Back to Journals » Cancer Management and Research » Volume 11
Differential gene expression identifies KRT7 and MUC1 as potential metastasis-specific targets in sarcoma
Authors Jiang L , Tolani B, Yeh CC, Fan Y , Reza JA , Horvai AE, Xia E, Kratz JR, Jablons DM, Mann MJ
Received 7 June 2019
Accepted for publication 7 August 2019
Published 9 September 2019 Volume 2019:11 Pages 8209—8218
DOI https://doi.org/10.2147/CMAR.S218676
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev K. Srivastava
Long Jiang*,1, Bhairavi Tolani*,1, Che-Chung Yeh1, Yanying Fan1, Joseph A Reza1, Andrew E Horvai2, Endi Xia1, Johannes R Kratz1, David M Jablons1, Michael J Mann1
1Thoracic Oncology Program, Helen Diller Family Comprehensive Cancer Center, University of California, San Francisco, San Francisco, CA 94115, USA; 2Department of Pathology, University of California, San Francisco, San Francisco, CA, USA
*These authors contributed equally to this work
Correspondence: Bhairavi Tolani; Michael J Mann
Thoracic Oncology Program, Helen Diller Family Comprehensive Cancer Center, University of California, San Francisco, San Francisco, CA 94115, USA
Tel +1 415 885 3882
Email [email protected]
[email protected]
Background: Despite numerous discoveries regarding the molecular genesis and progression of primary cancers, the biology of metastasis remains poorly understood. Compared to very large numbers of circulating tumor cells that are now known to accompany nearly all cancers, a relatively limited number of lesions actually develop in most patients with metastases. We hypothesized that phenotypic changes driven by differential gene expression in a finite subpopulation of tumor cells render those cells capable of metastasis and sought to identify key pathways through analysis of gene expression in primary and metastatic lesions from the same patients.
Methods: We compared whole-genome expression in 4 matched samples of primary and metastatic sarcoma, then evaluated candidate genes with differential expression via quantitative PCR in 30 additional matched sets, tumor tissue immunostaining, siRNA loss-of-function in a sarcoma cell migration assay, and clinical correlation with overall and disease-free survival after metastasectomy.
Results: Comparison of microarray signals identified differential expression of cell adhesion genes, including upregulation of KRT7 and MUC1 in metastases; KRT7 and MUC1 upregulation was confirmed in 22 (73%) and 20 (67%) matched sets of metastatic/primary tumors, respectively. Silencing of KRT7 and MUC1 via targeted siRNAs suppressed sarcoma cell migration in vitro, and a significant correlation (two-sided) was observed between both KRT7 and MUC1 expression in metastases and overall patient survival.
Conclusion: KRT7 and MUC1 may play a significant role in enabling sarcoma metastasis, and they may therefore be important prognostic biomarkers as well as potential targets for therapeutic prevention of metastasis.
Keywords: sarcomas, differential expression, metastasis, microarray, MUC1, KRT7
Introduction
Metastasis and invasion of adjacent vital structures are the primary causes of cancer-related deaths. Metastasis is often associated with advanced growth of primary tumors, but in some cancers, such as small cell lung cancer, they can also be seen commonly when primary tumors are still small. Despite the importance of metastasis in defining the virulence of cancer and determining patient prognosis, little is understood about the biological and molecular mechanisms responsible for the emergence of metastasis during the natural history of malignancies, nor has it been established whether metastatic potential is an early or late phenotypic element in most cancers.1–5
Malignant tumors are known to consist of heterogeneous subpopulations of cells harboring a range of genetic, biochemical, and immunological characteristics, and gene expression analysis is a relatively recent addition to the profiling of tumor specimens.6–8 It has also relatively recently been observed that nearly all cancers are associated with the release of thousands of tumor cells into the systemic circulation;9–12 yet the number of macroscopic, detectable metastases in most patients is much smaller. Given the establishment of these two elements in our evolving understanding of tumor biology, we therefore hypothesized that only a small subpopulation of the cells shed from a primary tumor into the systemic circulation bear a specific metastatic phenotype that supports the development of a successful metastasis in distant tissues. We further hypothesized that analysis of differential patterns of gene expression between primary tumors and metastatic lesions from the same patients could begin to yield important indicators of molecular elements that define and characterize that phenotype. Prior studies have begun to examine potentially meaningful patterns of differential gene expression between primary tumors and their associated metastatic lesions.13–21 These studies, however, have remained largely descriptive and few have attempted to relate patterns of expression to clinical correlates or demonstrations of functional significance.
