Back to Journals » Drug Design, Development and Therapy » Volume 17
Different Sedation Strategies in Older Patients Receiving Spinal Anesthesia for Hip Surgery on Postoperative Delirium: A Randomized Clinical Trial
Authors Zhu S , Liu Y, Wang X, Wang L, Li J, Xue X, Li Z, Liu J, Liu X , Zhao S
Received 15 September 2023
Accepted for publication 15 December 2023
Published 29 December 2023 Volume 2023:17 Pages 3845—3854
DOI https://doi.org/10.2147/DDDT.S439543
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Tin Wui Wong
Shuxing Zhu, Yaqing Liu, Xiuli Wang, Liang Wang, Jinru Li, Xiaoming Xue, Zhao Li, Jiaxin Liu, Xin Liu, Shuang Zhao
Department of Anesthesiology, The Third Hospital of Hebei Medical University, Shijiazhuang, Hebei, 050051, People’s Republic of China
Correspondence: Shuang Zhao, Department of Anesthesiology, The Third Hospital of Hebei Medical University, Shijiazhuang, Hebei, 050051, People’s Republic of China, Tel +86 13613210924, Email [email protected]
Background: Postoperative delirium (POD) is of great concern as a complication of surgery in older adult patients. Sedation strategies influence the development of POD. This study compared how sedation strategies administered during spinal anesthesia influenced POD in patients aged ≥ 65 years undergoing elective surgery for hip fracture repair.
Patients and Methods: A randomized clinical trial was conducted from 1 August 2021 to 30 June 2022 at a single academic medical center. Two hundred and twenty-six patients were randomly divided into four groups: lighter sedation with propofol (LP), heavier sedation with propofol (HP), lighter sedation with dexmedetomidine (LD), and heavier sedation with dexmedetomidine (HD). The incidence of delirium was the primary outcome and was assessed daily by the blinded Confusion Assessment Method.
Results: There was a significant association between dexmedetomidine (LD+HD group) and a lower incidence of delirium (11.9% [13/109] vs the propofol group (23.6% [26/110]; Risk ratio, 0.51; 95% CI, 0.274 to 0.929; p=0.024). In the propofol group, heavier sedation had a higher rate of POD (32.7% [18/55] vs the lighter sedation group (14.5% [8/55]; Risk ratio, 2.25; 95% CI, 1.069 to 4.736; p=0.025).
Conclusion: Dexmedetomidine was associated with a lower incidence of delirium than that with propofol among older patients with hip fractures. In patients that received propofol, heavier sedation was associated with high incidence of POD.
Keywords: aged, anesthetics, hip surgery, propofol, spinal anesthesia
Introduction
Delirium is a sophisticated neuropsychiatric syndrome marked by acute episodes of impairment in attention and other dimensions of cognition, and is linked with a multitude of independent and unfavorable consequences.1 Delirium incidence may be variable in terms of population and environment characteristics,1 and can involve up to 65% of older adults.2 With declining function of organs and concomitant diseases, the brain of older patients is more vulnerable. This vulnerability has been implicated in the greater incidence of perioperative cognitive complications, affecting survival outcomes in older patients. Strategies for effective detection, treatment, and prevention of delirium need to be explored.1 Currently, there is no specific drug for treating delirium; thus, prevention is better than a cure.
Sedation allows patients receiving spinal anesthesia to be more comfortable, but it could adversely affect postoperative cognitive function.3 The STRIDE trial showed a benefit of lighter sedation (higher bispectral index and lower dose of propofol) on postoperative delirium (POD) only in patients with a Charlson Comorbidity Index (CCI) score of 0, possibly because the benefits of lighter sedation were overshadowed by the patient’s own risk factors.4 However, in the ENGAGES trail, compared with usual care, electroencephalogram (EEG)-guided anesthesia did not lower the incidence of POD among older people undergoing major surgery.5 Therefore, we speculated that the depth of sedation may influence delirium.
