Back to Journals » Neuropsychiatric Disease and Treatment » Volume 11
Different presence of Chlamydia pneumoniae, herpes simplex virus type 1, human herpes virus 6, and Toxoplasma gondii in schizophrenia: meta-analysis and analytical study
Authors Gutiérrez-Fernández J , Luna del Castillo JDD, Mañanes-González S, Carrillo-Ávila JA, Gutiérrez B, Cervilla J, Sorlózano-Puerto A
Received 15 December 2014
Accepted for publication 27 January 2015
Published 27 March 2015 Volume 2015:11 Pages 843—852
DOI https://doi.org/10.2147/NDT.S79285
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Roger Pinder
José Gutiérrez-Fernández,1 Juan de Dios Luna del Castillo,2 Sara Mañanes-González,1 José Antonio Carrillo-Ávila,1 Blanca Gutiérrez,3 Jorge A Cervilla,3 Antonio Sorlózano-Puerto1
1Department of Microbiology, 2Department of Statistics and Operation Research, 3Department of Psychiatry, Institute of Neurosciences and CIBERSAM, School of Medicine and Biohealth Research Institute (Instituto de Investigación Biosanitaria) IBS-Granada, University of Granada, Granada, Spain
Abstract: In the present study we have performed both a meta-analysis and an analytical study exploring the presence of Chlamydia pneumoniae, herpes simplex virus type 1, human herpes virus 6, and Toxoplasma gondii antibodies in a sample of 143 schizophrenic patients and 143 control subjects. The meta-analysis was performed on papers published up to April 2014. The presence of serum immunoglobulin G and immunoglobulin A was performed by enzyme-linked immunosorbent assay test. The detection of microbial DNA in total peripheral blood was performed by nested polymerase chain reaction. The meta-analysis showed that: 1) C. pneumoniae DNA in blood and brain are more common in schizophrenic patients; 2) there is association with parasitism by T. gondii, despite the existence of publication bias; and 3) herpes viruses were not more common in schizophrenic patients. In our sample only anti-Toxoplasma immunoglobulin G was more prevalent and may be a risk factor related to schizophrenia, with potential value for prevention.
Keywords: meta-analysis, analytical study, Chlamydia pneumoniae, herpes simplex virus type 1,
human herpes virus 6, Toxoplasma gondii, schizophrenia
Introduction
The word “schizophrenia” refers to a group of disabling mental disorders characterized by alterations in perception, thought, affectivity, and behavior. Its etiology is unknown and there are no curative treatments.1 Although present worldwide, schizophrenia is more prevalent in developed countries, where the accumulated lifetime prevalence can reach more than 1% and an annual incidence of five cases per 10,000 people.2 A possible genetic vulnerability exists for schizophrenia, as supported by the increased familial morbid risk for schizophrenia found in first-degree relatives of affected subjects, possibly in conjunction with the influence of environmental factors. Prenatal and perinatal obstetric complications have also been related to schizophrenia, and some authors postulate that environmental factors (such as trauma, cannabis use, or neurotropic infections) might modulate the final impact of such complications on the early genetic alterations described for this illness.3
Among other environmental risk factors for schizophrenia, infectious factors have been posed, a hypothesis that would be supported by some studies describing an increased prevalence for the illness among those subjects born during winter and spring months in the northern hemisphere.4 Indeed, prevalence has been reported as being much higher following flu epidemics. Several infectious agents could harm a fetus. Being primarily neurotropic agents or not, acting directly or in an immune-mediated way, or perpetuating themselves or not, these agents could facilitate the occurrence of irreversible neurologic injuries associated with alterations in both neurotransmission expression and sensorial information processing,5 which may have a role in the development of schizophrenia later in life. These subjects may potentially benefit from antimicrobial treatment, which may improve their outcome.
Among all the infectious agents that have been studied in schizophrenia, Chlamydia pneumoniae, herpes simplex virus type 1 (HSV-1), human herpes virus 6 (HHV-6) and Toxoplasma gondii are especially interesting because they are neurotropic, they cause illnesses that could share symptoms with schizophrenia, and they can block neural function in a recurrent manner as they produce latent infections with potential reactivations. Although such possibilities are biologically plausible, the association between microbial agents and schizophrenia is not conclusive.6 The present study aimed to explore such associations via both a meta-analysis and an analytical study exploring the presence of the abovementioned infectious agents in a sample of patients with schizophrenia and controls.
Materials and methods
Meta-analysis
The meta-analysis was performed according to the same procedure used in a paper published by our group 3 years ago.6 Thus, in brief, we performed a systematic search of all articles published in English or Spanish in journals indexed on MEDLINE, psycINFO, ISI Web of Knowledge, and the Cochrane Library up to April 2014. The search terms used were “schizophrenia” and “herpes” or “Chlamydia pneumoniae” or “Chlamydophila pneumoniae” or “Toxoplasma gondii.” After that search, we excluded a group of papers including noncontrolled cohort studies, reviews, studies that did not present their results in a correct and/or explicit manner, animal studies, studies where the control group comprised patients with other psychiatric or neurological disorders, and those that did not assess the infectious processes. On the other hand, included studies were those that examined patients and healthy controls (characterized by absence of psychiatric disorders or any neurological disease), cohort studies of patients and controls, and case series.It was required that schizophrenic patients were included in the studies, and that the aim of the study was the direct or indirect exploration of the possible association between infection and schizophrenia.