Sarcomas are rare malignant tumors of mesenchymal origin. Localized sarcomas can often be treated effectively with surgery and radiation. However, sarcoma metastasis, which develops most commonly in the lungs and occurs in roughly 35–40% of sarcomas, generally responds poorly to systemic therapies. Metastatic sarcoma is therefore associated with relatively poor prognosis.22,23 As with other malignancies, a better understanding of the underlying molecular mechanisms that contribute to metastases is required in order to develop more effective treatment strategies for sarcoma. The propensity of sarcomas to metastasize chiefly to the lungs has justified a more aggressive surgical approach to pulmonary metastasis. In this study, we analyzed matched pairs of primary and metastatic sarcomas to identify candidate genes that play an important role in this critically important yet poorly understood element of cancer biology.
Materials and methods
Patient samples
Snap-frozen, banked tumor samples and formalin-fixed, paraffin-embedded (FFPE) samples were obtained from patients (age >18 years at the time of operation) who underwent resection of pulmonary metastatic sarcoma at the University of California San Francisco (UCSF) between 1997 and 2012. Demographics and pathologic characteristics were obtained through chart review. Vital status and date of death were determined by chart review, institutional cancer registry, California Death Records, and the Social Security Death Master File. The institutional review board at UCSF approved the study, UCSF IRB approval #10–01111, which was conducted in full accordance with the Declaration of Helsinki. Although the study was exempt from patient consent for de-identified, retrospective analysis of clinical data and FFPE tissue, patients provided written informed consent prior to intra-operative tissue specimen collection.
Microarray analyses
The mRNA expression level of samples was measured by Affymetrix GeneTitan Gene ST 1.1 array. Total RNA of tissues was extracted from tumor samples that had been snap frozen in the operating room in liquid nitrogen using Qiagen RNeasy Mini Kit. A total of 250 ng of total RNA was amplified into cRNA and made into cDNA using the Ambion WT Expression Kit. A total of 5.5 µg of the cDNA was then fragmented using the Affymetrix GeneChip WT Terminal Labeling kit and confirmed by running on the Agilent Bioanalyzer using the RNA 6000 kit. The labeled fragmented cDNA was added into the hybridization cocktail that was prepared according to the protocol included in the Affymetrix GeneTitan Hybridization Wash and Stain kit. The samples were then put onto hybridization trays and loaded into the Affymetrix GeneTitan MC at the UCSF Thoracic Oncology Laboratory for hybridization, washing, and scanning. The Log2-scale expression data were extracted using the built-in Robust Multi-array Average (RMA) algorithm in the Affymetrix software. mRNA expression profiles were compared between matched primary and metastatic sarcoma tissue to obtain gene expression changes.
Cell lines and reagents
Sarcoma cell lines U2OS (osteosarcoma), MES-SA (uterine sarcoma), and SKUT1 (leiomyosarcoma) were obtained from ATCC (Manassas, VA), as was rhabdoid tumor cell line A204 (rhabdoid tumors bear a SMARCB1 mutation and are mostly found in small children). Cells were cultured with 10% fetal bovine serum and 2% antibiotics (Penicillin-Streptomycin) by following the manufacturer’s recommendations for growth media.
Quantitative real-time PCR
Total RNA was extracted from FFPE tissue according to the manufacturer’s protocol by using a kit Ambion® (Life Technologies). Briefly, eight serial 10-μm FFPE sections were de-paraffinized, after which Proteinase K was added. After a few washes, total RNA was eluted and genomic DNA was digested by DNAse I. Subsequent RNA clean-up was performed by using an E.Z.N.A.® MicroElute RNA Clean Up Kit (Omega Bio-tek). RNA extracted from FFPE tissue samples was used only if a 260:280 ratio of 1.6 or greater was observed. The cDNA was transcribed by using an iScript cDNA Synthesis Kit (Bio-Rad) which served as a template for real-time PCR (Applied Biosystems 7000 sequence detection system). Real-time quantitative reverse transcript-polymerase chain reaction (qRT-PCR) for Keratin, type II cytoskeletal 7 (KRT7), and Mucin-1 (MUC1) was performed with SYBR Green, using gene-specific PCR primers (Table 1). GAPDH was used as a housekeeping gene and data were analyzed using Relative Quantification Software (Applied Biosystems). The qRT-PCR data were presented as fold change in target gene expression±standard deviation. Real-time PCR reactions were performed in triplicate and the data were used to calculate fold change in target gene expression (mean±SD) after normalization with GAPDH, followed by presentation of the ratio of metastatic/primary levels.