Dexmedetomidine is considered beneficial for postoperative cognition, and effects may be dose-related.6–9 Compared with the control group (saline or propofol), a lower incidence of POD was observed when intraoperative dexmedetomidine was continuously infused intravenously at a rate of 0.2–0.7 μg/kg/h, regardless of whether or not a loading dose was administered.6,8,10–13 However, for adults with sepsis who were being treated with recommended light-sedation approaches, there are no differences between effects of dexmedetomidine and propofol on cognition.3 Propofol, a classic sedative drug that has been widely used in operating rooms and intensive care units. The effects of propofol on cognition are currently inconclusive. Many of the current clinical trials have focused on the effects on cognition of propofol-based anesthesia rather than on propofol alone.14,15
A recent study has shown that propofol may lead to neurocognitive impairment.16 Liu et al found that propofol induced neuroinflammation and cognitive deficits in aged rats via the NF-κB pathway.17 Few studies have evaluated on the effects of treatment with propofol sedation alone on postoperative cognition in elderly patients. This study was performed to determine the effectiveness of propofol or dexmedetomidine on POD under different depths of sedation.
Materials and Methods
Study Design
This single-center parallel prospective randomized controlled trial was approved from Third Hospital of Hebei Medical University (W2021-021-1) and registered on ClinicalTrials.gov (NCT04891458). Written informed consent was provided from all participants.
Participants
The patients were approached prior to scheduled surgery in the morning by a research coordinator to assess eligibility and obtain informed consent. Patients (aged ≥ 65 years) scheduled for hip fracture surgery (total hip arthroplasty with/without cement, hemiarthroplasty with/without cement and Intramedullary nail) under spinal anesthesia, grades I to III according to the American Society of Anesthesiologists (ASA) classification were included. Exclusion criteria were new-onset cardiovascular and cerebrovascular adverse events within 6 months; severe organ failure; contraindications to spinal anesthesia; strong desire for general anesthesia; contraindications for dexmedetomidine or propofol; severe dementia or communication barriers; and other concomitant traumas requiring simultaneous surgery. Patients with failed spinal anesthesia or who developed intraoperative delirium were also excluded from the study.
Randomization and Masking
A researcher created a computer-generated simple randomization list with a 1:1:1:1 patient allocation prior to the study initiation. To identify patients in the target sedation group, experienced anesthetists were unblinded to the study allocation. Patients and research staff responsible for perioperative patient evaluations were unaware of the assignments for each group. Thus, this trial was single-blind in the sedation strategy administration phase and double-blinded in the outcome assessment phase.
Intervention and Control
No premedication was administrated. Venous access was established after the patient entered the operating room and hemodynamic monitoring was established. Using a face mask, oxygen was inhaled at 3 L/min. After placing the patient in the lateral position, a 25G Quincke needle with the bevel facing laterally was inserted into the L3-L4 space using either the median or paramedian approach. After confirming that the cerebrospinal fluid could flow freely, 2.0 mL of 0.25% bupivacaine was injected intrathecally. A cold swab was used for a sensory examination every 1–2 minutes. The plane was adjusted above T10.
After stabile anesthesia had been achieved, propofol or dexmedetomidine was administered intravenously for intraoperative sedation until the beginning of skin suture. Propofol was continuously infused at a rate of 0.5–3.0 mg/kg per hour and 0.3 μg/kg dexmedetomidine was administered for more than 10 minutes as the loading dose, followed by continuous administration of 0.2–0.7 μg/kg per hour.
Patients were assigned to four groups based on sedatives and the degree of intraoperative sedation according to the Modified Observer’s Assessment of Alertness and Sedation (MOAA/S) score. The target sedation level was <2 points (lighter sedation group) or >3 points (heavier sedation group), these targets were chosen based on previous published studies.5 The two MOAA/S scale ranges for sedation levels reflected the clinical situation more accurately, as accurate MOAA/S targets are not always achieved. An experienced anesthesiologist evaluated the depth of sedation every 5 minutes. If the depth of sedation was not in the target, the anesthesiologist would adjust the rate of drug infusion for target sedation level and conduct assessments at intervals of 2 minutes until the expected goal was reached, then resume the 5-minute assessment interval of sedation depth.