The following data were obtained for each serologic or molecular determination in each publication: odds ratios (OR) and their 95% confidence intervals (CI), population weights, and statistical significance of the analyses. The DerSimonian and Laird method was used as it produces overall estimates that are less affected by heterogeneity among studies.7 Heterogeneity among studies was determined using Cochran’s Q statistic method when the number of papers included was five or more. Additionally, Higgins I2 was also used as a measure of total OR variability given heterogeneity among studies. Hence, very high values of the latter measure, above 75%, would indicate a strong heterogeneity, suggesting the need to carry out an additional meta-regression using the restricted maximum likelihood method. In such cases, we also performed a more detailed subanalysis of the different subgroups, when the number of studies to be included in each subgroup was big enough (three or more). As a whole, we considered that no relationship existed between exposure to microorganisms and the presence of schizophrenia when the 95% CI included the one unit value.8
Begg’s and Egger’s tests were used when the number of studies included was five or more, in order to check for any particular publication biases and their magnitude.9,10 Study quality was assessed using the Newcastle-Ottawa Quality Assessment Scale (Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm; updated 15 June 2014). Data obtained from the various studies were analysed using the STATA Release 10.1 statistical package (StataCorp LP, College Station, TX, USA).
Analytical study of infectious agents
Sample characteristics: patients with schizophrenia
Patients with a diagnosis of schizophrenia and less than 5 years since their first episode (n=143) were invited to participate in the study. Patients were recruited from the Mental Health Services of Jerez de la Frontera and Jaen (south Spain). All patients fulfilled ICD10 (International Statistical Classification of Diseases and Related Health Problems, 10th Revision) criteria for schizophrenia. They were assessed using a Spanish validated version of the Schedules for Clinical Assessments in Neuropsychiatry. Stability in diagnosis during the last year and no changes in treatment for the last 2 months were also taken into account as inclusion criteria. All patients signed an informed consent prior to being included in the study.
Sample characteristics: control subjects
The control group comprised 143 unrelated individuals with no personal or family history of mental disorders or suicide and with no pharmacological or psychological treatment that could interfere with the neuropsychological assessment. A specifically trained psychologist interviewed each participant and used the Schedules for Clinical Assessments in Neuropsychiatry to check the absence of these conditions. They all came from different primary care centers belonging to the Primary Care catchment area of south Granada (Spain). All participants were nonimmunosuppressed and signed an informed consent prior to being included in the study.
Controls were stratified by age and sex with cases. In both groups, sex was equally distributed (62.24% of cases and 62.94% of controls were male subjects; chi-square =0.001, P=0.973) and mean age was also comparable between cases and controls (28.69 [standard deviation =5.41] versus 30.42 [standard deviation =5.62] respectively; t=1.771, P=0.079).
After losing some laboratory samples for different reasons, our final count was 271 blood samples (128 cases and 143 controls) and 284 plasma samples (142 cases and 142 controls). A microbiological study with these samples was made using the following procedures.
Specific serum immunoglobulins
Different commercial kits were used to detect different microbial immunoglobulin Gs (IgGs): the HERPES SIMPLEX 1 ELISA (enzyme-linked immunosorbent assay) IgG kit system developed by Vircell (Granada, Spain); the TOXOPLASMA ELISA IgG kit, also distributed by Vircell; the human HERPESVIRUS 6 ELISA IgG kit designed by Alere Healthcare (Barcelona, Spain); and the CHLAMYDOPHILA PNEUMONIAE ELISA IgG kit, developed by Savyon Diagnostics (Madrid, Spain). C. pneumoniae immunoglobulin A (IgA) was analyzed using a specific kit designed by Vircell (Granada, Spain). As for the rest of the IgA measurements, the assay was previously standardized by using the same solid phase previously used for IgG, a human anti-IgA conjugate (Siemens Lab, Barcelona, Spain), and a commercialized, specific IgA positive control with known concentration (Lab. Aviva Systems Biology, San Diego, CA, USA). Serum was diluted using anti-IgG. To determine the working dilution for both the conjugate and the positive control, dilutions of both were prepared. These were then doubly processed, comparing the average absorption result against the negative control. The sample was considered positive in detecting IgA when well absorbance was equal to or greater than double the threshold positive value. Samples were processed in duplicate according to our routine laboratory method. The mean absorbance value was used in the evaluation. The coefficients of variation of the ELISA were less than 10%.
Detection of peripheral-blood microbial DNA
DNA was extracted using ReliaPrep™ Blood gDNA Miniprep System (Promega Biotech Iberica, Madrid, Spain). Specific DNA was detected with nested polymerase chain reaction (PCR) of the C. pneumoniae pst1 region,11 HSV-1 gpD-1 region,12 VHH-6 U67 region,13 and T. gondii B1 region.14 We reached sensitivities up to 9 copies/μL (for American Type Culture Collection [ATCC] VR-1356 C. pneumoniae), 16 copies/μL (for ATCC VR-735 VHS-1), 6 copies/μL (for ATCC VR-1480 VHH-6), and 10 copies/μL (for ATCC 50174 T. gondii). We standardized PCR conditions to improve sensitivity (Table 1). We sequenced the amplified fragment in positive cases to demonstrate specificity. ACTB gene DNA was used as a control procedure (Gene-Link, Hawthorne, NY, USA).
Statistical analyses
Pearson’s chi-squared test was used to compare qualitative variables (serum IgG and IgA and blood DNA). A P-value of 0.05 or less was considered statistically significant.