 |
Table 1 Summary of genes and sequences of primers used for qRT-PCR |
Immunohistochemistry
Immunohistochemistry (IHC) staining was performed using a mouse anti-KRT7/17 monoclonal antibody (Invitrogen) and a mouse anti-MUC-1 monoclonal antibody (R&D Systems) at dilutions of 1:100–200. Five μm tissue sections from primary tumor, metastatic tumor, and adjacent normal lung tissue were de-paraffinized using xylene. Heat-mediated antigen retrieval was performed using sodium citrate buffer (10 mM sodium citrate, 0.05% Tween 20, pH 6.0). Antibody staining was visualized with DAB (Histostain Plus Broad Spectrum, Invitrogen) and hematoxylin counterstain (Fisher Scientific). Representative images at 400× were examined for positive staining in each primary sarcoma and matched metastatic sarcoma.
RNA interference (RNAi)
Cells were grown in 6-well plates and transiently transfected with KRT7 or MUC1-specific small interfering RNA (siRNA). The siRNA oligos were obtained from Sigma (KRT7 siRNA: SASI_Hs02_00339809 & SASI_Hs01_00243071; MUC1 siRNA: TRCN0000122938; siRNA Universal Negative Control #1). Lipofectamine RNAiMAX Transfection Reagent (Invitrogen) was used for siRNA transfection according to the manufacturer’s protocol. Twenty-four hours after siRNA transfection, confluent cells were subjected to wound-healing assay.
Wound-healing assay
Wound healing was assessed as previously described.24 Briefly, cells were seeded into culture dishes to obtain a confluent monolayer. After incubating overnight at 37°C, the cell monolayer was washed with PBS and replaced with reduced serum medium (2%). Cells were allowed to acclimatize to low serum conditions for 24 hrs. The cell monolayer was scratched in lines of equal width with a 200 μL pipette tip and debris was removed by washing with 500 μL of PBS followed by addition of reduced serum medium. This was designated as the 0 hr time-point and images were acquired using a light microscope. Cells were then incubated and images were acquired at 24 and 48 hrs. To quantify the migration, four wounds were made for each condition, and cell migration was calculated by the average of differences in distance between 48 hr and 0 hr time-points. Three measurements were made per scratch. All experiments were conducted more than three times, and representative results are presented.
Western blotting
Total protein from KRT7 or MUC1 siRNA transfected whole-cell lysates was prepared with M-PER buffer (ThermoFisher Scientific) and supplemented with protease and phosphatase inhibitors (Roche). Subsequently, protein concentrations were determined via a Bradford assay. Equal quantities of proteins were combined with 5X protein loading buffer and separated by SDS-PAGE followed by PVDF membrane transfer. Membranes were blocked with 5% milk followed by incubation with appropriate antibodies. Primary antibodies used in these experiments were: KRT7 (Thermo Scientific #MA1-19045), MUC1 (Cell Signaling #14161T), and GAPDH (Ambion). Blots were developed with ECL Reagents (Pierce).
Data analysis
For each gene of interest, expression levels in primary tumor and in metastatic tumor were normalized to mRNA from pooled normal lung tissue (Clontech). Gene expression in primary tumors, metastasis, and the ratio of gene expression in metastasis versus primary tumor were calculated. Gene–gene interactions were evaluated using the product of gene expression of two genes of interest (e.g., KRT7*MUC1 represented gene–gene interactions between KRT7 and MUC1). Overall and recurrence-free survival following surgical resection were assessed by Kaplan–Meier analysis using a right-censored dataset – high gene expression was defined as the upper quartile in each data set. Survival differences were evaluated using a log-rank test. Associations between gene expression and disease recurrence were evaluated using a Wilcoxon rank-sum test after testing for normalcy using a Shapiro–Wilk test.