The attending anesthesiologist selected the target range of mean arterial pressure (MAP) appropriate for each patient. Intraoperative fluid was administered with the 4-2-1 rule and condition of the patients. If hemodynamic instability continued after volume factors were removed, vasoactive drugs were used: a single intravenous dose of 0.3–0.5 mg atropine was administered for bradycardia, while 0.2 mg/kg esmolol hydrochloride was administered intravenously for tachycardia. A dose of 10–50 mg of urapidil hydrochloride was used for hypertension. If hypotension occurred, norepinephrine was administered intravenously at 8–12 mg per minute. If hemodynamics could not be stabilized within 10 minutes after treatment, patients were withdrawn from the trial. The type of sedative administered, hemodynamic variables, and adverse events were recorded during the perioperative period. The hemodynamic parameters were observed at three time points: (1) at the start of sedation (T1); (2) at the end of sedation (T2); and (3) in the ward (T3).
Outcomes
Daily assessment of delirium in the hospital was performed at 5 pm by the same trained researcher using the validated Confusion Assessment Method (sensitivity, 94–100%; specificity, 90–95%)18 on the day of surgery (PD0), and on the first postoperative day (PD1), on PD2, and on PD3. The primary outcome was the incidence of delirium 3 days postoperatively. Secondary outcomes included severity based on the Confusion Assessment Method Severity category and the time to onset after surgery of delirium.19 Information on potential risk variables associated with the POD was collected from baseline assessments, patient reports, and medical records. The adverse events, such as nausea, vomiting, pneumonia, hypotension, hypertension, hypoxemia, hypercarbia and myocardial ischemia were recorded.
Sample Size
Based on previous studies,4,6,14 we assumed a delirium incidence of 28% in the propofol group and 12% in the dexmedetomidine group. The number of patients estimated to provide a difference at a significance level of 0.05 with a power of 0.8 in the primary outcome was 219. Thus, with the expectation that 20% would drop out, 248 patients needed to be enrolled.
Statistical Analysis
Continuous variables were reported as mean (±SD) or median (25th, 75th percentile) and categorical data as frequency (percentage). Normally distributed continuous parameters such as BMI, hemoglobin and albumin were examined by independent sample t-test and other non-normally distributed variables like age, the years of educational level, homocysteine, the scores of MMSE and the Barthel index of ADL were examined using the Mann–Whitney U-test. Pearson’s chi-squared test, continuity correction, or Fisher’s exact test were used for categorical variables as appropriate. Risk ratio (RR) were reported with 95% confidence intervals (CIs). SPSS v26.0 for Windows software (SPSS Inc, USA) was chosen for all statistical analyses.
Results
Patient Characteristics
The study flow is displayed in Figure 1. A total of 248 patients were identified from 1 August 2021 to 30 August 2022, and 22 patients were excluded. Patient exclusion and random allocation are shown in Figure 1. When intraoperative delirium or persistent hypotension occurred, the trail was stopped. Overall, 219 patients received the scheduled intervention, and 7 patients were excluded from the analysis for the following reasons: surgery change (n=1), anaphylactic shock (n=1), contraindication to spinal anesthesia (n=1), intraoperative delirium (n=2), and persistent hypotension during surgery (n=2).
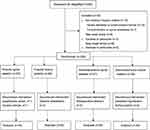 |
Figure 1 CONSORT 2010 Flow Diagram. Notes: Schulz KF, Altman DG, Moher D.CONSORT 2010 Statement: Updated Guidelines for Reporting Parallel Group Randomised Trials. PLoS Med. 2010;7(3): e1000251.20 Copyright: © 2010 Schulz et al. Creative Commons Attribution License. |
The mean age of the patients was 77 years (± SD 7), 45.2% were men; the median Mini-Mental State Examination (MMSE) score was 6 (± SD 3). No significant difference in baseline characteristics was observed between the propofol group and dexmedetomidine group (Table 1).