Results
Chlamydia pneumoniae
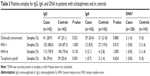
Finally, three studies were included in the meta-analysis15–17 (see Table 2). All of these studies showed similar quality and used blood and brain samples to detect bacterial DNA through PCR or nested-PCR. The meta-analysis revealed an association between schizophrenia and C. pneumoniae (OR =5.96; 95% CI =3.42–10.39; P<0.001). However, our own results found no significant differences between cases and controls for IgG (P=0.521), IgA (P=0.885), and specific DNA measures because DNA was scarcely represented (see Table 3).
Herpes simplex virus type 1 and human herpes virus 6
Fourteen studies were finally included in the meta-analysis for these two viruses (Table 2).18–31 The combined OR estimation involving the 14 studies comparing detection of different markers for infection by HSV-1 in schizophrenic patients and healthy controls was 1.17 (95% CI =0.88–1.54; P=0.273). When heterogeneity tests were done, we obtained a value of χ2exp=15.4 (df=13; P=0.312), after which we could state that the differences found between the studies were due to randomness. This fact was corroborated by an I2 coefficient of 15.4%, indicating that only 15.4% of the variability of the ORs was due to the heterogeneity among studies.
As shown, in spite of the absence of important and, if any, nonsignificant heterogeneity (<75%), a detailed study was carried out to consider the potential effect of some factors on the value of the overall OR through meta-regression, and it was concluded that the technique used to detect HSV-1 infection did not seem to affect OR value (P=0.274). Thus, we found no difference between risks arising from studies that detected DNA in brain tissue (OR =1.15; 95% CI =0.23–5.80) and that from studies in which serum antibodies were detected (OR =1.19; 95% CI =0.81–1.74).
Begg’s test was not significant (P=0.743), suggesting that there was a potential publishing bias. A similar result was obtained after Egger’s test (P=0.817). The quality of the studies examined was medium-high, and it was higher in the more-recent studies due to the introduction of quality validation scales, a fact that favored the control of the data included in the articles.
Finally, only three studies comparing infection by HHV-6 were included, all of them of acceptable quality (see Table 2).22,24,28 The statistical analysis could not confirm the existence of a significant association between infection by this virus and schizophrenia (OR =0.34; 95% CI =0.49–2.42; P=0.283). Our results about IgG, IgA, and specific DNA measures are detailed in Table 3. DNA was scarcely present. No significant differences were found between cases and controls for these markers (P=1.000 for HSV-1 IgG; P=0.133 for HHV-6 IgG; P=1.000 for HHV-6 IgA), with the only exception being HSV-1 IgA, which was more frequently detected in controls than in cases (P=0.015).
Toxoplasma gondii
We included eight studies centered on the potential relationship between T. gondii infection markers and schizophrenia as compared with healthy controls (Table 2).19,22,32–37 All these studies had similar weights, except for the one by Conejero-Goldberg et al22 that used postmortem brain tissue samples, and thus included a smaller number of cases and controls; this study was also of lower quality according to the Newcastle-Ottawa Scale. Due to their large sample size, the studies by Niebuhr et al35 and Mortensen et al34 stood out, the former being of the highest quality. The latter obtained the narrowest 95% CI range due to the large amount of participants included. After combining the different studies, we found a significant association between parasitation by T. gondii and schizophrenia (P=0.002); the combined OR was 2.50 (95% CI =1.40–4.47). We can therefore state that schizophrenia was 2.5 times more frequent in people in whom the marker for T. gondii was found when compared to people in whom it was not detected. The study by Conejero-Goldberg et al22 was the least-precise study and the one with the widest confidence interval. When heterogeneity tests were performed between the studies, a significant value was obtained: χ2exp=35.26 with df=8, P<0.0001. This result parallels an I2 coefficient equal to 77.3%, indicating that 77.3% of the variability of the ORs is due to the heterogeneity of the studies analyzed. Such heterogeneity is explained by significant differences between reported risk at the only publication that detected T. gondii DNA in brain biopsies (OR =1.83; 95% CI =0.03–97.01; P=0.001) and risk reported by studies using detected serum antibodies (OR =2.74; 95% CI =1.33–5.62). Finally, even though Begg’s test was not significant (P=0.711), Egger’s test was (P=0.03), indicating a tendency to publish studies where results were significant, according to this last test.
Our own sample results on IgG, IgA, and specific DNA measures are detailed in Table 3. DNA was scarcely present. No significant differences were found between cases and controls for IgA measures (P=0.624). However, IgG was significantly more frequently detected in cases than in controls (P=0.001).
Discussion
Chlamydia pneumoniae
Previous studies describing a significant association between C. pneumoniae infection and the origin of schizophrenia have been published15–17 (Table 2). Thus, Fellerhoff et al16,17 found DNA of C. pneumoniae in 15.3% of mononuclear blood cells from patients with schizophrenia and 11.8% in brain biopsies of these subjects. Finally, these authors described a significant improvement in psychotic symptoms in schizophrenic patients treated with azitromicine. Moreover, a study showed an improvement in cognitive functioning among schizophrenic patients treated with clozapine plus minocycline.38 The meta-analysis carried out in the present study reveals that C. pneumoniae is present in some patients and may be a potential etiological agent in schizophrenia.