For all statistical tests, a pre-specified two-sided α of 0.05 was regarded as statistically significant. All statistical analyses were conducted using STATA/MP 11 (StataCorp LP, College Station, TX, USA).
Results
Analysis of differential gene expression in sarcoma patient samples
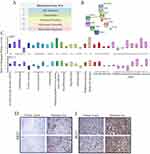
To identify differential gene expression in paired primary and metastatic sarcomas, mRNA samples from 4 sets of patients who underwent surgical resection of both primary and pulmonary metastatic lesions were subjected to genome-wide oligonucleotide microarray analysis using the Affymetrix GeneTitan Gene ST platform (Table S1). For this genome-wide exploration, we chose to limit our microarray analysis to RNA from patients whose primary tumor tissue and pulmonary metastasis samples were snap frozen in liquid nitrogen in the operating room to ensure the best preservation of RNA subjected to this extensive analysis. Although our sample size of four patients did not support a rigorous statistical analysis of the subsequent microarray data, the data derived from this higher quality RNA served as a screening exploration for genes with potentially large discrepancies in their expression between primary tumor and metastasis (Table S2).
Patient characteristics, sarcoma sub-type, and other clinical findings from these four patients with matched primary and metastatic tumor samples are summarized in Table 2. We did observe a pattern of differentially regulated gene sets. From our array of 48,226 genes, there were 593 whose expression differed between primary tumor and metastasis ≥2 fold (444 up- and 149 downregulated in metastatic samples). In particular, we observationally noted several subgroups of related genes falling into various functional groupings, including cell adhesion, chemotaxis, immune function, chromatin assembly, and blood development (Figure 1A). We focused on gene sets known to be regulated in response to adhesion modules specifically because aberration in cell–cell adhesion may be a critical factor contributing to tumor transformation, invasion, and metastasis. Among the top gene signatures identified by this analysis, we found 10 differentially regulated genes (Figure 1B). We decided to focus on KRT7 and MUC1 because they are commonly co-expressed based on functional protein association networks available on the STRING database; this functional co-expression, along with their identification among the differentially expressed genes in our exploratory microarray analysis, might reflect a functional metastatic phenotype that involved expression of both of these genes.25
 |
Table 2 Patient characteristics in included gene expression microarray studies |
Confirmation of KRT7 and MUC1 upregulation in sarcoma metastases compared to primary tumors
To confirm our microarray data, qRT-PCR analyses were performed on FFPE tissue from both metastatic and primary sarcoma tumors from 30 patients across 13 sarcoma sub-types. Twenty-four (80%) were identified histologically as high-grade (grade 3 of 3), and 25 were soft tissue sarcomas (Table 3). KRT7 expression was increased significantly in 22 metastatic sarcomas compared with matched primary tumors (73%), 5 showed no change and 3 had a slight reduction (Figure 1C top panel). The trend was similar for MUC1, with elevated expression in 20 tumors (67%), 3 that showed no change, and 7 that had a slight reduction (Figure 1C bottom panel). Eighteen (60%) of samples demonstrated relatively higher expression of both genes in metastases versus primary tumors. Immunohistochemical analysis of KRT7 and MUC1 protein expression in representative paired samples also gave consistent results indicating that both proteins were also upregulated at metastatic sites relative to the primary tumor (Figure 1D and E).
 |
Table 3 Summary of 30 patients characterized across13 sarcoma sub-types used for quantitative real-time PCR |
Clinical correlations of KRT7 and MUC1 expression
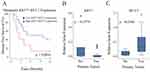
The potential clinical significance of increased metastatic KRT7 and MUC1 expression was supported by Kaplan–Meier analysis of overall survival from the time of metastasectomy in patients whose metastatic lesions were in the highest quartiles of either KRT7 or MUC1 expression (Figure 2A and B). Worse overall survival was associated with this high level of metastatic expression of either KRT7 (P=0.01) or MUC1 (P=0.05) compared to patients whose metastatic tumors had lower expression of these genes. Although there was no statistically significant association between the level of either KRT7 or MUC1 gene expression and recurrence of metastasis, interaction analysis suggested that an overlap of high expression of these 2 genes was associated with significantly more rapid recurrence within 12 months of resection (Figure 3A, P=0.0001). Interestingly, patients who experienced a recurrence of their sarcoma metastases also had higher expression of MUC1 (P=0.02), but not of KRT7 (P=0.28), in their primary tumors compared to patients who did not experience recurrent metastasis (Figure 3B and C).