 |
Table 1 Baseline Characteristics of Included Patients |
Intraoperative characteristics are summarized in Table 2. In general, the median duration of surgery was 84 min (IQR: 65 to 105) and the median estimated blood loss was 200 mL (IQR: 150 to 300). There were no differences in MAP, heart rate (HR), intraoperative fluid volume, blood loss, or duration of operation between the propofol sedation group and dexmedetomidine sedation group. Adverse events were not significantly different between the two groups (Table 3).
 |
Table 2 Intraoperative Characteristics by Randomization Group |
 |
Table 3 Adverse Events According to Sedation Group |
Efficacy of Propofol or Dexmedetomidine on Outcomes
In total, 39 patients developed delirium, corresponding to about 18% of the total study sample. A significantly lower incidence of delirium was observed in patients receiving dexmedetomidine than in those receiving propofol sedation (11.9% vs 23.6%; RR, 0.51; 95% CI, 0.274 to 0.929; p=0.024) (Table 4). Compared to the dexmedetomidine group, the incidence of hyperactive delirium was higher (11.8% vs 1.8%; RR, 0.16;95% CI, 0.036 to 0.672; p=0.006) (Table 4) in the propofol group. The difference between the two groups was not significant in terms of the maximum delirium severity score or the proportion of patients with POD on daily observation (Table 4).
 |
Table 4 Effect of Sedatives on Postoperative Delirium |
Considering that the depth of sedation may have influenced the results, we compared the effects of different depths of the same sedative on delirium. A higher incidence of delirium was observed in the propofol heavier sedation group (14.5% vs 32.7%; RR, 2.25; 95% CI, 1.069 to 4.736; p=0.025; Table 5). Patients with propofol heavier sedation had a higher rate of hypoactive delirium (1.8% vs 20.0%; RR, 11.0;95% CI, 1.470 to 82.319; p=0.002; Table 5), and a higher percentage of severe delirium (5.5% vs 23.6%; RR, 4.33;95% CI, 1.307 to 14.365; p=0.013; Table 5). However, in patients who received dexmedetomidine, there were no significant differences in the incidence, severity, or clinical phenotype of delirium between the lighter and heavier sedation groups (Table 6).
 |
Table 5 Effect of Sedation Strategies with Propofol on Postoperative Delirium |
 |
Table 6 Effect of Sedation Strategies with Dexmedetomidine on Postoperative Delirium |
Discussion
In this single-center clinical randomized trial, dexmedetomidine was associated with a lower incidence of delirium than that observed with propofol among older patients with hip fractures. Compared to the propofol lighter sedation group, more patients experiencing delirium in the propofol heavier sedation (MOAA/S ≤2), while there were no significant differences for patients between the dexmedetomidine lighter and heavier group.
The loading dose of the drug shortens onset time and extends the duration of action. Considering that the unilateral hip surgery time was short, we chose a loading dose of dexmedetomidine that would allow the patient to meet the anticipated depth of sedation more rapidly. Hypotension and bradycardia are common adverse effects of sedatives. To ensure the safety of the patients and taking into account the results of the pre-test, we administered a lower dose of dexmedetomidine to ensure stable hemodynamics while maintaining the depth of sedation. We found that the incidence of POD was lower in the dexmedetomidine group than that in the propofol group, and there were no significant differences between the subgroups of dexmedetomidine in terms of sedation depth. For the effect on POD, the administration of dexmedetomidine for intraoperative sedation is superior to propofol within a certain dose ranges.