This bacteria enters the organism via the respiratory system and it spreads using monocytes and lymphocytes reaching the central nervous system.39 Th1 lymphocytes are activated by exposure to bacterial antigens (in glial cells, for example), causing secretion of protoinflammatory cytokines (eg, interferon-γ), which, in turn, activate macrophages. Such macrophages increase indoleamine 2,3-dioxygenase (IDO) production, which converts tryptophan into kynurenic acid, hence diminishing tryptophan availability. In such a context, the pathogen cannot replicate itself. Intracellular kynurenic acid inhibits glutamine and nicotinic receptors, which are responsible for cognitive impairment. An increased activity of IDO, which may be caused by the infection, has been described in genetically vulnerable patients with schizophrenia.40 The failure of this defensive mechanism produces persistent and chronic infections with intermittent replication,41 along with both maintained and insufficient levels of kynurenic acid. Prenatal administration of lipopolysaccharide seemed to decrease dopamine concentration in rats’ frontal cortex whilst enhancing dopaminergic system activity in the striatum.42
Our own sample results do not show an association between C. pneumoniae infection and schizophrenia. The increased prevalence of C. pneumoniae infection in subjects without a respiratory illness may explain the lack of differences in antibody levels when comparing cases and controls.43 Moreover, because the infection is focused on the central nervous system, it seems reasonable to detect microbial DNA in brain (that could be responsible for the illness) but not in peripheral blood.16 Nonetheless, limited sample power cannot be ruled out for this negative finding.
Herpes simplex virus type 1 and human herpes virus 6
Different studies have explored the association between HSV-1/HHV-6 infections and schizophrenia, showing inconclusive results.6,18–29,34,35,44–52 Some of these studies have been included in our meta-analysis (Table 2). The lack of association found in the meta-analysis may reflect a real absence of association or a type 2 error due to the use of indirect measurements on brain samples. Only two previous studies have been able to find a significant association between HSV-1 and schizophrenia.26,31 In such studies, authors postulate that maternal HSV-1 antibodies may cause fetal brain damage even in the absence of a fetal infection.
However, HSV-1 is neurotropic and mainly spreads in frontal and temporal brain regions in which certain impairments could produce cognitive and memory alterations similar to those found in patients with schizophrenia.26,53 Moreover, a controlled trial showed an improvement in cognitive functioning among seropositive schizophrenic patients treated with one antipsychotic plus valacyclovir when compared to those treated with the antipsychotic agent only.54 Nevertheless, these results may be due to a simple clinical coincidence30 due to the high prevalence of this infection, as shown in other studies (Table 2). Also, regarding the possible role of the glutamate system, the association between mutations in the specific GluN2B N-methyl-D-aspartate (NMDA) receptor subunit and maternal herpes simplex virus infection and schizophrenia,55 a disorder where brain activation and cortical neurotoxicity of NMDA receptor antagonists, has been extensively reported.56,57 In addition, schizophrenia has also been related to an interaction of prenatal immune activation and subsequent peripubertal stress.58,59 Furthermore, schizophrenia has also been linked with the effect of stress on hippocampal neurogenesis,60 apoptosis, and parvalbumin expression.61,62
Our own sample results also failed to find any association, and the clinical meaning of the increased frequency of IgA anti-HSV-1 among controls is not easily explainable. However, the cause of medical consultation may be associated with a herpetic reactivation. This fact is difficult to demonstrate in a retrospective design and it is something that we did not take into account during our control recruitment. Because such herpes virus associations do not have to involve a primary effect, and they cannot be detected from cross-sectional studies63 due to the high prevalence of the infection, future longitudinal studies are needed to conclude if there are causal associations between virus infections and psychiatric illness.
Toxoplasma gondii
In the last years, several kinds of studies (serological, pharmacological, epidemiological, and behavioral studies) have been published on the association between T. gondii infection and changes in human behavior and neuropsychiatric disorders, including schizophrenia.19,22,32–35,37,44,46,51,64–74 There is clinical evidence supporting the interest of studying the role of T. gondii on such phenotypes: 1) higher titers of IgG anti-toxoplasma have been found in the serum and cerebrospinal fluid of patients with early-onset and late-onset diagnostics; 2) some antipsychotics (haloperidol, risperidone, fluphenazine) show anti-T. gondii activity; and 3) seropositive patients showed a predominance of positive symptoms.
Different meta-analyses on experimental studies focused on infection for T. gondii have been published. Results are congruent enough with the existence of an association between infection for T. gondii and schizophrenia. Our current meta-analysis OR values are very similar to those reported earlier, namely: OR =2.73 (95% CI =2.10–3.60; P<0.001) in the study by Torrey et al75 and OR =2.70 (95% CI =1.34–4.42; P=0.005) in our group’s previous meta-analysis.6 According to these results, we could say that schizophrenia was 2.7 times more frequent in people infected by T. gondii than in people with no signals of this infection. Thus, T. gondii may indeed play a key role in the etiology of schizophrenia.