 |
Figure 2 Overall survival and expression level. Kaplan–Meier curves for overall survival in patients with high versus low levels of expression of KRT7 (A) or MUC1 (B) in their metastatic lesions. |
Suppression of KRT7 and MUC1 reduces sarcoma cell migration
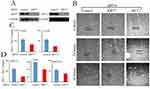
Epithelial–mesenchymal transition (EMT) occurs when cells lose their cell–cell adhesive properties followed by acquisition of migratory potential. EMT is required for wound healing, tumor invasion, metastasis, and cancer progression. Wound-healing assays can inform about EMT status and thus are used as a surrogate to assess metastatic potential in vitro.26 In the event that sarcoma metastases are supported by the contributions of upregulated KRT7 and MUC1 expression, then silencing KRT7 and MUC1 by siRNAs would be expected to result in reduced EMT and therefore reduced wound healing. To test this hypothesis, we transiently transfected sarcoma cell lines with siRNAs targeting KRT7 and MUC1. Administration of KRT7 and MUC1 siRNA in U2OS osteosarcoma cells, but not control siRNA, reduced the expression of these target genes as determined by Western blotting (Figure 4A). Importantly, these knockdowns of either KRT7 or MUC1 in U2OS osteosarcoma cells also resulted in a reduction in wound healing in an in vitro assay (Figure 4B). Furthermore, in addition to this finding which we quantified in U2OS osteosarcoma cells (Figure 4C), we observed the same trend in two soft tissue sarcoma cell lines, SKUT1 (leiomyosarcoma) and MES-SA (uterine sarcoma), and in rhabdoid tumor cell line A204 (Figure 4D). These results suggest that KRT7 and MUC1 may also contribute to cell migration during metastases.
Discussion
The goal of this study was to find potential prognostic biomarkers of tumor metastasis and corresponding targets that might be amenable to therapeutic intervention. We began with sets of matched primary and metastatic tumor from four patients that had all been snap frozen in the operating room, and in which the mRNA was most likely better preserved than RNA that could be extracted from FFPE tissues obtained after standard pathology processing. We subjected the RNA extracted from these higher quality tissue sources to genome-wide array analysis, and we observed relevant gene networks with a subset of gene sets belonging to cell adhesion modules and cytoskeletal components.
It has been suggested that the expression levels of several genes related to cell adhesion can be used to predict clinical outcomes in patients with metastases.27 Furthermore, recent studies revealed that MUC1 and KRT7 have been associated with tumor transformation, invasion, and metastasis in multiple other types of cancer.28–35 We therefore selected KRT7 and MUC1 from among the genes found to be relatively upregulated in metastatic tissues as initial target genes in this study.
Many genes involved in cell adhesion, chemotaxis, and immunity have previously been reported to be overexpressed in cancer cells.27 For example, MUC1 showed enhanced expression in colorectal, renal, squamous cell and gastric, pancreatic carcinomas and lung metastases from melanoma, prostate, breast, and ovarian carcinomas compared with matched normal controls, and was associated with cellular transformation, invasion, and metastatic potential.28–35 MUC1 has both cell adhesive as well as anti-adhesive properties, and influences the RAS/MAPK pathway in immune T-cells.36–41
KRT7 forms part of the cellular cytoskeleton and functions as a membrane-cytoskeletal linker which contributes to regulation of cell adhesion.42 KRT7 shows enhanced expression in two cancer types, colorectal and esophageal squamous cell carcinomas, and is also associated with cellular transformation.42 Cell adhesion molecules are thought to play important roles not only in preserving tissue architecture but also in tumor progression, which includes changes in morphology, invasion, and metastasis. Cytoskeletal component levels, such as the degree of keratinization, have been reported to be valuable in identifying patients at risk for developing regional metastases.27
Quantitative RT-PCR analysis of 30 patients’ matched samples across 13 sarcoma sub-types validated our microarray finding of KRT7 and MUC1 upregulation in metastases versus primary sarcoma. Immunohistochemical staining also confirmed elevated protein expression of KRT7 and MUC1 in samples of metastatic sarcoma compared to matched primary tumor from the same patient. Finally, we found that suppression of KRT7 and MUC1 reduces cell migration in an in vitro wound healing/EMT assay, suggesting that changes in these proteins that are related to cell adhesive properties and cytoskeletal function may contribute to sarcoma cell mobility, migration, and metastases.