Delirium is brain damage caused by multiple factors, the pathogenesis of which is still unclear. It is recognized that dexmedetomidine may prevent patients from experiencing POD more effectively than propofol.11,12,21 A total of 219 patients were included in our analysis, and the overall incidence of POD was 17.8%. Our results showed that the number of POD patients receiving dexmedetomidine was less than those receiving propofol. Both propofol and dexmedetomidine are sedative drugs, but their mechanisms of action are different. Propofol activates γ-aminobutyric acid (GABA-A) receptors directly and inhibits N-methyl-D-aspartate (NMDA) receptors,22 while dexmedetomidine produces hypnotic-sedative effects by activating α2-adrenergic receptors located in the coeruleus region of the brainstem.23 There are numerous mechanisms of delirium, some of which can be affected by dexmedetomidine.24–27 Wang et al found that dexmedetomidine stimulated astrocytes to release brain-derived neurotrophic factor, which acted as a paracrine factor to reduce excessive α5 subunit GABA-A receptor activation in neurons, and attenuated memory and problem-solving deficits following anesthesia.28 Furthermore, the neurophysiological mechanisms of dexmedetomidine, which replicate “restorative sleep” via activation of the brain stem and normal sleep pathways, instead of the cortical suppression achieved with GABAergic sedatives, make it clinically advantageous, especially in vulnerable patients. However, similar to the findings of the STRIDE trial, in our patients receiving propofol, heavier sedation achieved greater delirium, perhaps because the risks of propofol outweigh the benefits. Instead, in the dexmedetomidine group, there was no significant difference between the two levels of sedation. This could be due to the lower blood concentration of dexmedetomidine used in this study, which was not sufficient to achieve the full therapeutic efficacy of the drug.
Some studies have shown that dexmedetomidine is beneficial for the recovery of postoperative cognition,6,29,30 while other studies have not.31,32 This may be because the benefits of sedation are overshadowed by other risk factors. The MOAA/S score is a commonly used score of clinical sedation, which mainly evaluates the level of sedation through the patient’s responses to sound and touch. Compared to EEG monitoring, it is more cost-effective and feasible to use the MOAA/S score to assess the depth of sedation in economically underdeveloped countries and regions. Our findings may provide some reference for the optimization of sedation strategies in elderly patients with fragile brain functions.
There were several limitations in this study. First, at the beginning of the trial, we found that heavier sedation with dexmedetomidine was prone to hemodynamic instability in older patients. Therefore, considering its safety profile, the dose of dexmedetomidine we used was lower. The question of how to strike a balance between the dose of dexmedetomidine and the optimal sedative effect warrants further study. Second, this study did not conduct EEG monitoring, and thus, did not provide an objective indicator of sedation. Intraoperative anesthesia management was single-blind and outcome assessment phase was double-blinded, which is also the limitation of the study. Besides, delirium was not assessed after the fourth postoperative day, which might have missed some positive patients. In addition, this study was a single-center study with a small sample size, and its findings should be verified in multicenter, large-sample randomized trials.
Conclusions
Dexmedetomidine was associated with a lower incidence of delirium than that with propofol among older patients with hip fractures. In patients that received propofol, heavier sedation was associated with high incidence of POD.
Human and Animal Rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Data Sharing Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethical Approval
Approval for the research project was obtained from the Third Hospital of Hebei Medical University (W2021-021-1).
Informed Consent
Written informed consent was provided from all participants before randomization.
Funding
The work was supported by the Natural Science Foundation of Hebei Province (H2021206149) and by Health Commission of Hebei Province (20210025).
Disclosure
The authors have no relevant financial or non-financial interests to disclose.
