Back to Journals » Cancer Management and Research » Volume 14
Different Features of 18F-FAPI, 18F-FDG PET/CT and MRI in the Evaluation of Extrahepatic Metastases and Local Recurrent Hepatocellular Carcinoma (HCC): A Case Report and Review of the Literature
Authors Chen D, Chang C, Zhang Y, Yang S, Wang G, Lin L, Zhao X, Zhao K, Su X
Received 16 May 2022
Accepted for publication 23 August 2022
Published 5 September 2022 Volume 2022:14 Pages 2649—2655
DOI https://doi.org/10.2147/CMAR.S374916
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
Donghe Chen,1 Chengdong Chang,2 Yafei Zhang,1 Shuye Yang,1 Guolin Wang,1 Lili Lin,1 Xin Zhao,1 Kui Zhao,1 Xinhui Su1
1Department of Nuclear Medicine, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, People’s Republic of China; 2Department of Pathology, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, People’s Republic of China
Correspondence: Kui Zhao; Xinhui Su, Department of Nuclear Medicine, The First Affiliated Hospital, Zhejiang University School of Medicine, 79 Qingchun Road, Hangzhou, People’s Republic of China, Tel/Fax +86-571-87236428, Email [email protected]; [email protected]
Background: Recurrence and metastasis are important causes of postoperative death in most HCC patients. Conventional imaging modalities such as 18F-FDG PET/CT and enhanced MRI are still unsatisfactory in evaluating these patients in the clinical setting. PET/CT imaging with a radiolabeled fibroblast activation protein inhibitor (FAPI) has emerged as a new imaging technique for the diagnosis and radiotherapy of malignant tumors. While many studies have focused on the diagnostic accuracy of intrahepatic primary HCC, the evaluation of recurrent and metastatic HCC remains only poorly investigated.
Case Presentation: A 71-year-old man with a five-year history of HCC after radical resection underwent 18F-FDG PET/CT due to further surgery for tumor recurrence, which revealed two iso-metabolic lesions in the right peritoneum and a hypo-metabolic lesion in the right liver. 18F-FAPI PET/CT was performed to further complement 18F-FDG PET/CT in the detection of these suspected metastatic lesions. Importantly, multiple diffuse intense radioactivity was shown in the hepatic capsule, suggesting metastatic lesions, but a wedge-shaped elevated 18F-FAPI uptake disorder around the FDG-unavid necrotic lesion after radiofrequency ablation (RFA) demonstrated benign stromal fibrosis.
Conclusion: This case suggested that 18F-FAPI may have an advantage over 18F-FDG in detecting peritoneal metastasis even in tiny or early hepatic capsules of HCC, but its false positives due to postoperative stromal fibrosis should be noted. Wedge- or strip-shaped FAPI-avid lesions with sharp edges may be post-treatment stromal fibrosis.
Keywords: 18F-FAPI, 18F-FDG, PET/CT, magnetic resonance imaging, hepatocellular carcinoma, false-positive, stromal fibrosis
Introduction
Integrated 18F-FDG PET/CT has emerged as an important tool for diagnosis, staging, restaging and treatment monitoring for patients with various different types of tumors because it provides functional and morphological information in a single and noninvasive examination.1 However, it has some limitations in the diagnosis of HCC and detecting recurrent lesions during follow-up, with little change in treatment resulting.2,3 According to the literature reports,4,5 the sensitivity of 18F-FDG PET for detecting HCC is approximately 50–70% and has no obvious advantage compared with other imaging technologies, especially for well-differentiated HCC. Moreover, this type of disease generates a great difference between intra- and inter-operator during radiological evaluations.6 Fibroblast-activated protein is highly expressed in activated stromal fibroblasts in various cancers and at low levels throughout the body.7,8 FAPI-PET/CT is more sensitive than FDG PET/CT in detecting HCC lesions, including small (≤2 cm in diameter) and well or moderately differentiated tumors.9,10 Here, we discussed different features of 18F-FAPI, 18F-FDG PET/CT and MRI in the evaluation of extrahepatic metastases and local recurrent HCC in a typical patient.