Interestingly, high KRT7 and MUC1 expression levels in sarcoma metastases were correlated for the first time with worse overall survival in sarcoma. Our data may also suggest that the interaction of high expression of both of these genes may also be associated with more rapid recurrence of metastatic disease. The very high recurrence rate for metastatic sarcoma suggests that more accurate prognostic analysis based on the expression levels of key metastatic factors might enhance personalization of treatment regimens after metastasectomy, including the balance of aggressive chemotherapeutic versus repeated surgical approaches. Additional studies will focus more attention on expression profiles including KRT7, MUC1, and other metastasis-specific candidate genes.
Although accumulated evidence has previously shown that MUC1 and KRT7 are involved in the progression, invasion, and metastasis of human cancers, our study is one of the first attempts to characterize the difference in transcriptional profiles from metastatic sites in different patients with sarcoma and the first to implicate MUC1 and KRT7 in sarcoma metastasis. Our findings suggest that these differentially expressed genes may be important factors in tumor metastasis. Additional studies, with more tumor samples and additional clinical follow-up, as well as further corroboratory experiments, will help determine specific alternations at the molecular level in patients with metastasis. These studies, in turn, may provide a pathway for better prognostic tools and the identification of actionable biomarkers.
Conclusion
In conclusion, analysis of gene expression profiles by genome-wide microarray can provide useful information for clarifying the mechanism underlying the development and metastasis of sarcomas, not only revealing the differentially expressed genes, but also providing information for identifying novel diagnostic and therapeutic targets. Early evidence suggests that KRT7 and MUC1 maybe two such targets.
Abbreviations
KRT7, keratin, type II cytoskeletal 7; MUC1, Mucin-1; ASPS, alveolar soft part sarcoma; MFH, malignant fibrous histiocytoma; PNET, primitive neuroectodermal tumor; UPS, undifferentiated pleomorphic sarcoma; MPNST, malignant peripheral nerve sheath tumor; cDNA, complementary deoxyribonucleic acid; RNA, ribonucleic acid; mRNA, messenger ribonucleic acid; FFPE, formalin-fixed, paraffin-embedded; qRT-PCR, quantitative real-time PCR; siRNA, small interfering ribonucleic acid; IHC, immunohistochemistry; SD, standard deviation.
Acknowledgment
We thank the Department of Pathology at the University of California, San Francisco for assistance with this study. This work was supported by Cardiothoracic Translational Research Laboratory funds at UCSF (MJM) and Kazan McClain Partners’ Foundation (DMJ).
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Koh KH, Rhee H, Kang HJ, et al. Differential gene expression profiles of metastases in paired primary and metastatic colorectal carcinomas. Oncology. 2008;75(1–2):92–101. doi:10.1159/000155211
2. Ramaswamy S, Ross KN, Lander ES, Golub TR. A molecular signature of metastasis in primary solid tumors. Nat Genet. 2003;33:49–54. doi:10.1038/ng1060
3. Inamura K, Shimoji T, Ninomiya H, et al. A metastatic signature in entire lung adenocarcinomas irrespective of morphological heterogeneity. Hum Pathol. 2007;38(5):702–709. doi:10.1016/j.humpath.2006.11.019
4. Fidler IJ, Kripke ML. Metastasis results from preexisting variant cells within a malignant tumor. Science. 1977;197(4306):893–5.