References
1. Wilson JE, Mart MF, Cunningham C., et al. Delirium. Nat Rev Dis Primers. 2020;6(1):90. doi:10.1038/s41572-020-00223-4
2. Evered LA, Chan MTV, Han R, et al. Anaesthetic depth and delirium after major surgery: a randomised clinical trial. Br J Anaesth. 2021;127(5):704–712. doi:10.1016/j.bja.2021.07.021
3. Hughes CG, Mailloux PT, Devlin JW, et al. Dexmedetomidine or propofol for sedation in mechanically ventilated adults with sepsis. N Engl J Med. 2021;384(15):1424–1436. doi:10.1056/NEJMoa2024922
4. Sieber FE, Neufeld KJ, Gottschalk A, et al. Effect of depth of sedation in older patients undergoing hip fracture repair on postoperative delirium: the STRIDE randomized clinical trial. JAMA Surg. 2018;153(11):987–995. doi:10.1001/jamasurg.2018.2602
5. Wildes TS, Mickle AM, Ben Abdallah A, et al. Effect of electroencephalography-guided anesthetic administration on postoperative delirium among older adults undergoing major surgery: the ENGAGES randomized clinical trial. JAMA. 2019;321(5):473–483. doi:10.1001/jama.2018.22005
6. Li CJ, Wang BJ, Mu DL, et al. Randomized clinical trial of intraoperative dexmedetomidine to prevent delirium in the elderly undergoing major non-cardiac surgery. Br J Surg. 2020;107(2):e123–e132. doi:10.1002/bjs.11354
7. Cheng XQ, Mei B, Zuo YM, et al. A multicentre randomised controlled trial of the effect of intra-operative dexmedetomidine on cognitive decline after surgery. Anaesthesia. 2019;74(6):741–750. doi:10.1111/anae.14606
8. van Norden J, Spies CD, Borchers F, et al. The effect of peri-operative dexmedetomidine on the incidence of postoperative delirium in cardiac and non-cardiac surgical patients: a randomised, double-blind placebo-controlled trial. Anaesthesia. 2021;76(10):1342–1351. doi:10.1111/anae.15469
9. Duan X, Coburn M, Rossaint R, Sanders RD, Waesberghe JV, Kowark A. Efficacy of perioperative dexmedetomidine on postoperative delirium: systematic review and meta-analysis with trial sequential analysis of randomised controlled trials. Br J Anaesth. 2018;121(2):384–397. doi:10.1016/j.bja.2018.04.046
10. Xin X, Chen J, Hua W, Wang H. Intraoperative dexmedetomidine for prevention of postoperative delirium in elderly patients with mild cognitive impairment. Int J Geriatr Psychiatry. 2021;36(1):143–151. doi:10.1002/gps.5406
11. Park JW, Kim EK, Lee HT, Park S, Do SH. The effects of propofol or dexmedetomidine sedation on postoperative recovery in elderly patients receiving lower limb surgery under spinal anesthesia: a retrospective propensity score-matched analysis. J Clin Med. 2021;10:1.
12. Shin HJ, Woo Nam S, Kim H, et al. Postoperative delirium after dexmedetomidine versus propofol sedation in healthy older adults undergoing orthopedic lower limb surgery with spinal anesthesia: a randomized controlled trial. Anesthesiology. 2023;138(4):456.
13. Zhang W, Wang T, Wang G, Yang M, Zhou Y, Yuan Y. Effects of dexmedetomidine on postoperative delirium and expression of IL-1beta, IL-6, and TNF-alpha in elderly patients after hip fracture operation. Front Pharmacol. 2020;11:678. doi:10.3389/fphar.2020.00678
14. Zhang Y, Shan GJ, Zhang YX, et al. Propofol compared with sevoflurane general anaesthesia is associated with decreased delayed neurocognitive recovery in older adults. Br J Anaesth. 2018;121(3):595–604. doi:10.1016/j.bja.2018.05.059
15. Miller D, Lewis SR, Pritchard MW, et al. Intravenous versus inhalational maintenance of anaesthesia for postoperative cognitive outcomes in elderly people undergoing non-cardiac surgery. Cochrane Database Syst Rev. 2018;8(8):CD012317. doi:10.1002/14651858.CD012317.pub2
16. Zhang T, Ji D, Sun J, Song J, Nie L, Sun N. NPAS4 suppresses propofol-induced neurotoxicity by inhibiting autophagy in hippocampal neuronal cells. Arch Biochem Biophys. 2021;711:109018. doi:10.1016/j.abb.2021.109018
17. Liu PF, Gao T, Li TZ, et al. Repeated propofol exposure-induced neuronal damage and cognitive impairment in aged rats by activation of NF-kappaB pathway and NLRP3 inflammasome. Neurosci Lett. 2021;740:135461. doi:10.1016/j.neulet.2020.135461
18. Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941–948. doi:10.7326/0003-4819-113-12-941
19. Breitbart W, Rosenfeld B, Roth A, Smith MJ, Cohen K, Passik S. The memorial delirium assessment scale. J Pain Symptom Manage. 1997;13(3):128–137. doi:10.1016/S0885-3924(96)00316-8
20. Schulz KF, Altman DG, Moher D CONSORT statement: updated guidelines for reporting parallel group randomised trials. PLoS Med. 2010;7(3):e1000251.