Case Presentation
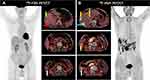
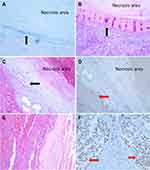
A 75-year-old man, with a history of hepatitis B virus-related cirrhosis, underwent radical resection of right liver cancer 5 years ago. The pathological diagnosis was moderately differentiated hepatocellular carcinoma (HCC). Half a year ago, he presented to our hospital with abdominal pain. Abdominal enhanced CT suggested recurrent hepatocellular carcinoma and right peritoneal metastasis. He underwent comprehensive treatment, including radiofrequency ablation (RFA), radiotherapy and lenvatinib. Now, he came to our hospital for further surgery treatment. Follow-up laboratory examinations showed that the serum alpha-fetoprotein (AFP) level increased progressively to 71.5 ug/L (reference range: 0–3 ug/L). 18F-FDG PET/CT and 18F-FAPI PET/CT were performed to further evaluate the nature of the lesion (Figure 1). 18F-FDG PET/CT revealed multiple iso-metabolic metastases in the right mesh membrane, hypo-metabolic tumor necrosis after RFA in the right liver, and low uptake in peritoneal metastases (Figure 1A). Astonishingly, 18F-FAPI PET/CT showed increased diffuse uptake of metastases in the hepatic capsule, which were negative in 18F-FDG PET/CT (Figure 1B). Furthermore, we accidentally observed wedge-shaped high 18F-FAPI uptake disorders around the tumor necrosis lesion after RFA in the right liver (Figure 1B, blue allow), consistent with the shape shown on MRI enhanced phase (Figure 2). Then, the patient was operated after exclusion of contraindications. Nodules in sub-hepatic capsule of segment 5 and right peritoneal nodules were resected, and HCC metastasis was confirmed by histopathology. RFA lesions with peripheral liver tissue were also resected, and histopathology confirmed complete necrosis of the tumor and surrounding benign hepatocyte degeneration and stromal fibrosis after RFA (Figure 3).
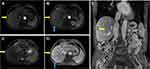 |
Figure 2 Enhanced liver MRI showed low signal intensity of hepatic capsular nodules on the arterial enhanced phase (A and B; yellow arrows) with high signal intensity on T2-phase (C, yellow arrow) and mild enhancement of the edge on the venous enhanced phase (D and E, yellow arrows). These lesions were resected, and metastases were confirmed by pathology (Figure 3). MRI on axial slices also showed a wedge-shaped high signal intensity around the tumor necrosis lesion after RFA in the right liver in the arterial and venous enhanced phases (B and D, blue allows). |
Discussion
The clinical diagnosis and treatment of hepatocellular carcinoma (HCC) has developed rapidly in recent years. Wide application of various imaging techniques, improvement of surgical techniques, the progress of nonsurgical local treatment and development of liver transplantation have significantly improved the short-term efficacy of HCC surgery. However, recurrence and metastasis are still important causes of postoperative death in most HCC patients. The 5-year recurrence rate of HCC is 32.5%–61.5%, and disease-free survival is 16%–38.6%.10–12
Ultrasonography (US), computed tomography (CT) and magnetic resonance imaging (MRI) in combination with measurement of serum alpha-fetoprotein (AFP) levels are routinely used for evaluating recurrence and metastasis of HCC in the clinical setting. Common limitations of all the above-mentioned methods were simply based on morphological abnormalities and inability to reflect the whole-body models.
Positron emission tomography (PET) using 18F-fluorodeoxyglucose (18F-FDG) generates hybrid and simultaneous images of both anatomy and glucose metabolism, allowing for an integrated comprehensive body oncology imaging technique. It is widely used for staging and follow-up of a variety of malignancies; however, it has unsatisfactory sensitivity in the evaluation of HCC, especially in well-differentiated and small HCC.4,13
Therefore, there is a clinical need for better PET radiotracers for detecting recurrence and metastasis of HCC after treatment. Other radiotracers, such as 11 C-acetate and 11 C-choline, improved sensitivity in terms of detection and therapeutic response. However, the high overall radiation dose delivered to the patient as well as the short half-life (20.4 min) and relatively low image resolution of PET/CT with 11C-labeled radiopharmaceuticals are limited routine clinical use.5,14,15
Fibroblast activation protein (FAP) is a membrane-bound serine protease overexpressed in cancer-associated fibroblasts. PET/CT imaging with radiolabeled FAP inhibitor (FAPI) has emerged as a new imaging technique for the diagnosis and radiotherapy of malignant tumors because of its advantages of simple preparation (no need for rest and fasting) and high tumor to non-tumor blood flow ratio (T/NT).16,17 In recent years, the number of studies18–27 on the clinical application of FAPI PET/CT imaging has constantly increased, especially in HCC.23–26 While these studies have focused on the diagnostic accuracy of intrahepatic primary HCC, the evaluation of recurrent and metastatic HCC remains poorly investigated (5 cases in Table 1).