5. van ‘t Veer LJ, Dai H, van de Vijver MJ, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–536. doi:10.1038/415530a
6. Park KJ, Zivanovic O, Konner J, et al. Heterogeneous tumor-immune microenvironments among differentially growing metastases in an ovarian cancer patient. Cell. 2017;170(5):927–938. doi:10.1016/j.cell.2017.07.025
7. Andor N, Harness JV, Müller S, Mewes HW, Petritsch C. EXPANDS: expanding ploidy and allele frequency on nested subpopulations. Bioinformatics. 2014;30(1):50–60. doi:10.1093/bioinformatics/btt622
8. Tse E, Kwong YL. T-cell lymphoma: microenvironment-related biomarkers. Semin Cancer Biol. 2015;34:46–51. doi:10.1016/j.semcancer.2015.06.001
9. Pantel K, Speicher MR. The biology of circulating tumor cells. Oncogene. 2016;35(10):1216–1224. doi:10.1038/onc.2015.192
10. Thierry AR, El Messaoudi S, Gahan PB, Anker P, Stroun M. Origins, structures, and functions of circulating DNA in oncology. Cancer Metastasis Rev. 2016;35(3):347–376. doi:10.1007/s10555-016-9629-x
11. Chang Y, Tolani B, Nie X, Zhi X, Hu M, He B. Review of the clinical applications and technological advances of circulating tumor DNA in cancer monitoring. Ther Clin Risk Manag. 2017;13:1363–1374. doi:10.2147/TCRM.S141991
12. Conti A, Fredolini C, Tamburro D, et al. Identification of novel candidate circulating biomarkers for malignant soft tissue sarcomas: correlation with metastatic progression. Proteomics. 2016;16(4):689–697. doi:10.1002/pmic.201500164
13. Wang N, Zhou F, Xiong H, et al. Screening and identification of distant metastasis-related differentially expressed genes in human squamous cell lung carcinoma. Anat Rec (Hoboken). 2012;295(5):748–757. doi:10.1002/ar.22441
14. Xie HL, Li ZY, Gan RL, et al. Differential gene and protein expression in primary gastric carcinomas and their lymph node metastases as revealed by combined cDNA microarray and tissue microarray analysis. J Dig Dis. 2010;11(3):167–175. doi:10.1111/j.1751-2980.2010.00432.x
15. Ohno Y, Izumi M, Tachibana M, et al. Characterization and gene expression analysis of novel matched primary and metastatic renal cell carcinoma cell lines. Oncol Rep. 2008;20(3):501–509.
16. Wang L, Zhu JS, Song MQ, Chen GQ, Chen JL. Comparison of gene expression profiles between primary tumor and metastatic lesions in gastric cancer patients using laser microdissection and cDNA microarray. World J Gastroenterol. 2006;12(43):6949–6954. doi:10.3748/wjg.v12.i43.6949-Bellvis
17. Unique gMuñoz L, Fontanillo C, González-González M, et al. Unique genetic profile of sporadic colorectal cancer liver metastasis versus primary tumors as defined by high-density single-nucleotide polymorphism arrays. Mod Pathol. 2012;25(4):590–601. doi:10.1038/modpathol.2011.195
18. Belbin TJ, Singh B, Smith RV, et al. Molecular profiling of tumor progression in head and neck cancer. Arch Otolaryngol Head Neck Surg. 2005;131(1):10–18. doi:10.1001/archotol.131.1.10
19. Ohali A, Avigad S, Zaizov R, et al. Prediction of high risk ewing’s sarcoma by gene expression profiling. Oncogene. 2004;23(55):8997–9006. doi:10.1038/sj.onc.1208060
20. Naruke A, Azuma M, Takeuchi A, et al. Comparison of site-specific gene expression levels in primary tumors and synchronous lymph node metastases in advanced gastric cancer. Gastric Cancer. 2015;18(2):262–270. doi:10.1007/s10120-014-0357-z
21. Del Valle PR, Milani C, Brentani MM, et al. Transcriptional profile of fibroblasts obtained from the primary site, lymph node and bone marrow of breast cancer patients. Genet Mol Biol. 2014;37(3):480–489.