In our present meta-analysis, which includes the most-recent publications on the field, schizophrenia was found to be 2.5 times more frequent in people with a previous T. gondii infection than in subjects with no signals of having been under the effects of a T. gondii infection. However, we have to point out that heterogeneity tests and publication bias were also significant. Studies supporting this positive finding were: 1) larger studies, where it is more difficult to detect differences, and 2) studies involving soldiers who showed an increased seroprevalence, probably due to their lifestyles. But the significant OR may also be due to a higher prevalence of antibodies acquired after the onset of the disease, due to, for instance, bad hygienic habits.69
In hypothesis, a previous neurological injury caused by the parasite, and the consequent inflammatory response,76 may activate astrocyte function, increasing levels of intracerebral kynurenic acid the toxicity of which acts to inhibit glutamine and nicotinic receptors. These changes are believed to be related to serotonin and melatonin deficits, which would contribute to increased cognitive impairment.67,77 Such mechanism has also been suggested for C. pneumoniae. Increased levels of dopamine in the central nervous system is another consequence of T. gondii infection.78 This effect would be modulated through genes involved in tyrosin–hydroxilase synthesis, which metabolizes L-tyrosine into dihydroxyphenylalanine (a dopamine precursor) and could explain some behavioral and motor symptoms.79 Also, T. gondii has been shown to induce elevated levels of central nervous system dopamine in experimentally infected animals.80
Since most of the previous studies have not detected an increase of anti-toxoplasma immunoglobulin M, it is thought that manifestations are a consequence of a parasite reactivation. However, the study of parasitism is especially important because the first infection occurs in the fetus and produces a congenital toxoplasmosis with neurodevelopmental consequences.81 These alterations might be evident only later in life, maybe coinciding with the start of gray matter degeneration after puberty.82 Some disorders, such as chorioretinitis, also appear in subjects around 20–30 years old and could have a similar pathogenesis.77 This fact is supported by serological studies34,65 showing increased levels of IgG in mothers and newborns who are at higher risk for schizophrenia in adult life. Finally, Xiao et al74 described an association between T. gondii genotype I fetal infection and schizophrenia. This genotype is responsible for up to 75% of congenital toxoplasmosis in Spain.83 Thus, there seems to be a plausible connection between primary maternal parasitism and increased risk for schizophrenia in the offspring.69
We also found that anti-T. gondii IgG was more frequent in patients with schizophrenia than in controls, supporting the possibility of a previous infection with a possible intracerebral inflammatory response. DNA studies using blood samples were not significant. This would be in agreement with poor systemic consequences in the process.
Conclusion
The present meta-analysis shows that C. pneumoniae DNA (measured both in blood and brain) is significantly more frequent in patients with schizophrenia than in controls. A significant association was also detected between T. gondii parasitism and schizophrenia, even after controlling for publication bias. In addition, in the analysis performed in our own sample, we found an important relationship between the illness and the presence of IgG anti-T. gondii, which may suggest the potential interest of detecting such parasitism to develop potential prevention strategies. Finally, we think that new studies are needed to have robust, and more conclusive results. We need prospective and comparative studies, with larger patient and control samples and using combined microbiological techniques for the analysis of the same subject and sample. Future studies should also take into account the illness phase and analyze blood, cerebrospinal fluid, and brain tissues simultaneously using standardized, sensitive, and appropriate techniques, including, if possible, pregnant women and their offspring as subjects under analysis.
Acknowledgment
Part of this work was presented at the Royal Academy of Medicine of Spain.
Disclosure
The authors report no conflicts of interest in this work.
References
Tardy M, Huhn M, Engel RR, Leucht S. Fluphenazine versus low-potency first-generation antipsychotic drugs for schizophrenia. Cochrane Database Syst Rev. 2014;8:CD009230. | ||
American Psychiatric Association. DSM-5 development [homepage on the Internet]. Arlington, VA: American Psychiatric Association; 2014. Available from: http://www.dsm5.org/Pages/Default.aspx. Accessed September 4, 2014. | ||
Jones PB, Rantakallio P, Hartikainen AL, Isohanni M, Sipila P. Schizophrenia as a long-term outcome of pregnancy, delivery, and perinatal complications: a 28-year follow-up of the 1966 north Finland general population birth cohort. Am J Psychiatry. 1998;155(3):355–364. | ||