 |
Table 1 Summary Table of the Clinical Application of Recurrence and Metastasis of HCC in FAPI PET/CT and Other Imaging Methods |
Firstly, our case highlighted that 18F-FAPI-PET/CT had high sensitivity in detecting peritoneal metastases, especially in the hepatic capsule. According to previous studies, the detection rate of 18F-FDG PET was low (13%) for extrahepatic metastases of HCC less than or equal to 1 cm, perhaps related to poor spatial resolution for small lesions.30 In our case, both 18F-FDG PET/CT and 18F-FAPI PET/CT revealed two peritoneal metastases larger than 1 cm in greatest diameter. However, the tumor-to-background contrast of these lesions in 18F-FAPI PET/CT (SUVmax = 5.0; SUVmax of nearby tissue = 1.21) was higher than that in 18F-FDG PET/CT (SUVmax = 3.51; SUVmax of nearby tissue = 1.98). Moreover, although local morphological abnormalities of the hepatic capsule were found in enhanced liver MRI, oncological evaluation was still inconclusive due to FDG-negative findings in these lesions (SUVmax = 2.25; liver SUVmax = 3.47). Interestingly, these paired lesions demonstrated increased 18F-FAPI uptake with favorable tumor-to-background contrast (SUVmax = 7.77, liver SUVmax = 0.62) in further 18F-FAPI PET/CT. The liver capsule is composed of two tightly bound layers of tissue: the inner layer is Glisson’s capsule, and the outer serosa is derived from the peritoneum.31 Liver hepatic capsule implant metastasis can be the result of exfoliation of tumor cells from primary lesions or medical manipulations such as surgery, RFA or transcatheter arterial chemoembolization (TACE) and is often missed on conventional imaging, even 18F-FDG PET/CT. From this case, 18F-FAPI-PET/CT had superior detection of peritoneal metastases than MRI and FDG PET/CT, especially for tiny or early signs of hepatic capsular metastases.
Secondly, our case also highlighted false-positive findings due to stromal fibrosis after RFA in 18F-FAPI-PET/CT imaging. Some previous studies have discovered that inflammatory diseases (angiocholitis, pancreatitis and IgG4-related disease, infectious, granulomatous disease) and diseases in which a fibrotic reaction was activated (myelofibrosis, fibrotic nodules and liver cirrhosis) can cause false-positive uptake of FAPI.20,29,32 In our case, a wedge-shaped high 18F-FAPI uptake disorder around the tumor necrosis lesion after RFA in 18F-FAPI-PET/CT was confirmed as benign fibrosis by pathology and FAP staining, thus indicating a false positive. Therefore, we consider that interventional therapy, such as RFA and even TACE, can also induce inflammation and fibrosis in surrounding areas. Previous articles29 indicated that FAPI-04 PET/CT has difficulty differentiating between residual/recurrent disease and inflammatory reactions after radiation, operative and interventional therapy. In our opinion, wedge or strip shaped FAPI-avid lesions with sharp edges may be a post-operative change, while runner, oval or round-shaped FAPI-avid lesions with wave edges may be residual or recurrent of HCC. A further well-designed study with a large number of patients is needed to confirm this point.
This case suggested that 18F-FAPI PET/CT had more significant advantages in detecting and evaluating tumor activity of peritoneal metastasis even tiny or early hepatic capsular metastases than conventional modalities (such as 18F-FDG PET/CT and enhanced MRI) and contributed to the clinical management of HCC patients after operation or interventional therapy. However, false-positive 18F-FAPI PET findings due to benign fibrosis should be interpreted with more caution, and wedge- or strip-shaped FAPI-avid lesions with sharp edges may be post-treatment stromal fibrosis. Therapeutic decisions should be made on the basis of clinical and even histologic confirmation.
Abbreviations
HCC, Hepatocellular Carcinoma; FAPI, Fibroblast activation protein inhibitor; US, Ultrasonography; CT, Computed tomography; MRI, Magnetic resonance imaging; AFP, Alpha-fetoprotein; RFA, Radiofrequency ablation; 18F-FDG, 18F-2-deoxy-2-fluoro-D-glucose; PET/CT, positron emission tomography/computed tomography; SUVmax, The maximum standardized uptake value; T/NT, Tumor to non-tumor blood flow ratio; TACE, Transcatheter arterial chemoembolization.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
The study was approved by the Ethics Committee of The first Affiliated Hospital, Zhejiang University School of Medicine.
Consent for Publication
Written informed consent was obtained from the patient’s next of kin for publication of this case report and any accompanying images.