22. Siegel R, Naishadham D, Jemal A. Cancer statistics. CA Cancer J Clin. 2012;62(1):10–29. doi:10.3322/caac.20138
23. Hoang NT, Acevedo LA, Mann MJ, Tolani B. A review of soft-tissue sarcomas: translation of biological advances into treatment measures. Cancer Manag Res. 2018;10:1089–1114. doi:10.2147/CMAR.S159641
24. Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2(2):329–333. doi:10.1038/nprot.2007.30
25. Szklarczyk D, Franceschini A, Wyder S, et al. STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015;43(Database issue):D447–52. Available from: https://string-db.org
26. Jolly MK, Ware KE, Gilja S, Somarelli JA, Levine H. EMT and MET: necessary or permissive for metastasis? Mol Oncol. 2017;11(7):755–769. doi:10.1002/1878-0261.12083
27. Martin TA, Ye L, Sanders AJ, et al. Madame curie bioscience database cancer. invasion and metastasis: molecular and cellular perspective. Landes Biosci. 2000-2013. Available from: https://www.ncbi.nlm.nih.gov/books/NBK164700. Accesses Jan 30, 2019
28. Nakamori S, Ota DM, Cleary KR, Shirotani K, Irimura T. MUC1 mucin expression as a marker of progression and metastasis of human colorectal carcinoma. Gastroenterology. 1994;106(2):353–361. doi:10.1016/0016-5085(94)90592-4
29. Roy LD, Sahraei M, Subramani DB, et al. MUC1 enhances invasiveness of pancreatic cancer cells by inducing epithelial to mesenchymal transition. Oncogene. 2011;30(12):1449–1459. doi:10.1038/onc.2010.526
30. Wang X, Lan H, Li J, Su Y, Xu L. Muc1 promotes migration and lung metastasis of melanoma cells. Am J Cancer Res. 2015;5(9):2590–2604.
31. Shi M, Chen D, Yang D, Liu XY. CCL21-CCR7 promotes the lymph node metastasis of esophageal squamous cell carcinoma by up-regulating MUC1. J Exp Clin Cancer Res. 2015;34:149. doi:10.1186/s13046-015-0268-9
32. Deng J, Wang L, Chen H, et al. The role of tumour-associated MUC1 in epithelial ovarian cancer metastasis and progression. Cancer Metastasis Rev. 2013;32(3–4):535–551. doi:10.1007/s10555-013-9423-y
33. Lee SH, Park HK, Kim JH, Han HS. Significance of MUC1 expression in biopsy specimens of submucosal invasive gastric carcinoma: the association with lymph node metastasis. Oncol Lett. 2015;10(3):1437–1443. doi:10.3892/ol.2015.3483
34. Rausch S, Beermann J, Scharpf M, et al. Differential expression and clinical relevance of MUC1 in renal cell carcinoma metastasis. World J Urol. 2016;34(12):1635–1641. doi:10.1007/s00345-016-1804-8
35. Ciborowski P, Finn OJ. Non-glycosylated tandem repeats of MUC1 facilitate attachment of breast tumor cells to normal human lung tissue and immobilized extracellular matrix proteins (ECM) in vitro: potential role in metastasis. Clin Exp Metastasis. 2002;19(4):339–345.
36. Kumar P, Ji J, Thirkill TL, Douglas GC. MUC1 is expressed by human skin fibroblasts and plays a role in cell adhesion and migration. Biores Open Access. 2014;3(2):45–52. doi:10.1089/biores.2013.0045
37. Makiguchi Y, Hinoda Y, Imai K. Effect of MUC1 mucin, an anti-adhesion molecule, on tumor cell growth. Jpn J Cancer Res. 1996;87(5):505–511. doi:10.1111/j.1349-7006.1996.tb00252.x
38. Singh PK, Hollingsworth MA. Cell surface-associated mucins in signal transduction. Trends Cell Biol. 2006;16(9):467–476. doi:10.1016/j.tcb.2006.07.006
39. Aaboe M, Marcussen N, Jensen KM, Thykjaer T, Dyrskjøt L, Orntoft TF. Gene expression profiling of noninvasive primary urothelial tumours using microarrays. Br J Cancer. 2005;93(10):1182–1190. doi:10.1038/sj.bjc.6602813
40. Lim S, Nam H, Jeon JS. Chemotaxis model for breast cancer cells based on signal/noise ratio. Biophys J. 2018;S0006-3495(18):31110–X.
41. Verdú M, Román R, Calvo M, et al. Clinicopathological and molecular characterization of colorectal micropapillary carcinoma. Mod Pathol. 2011;24(5):729–738. doi:10.1038/modpathol.2011.1
42. Sano M, Aoyagi K, Takahashi H, et al. Forkhead box A1 transcriptional pathway in KRT7-expressing esophageal squamous cell carcinomas with extensive lymph node metastasis. Int J Oncol. 2010;36(2):321–330.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.