Boksa P. Maternal infection during pregnancy and schizophrenia. J Psychiatry Neurosci. 2008;33(3):183–185. | ||
Gomez-Sintes R, Bortolozzi A, Artigas F, Lucas JJ. Reduced striatal dopamine DA D2 receptor function in dominant-negative GSK-3 transgenic mice. Eur Neuropsychopharmacol. 2014;24(9):1524–1533. | ||
Arias I, Sorlozano A, Villegas E, et al. Infectious agents associated with schizophrenia: a meta-analysis. Schizophr Res. 2012;136(1–3):128–136. | ||
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. | ||
Egger M, Smith GD, Altman DG. Systematic reviews in health care: Metaanalysis in context. 2nd ed. London, UK: BMJ Books; 1995. | ||
Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. | ||
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. | ||
Campbell LA, Perez Melgosa M, Hamilton DJ, Kuo CC, Grayston JT. Detection of Chlamydia pneumoniae by polymerase chain reaction. J Clin Microbiol. 1992;30(2):434–439. | ||
Coyle PV, Jain S, Wyatt D, McCaughey C, O’Neill HJ. Description of a nonlethal herpes simplex virus type 1 glycoprotein D deletion mutant affecting a site frequently used for PCR. Clin Diagn Lab Immunol. 2000;7(2):322–324. | ||
Bandobashi K, Daibata M, Kamioka M, et al. Human herpesvirus 6 (HHV-6)-positive Burkitt’s lymphoma: establishment of a novel cell line infected with HHV-6. Blood. 1997;90(3):1200–1207. | ||
Ponce Zapata N, Gómez Marín J. Estandarización y validación clínica de la prueba de reacción en cadena de la polimerasa (PCR) para diagnóstico de toxoplasmosis cerebral en pacientes infectados por el VIH. [Standardization and clinical validation of the polymerase chain reaction (PCR) for diagnosis of cerebral toxoplasmosis in HIV-infected patients]. Infectio. 2003;7(1):8–14. Spanish. | ||
Fellerhoff B, Laumbacher B, Wank R. High risk of schizophrenia and other mental disorders associated with chlamydial infections: hypothesis to combine drug treatment and adoptive immunotherapy. Med Hypotheses. 2005;65(2):243–252. | ||
Fellerhoff B, Wank R. Increased prevalence of Chlamydophila DNA in post-mortem brain frontal cortex from patients with schizophrenia. Schizophr Res. 2011;129(2–3):191–195. | ||
Fellerhoff B, Laumbacher B, Mueller N, Gu S, Wank R. Associations between Chlamydophila infections, schizophrenia and risk of HLA-A10. Mol Psychiatry. 2007;12(3):264–272. | ||
Alexander RC, Cabirac G, Lowenkopf T, et al. Search for evidence of herpes simplex virus, type 1, or varicella-zoster virus infection in postmortem brain tissue from schizophrenic patients. Acta Psychiatr Scand. 1992;86(5):418–420. | ||
Blomström A, Karlsson H, Wicks S, Yang S, Yolken RH, Dalman C. Maternal antibodies to infectious agents and risk for non-affective psychoses in the offspring – a matched case-control study. Schizophr Res. 2012;140(1–3):25–30. | ||
Brown AS. Prenatal infection as a risk factor for schizophrenia. Schizophr Bull. 2006;32(2):200–202. | ||
Carter GI, Taylor GR, Crow TJ. Search for viral nucleic acid sequences in the post mortem brains of patients with schizophrenia and individuals who have committed suicide. J Neurol Neurosurg Psychiatry. 1987;50(3):247–251. | ||
Conejero-Goldberg C, Torrey EF, Yolken RH. Herpesviruses and Toxoplasma gondii in orbital frontal cortex of psychiatric patients. Schizophr Res. 2003;60(1):65–69. | ||
Delisi LE, Smith SB, Hamovit JR, et al. Herpes simplex virus, cytomegalovirus and Epstein-Barr virus antibody titres in sera from schizophrenic patients. Psychol Med. 1986;16(4):757–763. | ||
Fukuda R, Sasaki T, Kunugi H, Nanko S. No changes in paired viral antibody titers during the course of acute schizophrenia. Neuropsychobiology. 1999;40(2):57–62. | ||
Gotlieb-Stematsky T, Zonis J, Arlazoroff A, Mozes T, Sigal M, Szekely AG. Antibodies to Epstein-Barr virus, herpes simplex type 1, cytomegalovirus and measles virus in psychiatric patients. Arch Virol. 1981;67(4):333–339. | ||
Prasad KM, Shirts BH, Yolken RH, Keshavan MS, Nimgaonkar VL. Brain morphological changes associated with exposure to HSV1 in first-episode schizophrenia. Mol Psychiatry. 2007;12(1):105–113,1. | ||
Stevens JR, Langloss JM, Albrecht P, Yolken R, Wang YN. A search for cytomegalovirus and herpes viral antigen in brains of schizophrenic patients. Arch Gen Psychiatry. 1984;41(8):795–801. | ||
Taller AM, Asher DM, Pomeroy KL, et al. Search for viral nucleic acid sequences in brain tissues of patients with schizophrenia using nested polymerase chain reaction. Arch Gen Psychiatry. 1996;53(1):32–40. | ||
Taylor GR, Crow TJ. Viruses in human brains: a search for cytomegalovirus and herpes virus 1 DNA in necropsy tissue from normal and neuropsychiatric cases. Psychol Med. 1986;16(2):289–295. | ||
Thomas P, Bhatia T, Gauba D, et al. Exposure to herpes simplex virus, type 1 and reduced cognitive function. J Psychiatr Res. 2013;47(11):1680–1685. | ||
Watson AM, Prasad KM, Klei L, et al. Persistent infection with neurotropic herpes viruses and cognitive impairment. Psychol Med. 2013;43(5):1023–1031. | ||