21. Djaiani G, Silverton N, Fedorko L, et al. Dexmedetomidine versus propofol sedation reduces delirium after cardiac surgery: a randomized controlled trial. Anesthesiology. 2016;124(2):362–368. doi:10.1097/ALN.0000000000000951
22. Kotani Y, Shimazawa M, Yoshimura S, Iwama T, Hara H. The experimental and clinical pharmacology of propofol, an anesthetic agent with neuroprotective properties. CNS Neurosci Ther. 2008;14(2):95–106. doi:10.1111/j.1527-3458.2008.00043.x
23. Nelson LE, Lu J, Guo T, Saper CB, Franks NP, Maze M. The alpha2-adrenoceptor agonist dexmedetomidine converges on an endogenous sleep-promoting pathway to exert its sedative effects. Anesthesiology. 2003;98(2):428–436. doi:10.1097/00000542-200302000-00024
24. He H, Zhou Y, Zhou Y, et al. Dexmedetomidine mitigates microglia-mediated neuroinflammation through upregulation of programmed cell death protein 1 in a rat spinal cord injury model. J Neurotrauma. 2018;35(21):2591–2603. doi:10.1089/neu.2017.5625
25. Nelson S, Muzyk AJ, Bucklin MH, Brudney S, Gagliardi JP. Defining the role of dexmedetomidine in the prevention of delirium in the intensive care unit. Biomed Res Int. 2015;2015:635737. doi:10.1155/2015/635737
26. Jin Z, Hu J, Ma D. Postoperative delirium: perioperative assessment, risk reduction, and management. Br J Anaesth. 2020;125(4):492–504. doi:10.1016/j.bja.2020.06.063
27. Yamazaki S, Yamaguchi K, Someya A, Nagaoka I, Hayashida M. Anti-inflammatory action of dexmedetomidine on human microglial cells. Int J Mol Sci. 2022;23(17):10096. doi:10.3390/ijms231710096
28. Wang DS, Kaneshwaran K, Lei G, et al. Dexmedetomidine prevents excessive gamma-aminobutyric acid type a receptor function after anesthesia. Anesthesiology. 2018;129(3):477–489. doi:10.1097/ALN.0000000000002311
29. Shin HJ, Woo Nam S, Kim H, et al. Postoperative delirium after dexmedetomidine versus propofol sedation in healthy older adults undergoing orthopedic lower limb surgery with spinal anesthesia: a randomized controlled trial. Anesthesiology. 2023;138(2):164–171. doi:10.1097/ALN.0000000000004438
30. Pereira JV, Sanjanwala RM, Mohammed MK, Le ML, Arora RC. Dexmedetomidine versus propofol sedation in reducing delirium among older adults in the ICU: a systematic review and meta-analysis. Eur J Anaesthesiol. 2020;37(2):121–131. doi:10.1097/EJA.0000000000001131
31. Turan A, Duncan A, Leung S, et al. Dexmedetomidine for reduction of atrial fibrillation and delirium after cardiac surgery (DECADE): a randomised placebo-controlled trial. Lancet. 2020;396(10245):177–185. doi:10.1016/S0140-6736(20)30631-0
32. Deiner S, Luo X, Lin HM, et al. Intraoperative infusion of dexmedetomidine for prevention of postoperative delirium and cognitive dysfunction in elderly patients undergoing major elective noncardiac surgery: a randomized clinical trial. JAMA Surg. 2017;152(8):e171505. doi:10.1001/jamasurg.2017.1505
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