Acknowledgments
We are thankful to the parents of patient for the support given in providing the data.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by grants from the National Natural Science Foundation of China (NSFC) (82071965).
Disclosure
The authors declare that they have no competing interests.
References
1. Maldonado A, González-Alenda FJ, Alonso M, Sierra JM. PET-CT in clinical oncology. Clin Transl Oncol. 2007;9(8):494–505. doi:10.1007/s12094-007-0093-5
2. Lee SM, Kim HS, Lee S, Lee JW. Emerging role of 18F-fluorodeoxyglucose positron emission tomography for guiding management of hepatocellular carcinoma. World J Gastroenterol. 2019;25(11):1289–1306. doi:10.3748/wjg.v25.i11.1289
3. Haug AR. Imaging of primary liver tumors with positron-emission tomography. Q J Nucl Med Mol Imaging. 2017;61(3):292–300. doi:10.23736/S1824-4785.17.02994-6
4. Hayakawa N, Nakamoto Y, Nakatani K, et al. Clinical utility and limitations of FDG PET in detecting recurrent hepatocellular carcinoma in postoperative patients. Int J Clin Oncol. 2014;19(6):1020–1028. doi:10.1007/s10147-013-0653-3
5. Dubash SR, Idowu OA, Sharma R. The emerging role of positron emission tomography in hepatocellular carcinoma. Hepat Oncol. 2015;2(2):191–200. doi:10.2217/hep.15.6
6. Tovoli F, Renzulli M, Negrini G, et al. Inter-operator variability and source of errors in tumour response assessment for hepatocellular carcinoma treated with sorafenib. Eur Radiol. 2018;28(9):3611–3620. doi:10.1007/s00330-018-5393-3
7. Talbot JN, Fartoux L, Balogova S, et al. Detection of hepatocellular carcinoma with PET/CT: a prospective comparison of 18F-fluorocholine and 18F-FDG in patients with cirrhosis or chronic liver disease. J Nucl Med. 2010;51(11):1699–1706. doi:10.2967/jnumed.110.075507
8. Khan MA, Combs CS, Brunt EM, et al. Positron emission tomography scanning in the evaluation of hepatocellular carcinoma. J Hepatol. 2000;32(5):792–797. doi:10.1016/S0168-8278(00)80248-2
9. Verhoef C, Valkema R, de Man RA, Krenning EP, Yzermans JN. Fluorine-18 FDG imaging in hepatocellular carcinoma using positron coincidence detection and single photon emission computed tomography. Liver. 2002;22(1):51–56. doi:10.1046/j.0106-9543.2001.01593.x
10. Sun HC, Tang ZY. Preventive treatments for recurrence after curative resection of hepatocellular carcinoma – a literature review of randomized control trials. World J Gastroenterol. 2003;9(4):635–640. doi:10.3748/wjg.v9.i4.635
11. Guiu B, Minello A, Cottet V, et al. A 30-year, population-based study shows improved management and prognosis of hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2010;8(11):986–991. doi:10.1016/j.cgh.2010.07.018
12. Zhou XD. Recurrence and metastasis of hepatocellular carcinoma: progress and prospects. Hepatobiliary Pancreat Dis Int. 2002;1(1):35–41.
13. Teefey SA, Hildeboldt CC, Dehdashti F, et al. Detection of primary hepatic malignancy in liver transplant candidates: prospective comparison of CT, MR imaging, US, and PET. Radiology. 2003;226(2):533–542. doi:10.1148/radiol.2262011980
14. Cheung TT, Chan SC, Ho CL, et al. Can positron emission tomography with the dual tracers [11 C]acetate and [18 F]fludeoxyglucose predict microvascular invasion in hepatocellular carcinoma? Liver Transpl. 2011;17(10):1218–1225. doi:10.1002/lt.22362
15. Chen H, Teng M, Zhang H, Liang X, Cheng H, Liu G. Advanced radionuclides in diagnosis and therapy for hepatocellular carcinoma. Chin Chem Lett. 2022;33(7):3371–3383. doi:10.1016/j.cclet.2022.03.079
16. Loktev A, Lindner T, Mier W, et al. A tumor-imaging method targeting cancer-associated fibroblasts. J Nucl Med. 2018;59(9):1423–1429. doi:10.2967/jnumed.118.210435