Alvarado-Esquivel C, Alanis-Quiñones OP, Arreola-Valenzuela MA, et al. Seroepidemiology of Toxoplasma gondii infection in psychiatric inpatients in a northern Mexican city. BMC Infect Dis. 2006;6:178. | ||
Cetinkaya Z, Yazar S, Gecici O, Namli MN. Anti-Toxoplasma gondii antibodies in patients with schizophrenia – preliminary findings in a Turkish sample. Schizophr Bull. 2007;33(3):789–791. | ||
Mortensen PB, Nørgaard-Pedersen B, Waltoft BL, et al. Toxoplasma gondii as a risk factor for early-onset schizophrenia: analysis of filter paper blood samples obtained at birth. Biol Psychiatry. 2007;61(5):688–693. | ||
Niebuhr DW, Millikan AM, Cowan DN, Yolken R, Li Y, Weber NS. Selected infectious agents and risk of schizophrenia among U.S. military personnel. Am J Psychiatry. 2008;165(1):99–106. | ||
Tamer GS, Dundar D, Yalug I, Caliskan S, Yazar S, Aker A. The schizophrenia and Toxoplasma gondii connection: infectious, immune or both? Adv Ther. 2008;25(7):703–709. | ||
Yolken RH, Bachmann S, Ruslanova I, et al. Antibodies to Toxoplasma gondii in individuals with first-episode schizophrenia. Clin Infect Dis. 2001;32(5):842–844. | ||
Kelly DL, Vyas G, Richardson CM, et al. Adjunct minocycline to clozapine treated patients with persistent schizophrenia symptoms. Schizophr Res. 2011;133(1–3):257–258. | ||
Contini C, Seraceni S, Cultrera R, Castellazzi M, Granieri E, Fainardi E. Chlamydophila pneumoniae Infection and Its Role in Neurological Disorders. Interdiscip Perspect Infect Dis. 2010;2010:273573. | ||
Na KS, Jung HY, Kim YK. The role of pro-inflammatory cytokines in the neuroinflammation and neurogenesis of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:277–286. | ||
Villegas E, Sorlózano A, Camacho A, Gutiérrez J. [Chlamydophila pneumoniae: from its proteomics to arteriosclerosis]. Enferm Infecc Microbiol Clin. 2008;26(10):629–627. Spanish. | ||
Basta-Kaim A, Fijał K, Budziszewska B, et al. Prenatal lipopolysaccharide treatment enhances MK-801-induced psychotomimetic effects in rats. Pharmacol Biochem Behav. 2011;98(2):241–249. | ||
Gutiérrez J, Mendoza J, Fernández F, Linares-Palomino J, Soto MJ, Maroto MC. ELISA test to detect Chlamydophila pneumoniae IgG. J Basic Microbiol. 2002;42(1):13–18. | ||
Buka SL, Tsuang MT, Torrey EF, Klebanoff MA, Bernstein D, Yolken RH. Maternal infections and subsequent psychosis among offspring. Arch Gen Psychiatry. 2001;58(11):1032–1037. | ||
Fux M, Sarov I, Ginot Y, Sarov B. Herpes simplex virus and cytomegalovirus in the serum of schizophrenic patients versus other psychosis and normal controls. Isr J Psychiatry Relat Sci. 1992;29(1):33–35. | ||
Leweke FM, Gerth CW, Koethe D, et al. Antibodies to infectious agents in individuals with recent onset schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2004;254(1):4–8. | ||
Mesa-Castillo S. [An ultrastructural study of the temporal lobe and peripheral blood in schizophrenic patients]. Rev Neurol. 2001;33(7):619–623. Spanish. | ||
Niebuhr DW, Millikan AM, Yolken R, Li Y, Weber NS. Results from a hypothesis generating case-control study: herpes family viruses and schizophrenia among military personnel. Schizophr Bull. 2008;34(6):1182–1188. | ||
Prasad KM, Eack SM, Goradia D, et al. Progressive gray matter loss and changes in cognitive functioning associated with exposure to herpes simplex virus 1 in schizophrenia: a longitudinal study. Am J Psychiatry. 2011;168(8):822–830. | ||
Schretlen DJ, Vannorsdall TD, Winicki JM, et al. Neuroanatomic and cognitive abnormalities related to herpes simplex virus type 1 in schizophrenia. Schizophr Res. 2010;118(1–3):224–231. | ||
Wang H, Yolken RH, Hoekstra PJ, Burger H, Klein HC. Antibodies to infectious agents and the positive symptom dimension of subclinical psychosis: The TRAILS study. Schizophr Res. 2011;129(1):47–51. | ||
Whitford TJ, Wood SJ, Yung A, et al. Structural abnormalities in the cuneus associated with Herpes Simplex Virus (type 1) infection in people at ultra high risk of developing psychosis. Schizophr Res. 2012;135(1–3):175–180. | ||
Dickerson F, Stallings C, Origoni A, Vaughan C, Khushalani S, Yolken R. Additive effects of elevated C-reactive protein and exposure to Herpes Simplex Virus type 1 on cognitive impairment in individuals with schizophrenia. Schizophr Res. 2012;134(1):83–88. | ||
Prasad KM, Eack SM, Keshavan MS, Yolken RH, Iyengar S, Nimgaonkar VL. Antiherpes virus-specific treatment and cognition in schizophrenia: a test-of-concept randomized double-blind placebo-controlled trial. Schizophr Bull. 2013;39(4):857–866. | ||
Demontis D, Nyegaard M, Buttenschøn HN, et al. Association of GRIN1 and GRIN2A-D with schizophrenia and genetic interaction with maternal herpes simplex virus-2 infection affecting disease risk. Am J Med Genet B Neuropsychiatr Genet. 2011;156B(8):913–922. | ||
Inta D, Trusel M, Riva MA, Sprengel R, Gass P. Differential c-Fos induction by different NMDA receptor antagonists with antidepressant efficacy: potential clinical implications. Int J Neuropsychopharmacol. 2009;12(8):1133–1136. | ||
