements and systemic inflammation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187(7):728–735. doi:10.1164/rccm.201209-1665OC
12. O’Donnell DE, Lam M, Webb KA. Measurement of symptoms, lung hyperinflation, and endurance during exercise in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;158(5 Pt 1):1557–1565. doi:10.1164/ajrccm.158.5.9804004
13. Roca J, Whipp BJ, Agusti AG. Clinical exercise testing with reference to lung diseases: indications, standardization and interpretation strategies. ERS task force on standardization of clinical exercise testing. European respiratory society. Eur Respir J. 1997;10(11):2662–2689.
14. Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi:10.1183/09031936.05.00034805
15. O’Donnell DE, Fluge T, Gerken F, et al. Effects of tiotropium on lung hyperinflation, dyspnoea and exercise tolerance in COPD. Eur Respir J. 2004;23(6):832–840. doi:10.1183/09031936.04.00116004
16. Puente-Maestu L, Villar F, de Miguel J, et al. Clinical relevance of constant power exercise duration changes in COPD. Eur Respir J. 2009;34(2):340–345. doi:10.1183/09031936.00078308
17. O’Donnell DE, Lam M, Webb KA. Spirometric correlates of improvement in exercise performance after anticholinergic therapy in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160(2):542–549. doi:10.1164/ajrccm.160.2.9901038
18. Newton MF, O’Donnell DE, Forkert L. Response of lung volumes to inhaled salbutamol in a large population of patients with severe hyperinflation. Chest. 2002;121(4):1042–1050. doi:10.1378/chest.121.4.1042
19. Cazzola M, MacNee W, Martinez FJ, et al. Outcomes for COPD pharmacological trials: from lung function to biomarkers. Eur Respir J. 2008;31(2):416–469. doi:10.1183/09031936.00099306
20. Sidhaye VK, Nishida K, Martinez FJ. Precision medicine in COPD: where are we and where do we need to go? Eur Respir Rev. 2018;27(149):180022. doi:10.1183/16000617.0022-2018
21. Singh D, Donohue JF, Boucot IH, Barnes NC, Compton C, Martinez FJ. Future concepts in bronchodilation for COPD: dual- versus monotherapy. Eur Respir Rev. 2021;30(160):210023. doi:10.1183/16000617.0023-2021
22. Han MK, Criner GJ, Dransfield MT, et al. Prognostic value of clinically important deterioration in COPD: IMPACT trial analysis. ERJ Open Res. 2021;7(1):00663–2020. doi:10.1183/23120541.00663-2020
23. Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 Report. GOLD executive summary. Am J Respir Crit Care Med. 2017;195(5):557–582.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.