17. Giesel FL, Kratochwil C, Lindner T, et al. 68Ga-FAPI PET/CT: biodistribution and preliminary dosimetry estimate of 2 DOTA-containing FAP-targeting agents in patients with various cancers. J Nucl Med. 2019;60(3):386–392. doi:10.2967/jnumed.118.215913
18. Hathi DK, Jones EF. 68Ga FAPI PET/CT: tracer uptake in 28 different kinds of cancer. Radiol Imaging Cancer. 2019;1(1):e194003. doi:10.1148/rycan.2019194003
19. Röhrich M, Loktev A, Wefers AK, et al. IDH-wildtype glioblastomas and grade III/IV IDH-mutant gliomas show elevated tracer uptake in fibroblast activation protein-specific PET/CT. Eur J Nucl Med Mol Imaging. 2019;46(12):2569–2580. doi:10.1007/s00259-019-04444-y
20. Chen H, Pang Y, Wu J, et al. Comparison of [68Ga]Ga-DOTA-FAPI-04 and [18F] FDG PET/CT for the diagnosis of primary and metastatic lesions in patients with various types of cancer. Eur J Nucl Med Mol Imaging. 2020;47(8):1820–1832. doi:10.1007/s00259-020-04769-z
21. Windisch P, Röhrich M, Regnery S, et al. Fibroblast Activation Protein (FAP) specific PET for advanced target volume delineation in glioblastoma. Radiother Oncol. 2020;150:159–163. doi:10.1016/j.radonc.2020.06.040
22. Ristau J, Giesel FL, Haefner MF, et al. Impact of primary staging with fibroblast activation protein specific enzyme inhibitor (FAPI)-PET/CT on radio-oncologic treatment planning of patients with esophageal cancer. Mol Imaging Biol. 2020;22(6):1495–1500. doi:10.1007/s11307-020-01548-y
23. Shi X, Xing H, Yang X, et al. Fibroblast imaging of hepatic carcinoma with 68Ga-FAPI-04 PET/CT: a pilot study in patients with suspected hepatic nodules. Eur J Nucl Med Mol Imaging. 2021;48(1):196–203. doi:10.1007/s00259-020-04882-z
24. Shi X, Xing H, Yang X, et al. Comparison of PET imaging of activated fibroblasts and 18F-FDG for diagnosis of primary hepatic tumours: a prospective pilot study. Eur J Nucl Med Mol Imaging. 2021;48(5):1593–1603. doi:10.1007/s00259-020-05070-9
25. Guo W, Pang Y, Yao L, et al. Imaging fibroblast activation protein in liver cancer: a single-center post hoc retrospective analysis to compare [68Ga]Ga-FAPI-04 PET/CT versus MRI and [18F]-FDG PET/CT. Eur J Nucl Med Mol Imaging. 2021;48(5):1604–1617. doi:10.1007/s00259-020-05095-0
26. Geist BK, Xing H, Wang J, et al. A methodological investigation of healthy tissue, hepatocellular carcinoma, and other lesions with dynamic 68Ga-FAPI-04 PET/CT imaging. EJNMMI Phys. 2021;8(1):8. doi:10.1186/s40658-021-00353-y
27. Röhrich M, Naumann P, Giesel FL, et al. Impact of 68Ga-FAPI PET/CT imaging on the therapeutic management of primary and recurrent pancreatic ductal adenocarcinomas. J Nucl Med. 2021;62(6):779–786. doi:10.2967/jnumed.120.253062
28. Wang H, Zhu W, Ren S, et al. 68Ga-FAPI-04 versus 18F-FDG PET/CT in the detection of hepatocellular carcinoma. Front Oncol. 2021;11:693640. doi:10.3389/fonc.2021.693640
29. Chen H, Zhao L, Ruan D, et al. Usefulness of [68Ga]Ga-DOTA-FAPI-04 PET/CT in patients presenting with inconclusive [18F]FDG PET/CT findings. Eur J Nucl Med Mol Imaging. 2021;48(1):73–86. doi:10.1007/s00259-020-04940-6
30. Sugiyama M, Sakahara H, Torizuka T, et al. 18F-FDG PET in the detection of extrahepatic metastases from hepatocellular carcinoma. J Gastroenterol. 2004;39(10):961–968. doi:10.1007/s00535-004-1427-5
31. Bayraktutan U, Ogul H, Kantarci M, Karaca L, Pirimoglu B. Education and imaging. Hepatobiliary and pancreatic: calcified perihepatic and capsular metastases. J Gastroenterol Hepatol. 2014;29(4):664. doi:10.1111/jgh.12533
32. Peng D, He J, Liu H, Cao J, Wang Y, Chen Y. FAPI PET/CT research progress in digestive system tumours. Dig Liver Dis. 2022;54(2):164–169. doi:10.1016/j.dld.2021.07.011
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.