Lima-Ojeda JM, Vogt MA, Pfeiffer N, et al. Pharmacological blockade of GluN2B-containing NMDA receptors induces antidepressant-like effects lacking psychotomimetic action and neurotoxicity in the perinatal and adult rodent brain. Prog Neuropsychopharmacol Biol Psychiatry. 2013;45:28–33. | ||
Giovanoli S, Engler H, Engler A, et al. Stress in puberty unmasks latent neuropathological consequences of prenatal immune activation in mice. Science. 2013;339(6123):1095–1099. | ||
Yoshimi N, Futamura T, Hashimoto K. Prenatal immune activation and subsequent peripubertal stress as a new model of schizophrenia. Expert Rev Neurother. 2013;13(7):747–750. | ||
Li M, Li X, Zhang X, et al. Effects of prenatal chronic mild stress exposure on hippocampal cell proliferation, expression of GSK-3α, β and NR2B in adult offspring during fear extinction in rats. Int J Dev Neurosci. 2014;35:16–24. | ||
Hu W, Zhang M, Czéh B, Flügge G, Zhang W. Stress impairs GABAergic network function in the hippocampus by activating nongenomic glucocorticoid receptors and affecting the integrity of the parvalbumin-expressing neuronal network. Neuropsychopharmacology. 2010;35(8):1693–1707. | ||
Filipović D, Zlatković J, Gass P, Inta D. The differential effects of acute vs chronic stress and their combination on hippocampal parvalbumin and inducible heat shock protein 70 expression. Neuroscience. 2013;236:47–54. | ||
Prasad KM, Watson AM, Dickerson FB, Yolken RH, Nimgaonkar VL. Exposure to herpes simplex virus type 1 and cognitive impairments in individuals with schizophrenia. Schizophr Bull. 2012;38(6):1137–1148. | ||
Amminger GP, McGorry PD, Berger GE, et al. Antibodies to infectious agents in individuals at ultra-high risk for psychosis. Biol Psychiatry. 2007;61(10):1215–1217. | ||
Brown AS, Schaefer CA, Quesenberry CP, Liu L, Babulas VP, Susser ES. Maternal exposure to toxoplasmosis and risk of schizophrenia in adult offspring. Am J Psychiatry. 2005;162(4):767–773. | ||
Dickerson F, Boronow J, Stallings C, Origoni A, Yolken R. Toxoplasma gondii in individuals with schizophrenia: association with clinical and demographic factors and with mortality. Schizophr Bull. 2007;33(3):737–740. | ||
Hinze-Selch D, Däubener W, Eggert L, Erdag S, Stoltenberg R, Wilms S. Acontrolled prospective study of toxoplasma gondii infection in individuals with schizophrenia: beyond seroprevalence. Schizophr Bull. 2007;33(3):782–788. | ||
Mortensen PB, Nørgaard-Pedersen B, Waltoft BL, Sørensen TL, Hougaard D, Yolken RH. Early infections of Toxoplasma gondii and the later development of schizophrenia. Schizophr Bull. 2007;33(3):741–744. | ||
Pedersen MG, Stevens H, Pedersen CB, Nørgaard-Pedersen B, Mortensen PB. Toxoplasma infection and later development of schizophrenia in mothers. Am J Psychiatry. 2011;168(8):814–821. | ||
Selten JP, Kahn RS. Schizophrenia after prenatal exposure to Toxoplasma gondii? Clin Infect Dis. 2002;35(5):633–634. | ||
Torrey EF, Yolken RH. Schizophrenia and toxoplasmosis. Schizophr Bull. 2007;33(3):727–728. | ||
Wang HL, Wang GH, Li QY, Shu C, Jiang MS, Guo Y. Prevalence of Toxoplasma infection in first-episode schizophrenia and comparison between Toxoplasma-seropositive and Toxoplasma-seronegative schizophrenia. Acta Psychiatr Scand. 2006;114(1):40–48. | ||
Webster JP, Kaushik M, Bristow GC, McConkey GA. Toxoplasma gondii infection, from predation to schizophrenia: can animal behaviour help us understand human behaviour? J Exp Biol. 2013;216(Pt 1):99–112. | ||
Xiao J, Buka SL, Cannon TD, et al. Serological pattern consistent with infection with type I Toxoplasma gondii in mothers and risk of psychosis among adult offspring. Microbes Infect. 2009;11(13):1011–1018. | ||
Torrey EF, Bartko JJ, Lun ZR, Yolken RH. Antibodies to Toxoplasma gondii in patients with schizophrenia: a meta-analysis. Schizophr Bull. 2007;33(3):729–736. | ||
Fabiani S, Pinto B, Bruschi F. Toxoplasmosis and neuropsychiatric diseases: can serological studies establish a clear relationship? Neurol Sci. 2013;34(4):417–425. | ||
Carruthers VB, Suzuki Y. Effects of Toxoplasma gondii infection on the brain. Schizophr Bull. 2007;33(3):745–751. | ||
Prandovszky E, Gaskell E, Martin H, Dubey JP, Webster JP, McConkey GA. The neurotropic parasite Toxoplasma gondii increases dopamine metabolism. PLoS One. 2011;6(9):e23866. | ||
Flegr J. Effects of toxoplasma on human behavior. Schizophr Bull. 2007;33(3):757–760. | ||
Yolken RH, Dickerson FB, Fuller Torrey E. Toxoplasma and schizophrenia. Parasite Immunol. 2009;31(11):706–715. | ||
Malinger G, Werner H, Rodriguez Leonel JC, et al. Prenatal brain imaging in congenital toxoplasmosis. Prenat Diagn. 2011;31(9):881–886. | ||
Stiles J, Jernigan TL. The basics of brain development. Neuropsychol Rev. 2010;20(4):327–348. | ||
Fuentes I, Rubio JM, Ramírez C, Alvar J. Genotypic characterization of Toxoplasma gondii strains associated with human toxoplasmosis in Spain: direct analysis from clinical samples. J Clin Microbiol. 2001;39(4):1566–1570. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.