Back to Journals » International Journal of General Medicine » Volume 15
Different Aspects of Diabetes in Hospitalized Patients with COVID-19
Authors Sayed AA, Abdelfatah HH, Abdelhameid MA, Ali OM
Received 7 February 2022
Accepted for publication 18 May 2022
Published 21 June 2022 Volume 2022:15 Pages 5729—5740
DOI https://doi.org/10.2147/IJGM.S360160
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Aml Ahmed Sayed, Hossam Hassan Abdelfatah, Marwa Ahmed Abdelhameid, Omaima Mohamed Ali
Internal Medicine Department, Faculty of Medicine, Aswan University, Aswan, Egypt
Correspondence: Aml Ahmed Sayed, Internal Medicine Department, Faculty of Medicine, Aswan University, Aswan, 81528, Egypt, Tel +20 01064737884, Email [email protected]
Background: The novel severe acute respiratory syndrome coronavirus (SARS-CoV-2) causes COVID-19, a recent infectious disease that aggravates the underlying pathophysiology of hyperglycemia in diabetic individuals. This study aimed to detect how diabetes mellitus (DM) affected COVID-19 patients’ morbidity and mortality, and the incidence of neonset DM.
Patients and Methods: The present study was a cross-sectional study done at Aswan Isolation Hospitals, Egypt. It comprised 200 individuals who had been tested positive for COVID-19. They were divided into two groups: group 1 (pre-existing diabetes = 143 patients) and group 2 (new-onset diabetes = 57 patients), and all patients were subjected to general examinations, hospital stay duration, and investigations, such as (complete blood count, urea, creatinine, HBA1c, fasting, postprandial, and random blood sugar, D-Dimer, ferritin, C-reactive protein, PCR for SARS COV-2 RNA, and CT chest.
Results: The current study consisted of 94 males and 106 females. According to disease severity, they were 96 (48.0%) critical cases, 57 (28.5%) severe cases, and 47 (23.5%) non-severe cases. The incidence of new-onset DM in COVID-19 patients was 28.5% (57 new cases), with a mortality rate of 42.0% (84 cases). Regarding glycemic control, we found a significant difference in fasting blood sugar (FBS) between the two groups, with a significant increase of FBS in the dead group than in the survived group. We also found a significant age difference in critical than in severe and non-severe groups, with a high mortality rate in older patients. Inflammatory markers, such as ferritin, CRP, and D-dimer, were higher in critical than in severe and non-severe groups.
Conclusion: The prevalence of new-onset DM is significant among hospitalized COVID-19 patients. Older patients were more prone to disease severity with high mortality rate. Inflammatory markers such as CRP and ferritin were significantly related to the COVID-19 severity and outcome.
Keywords: COVID-19, diabetes mellitus, morbidity, mortality rate, disease severity
Introduction
COVID-19 is a newly discovered infectious disease caused by the novel severe SARS-CoV-2. The high infectivity and asymptomatic transmission of COVID-19 made it different from previous coronavirus infections (severe acute respiratory syndrome and the Middle East respiratory syndrome (SARS and MERS), which led to the present global COVID-19 pandemic. COVID-19 infection appears to worsen the underlying pathophysiology of hyperglycemia in diabetic patients, according to new facts.1
Diabetes is identified by chronic hyperglycemia that impairs the immune response and makes patients more susceptible to develop infectious diseases. On the other hand, acute and stress hyperglycemia can cause more complications in COVID-19 patients, regardless of past history of diabetes. Hyperglycemia was reported in 51% of COVID-19 individuals in a recent study from Wuhan, China.2 The US Centers for Disease Control and Prevention reported diabetes prevalence rising with increasing severity of COVID-19, from 6.4% in non-hospitalized patients to 24.2% in hospitalized patients and 32.4% in ICU patients.3
There is a bidirectional relationship between COVID-19 and diabetes. On the one hand, diabetes is associated with an increased risk of severe COVID-19. On the other hand, new-onset diabetes and severe metabolic complications of pre existing diabetes, including diabetic ketoacidosis and hyperosmolarity have been observed in patients with COVID-19.4
COVID-19 causes diabetic keto-acidosis (DKA) by the mechanism, which has yet to be fully explained; nevertheless, it has been shown that it seems to use the receptor for angiotensin-converting enzyme 2 as a gate that is expressed in the intestine, kidney, and pancreas. Consequently, the virus can trigger cellular destruction of the islets of Langerhans, which may explain the increased prevalence of DKA in diabetic and non-diabetic persons. This damage may be expressed by the rising of pancreatic enzyme levels. Similarly, COVID-19-induced insulin resistance has been observed, which, in addition to pancreatic damage, elevate the risk of hyper-glycemic crisis in diabetic patients.5
In human monocytes, increased blood sugar levels directly increase COVID-19 replication, and glycolysis maintains COVID-19 replication by the production of mitochondrial reactive oxygen species and activation of hypoxia-inducible factor 1α. Therefore, high blood glucose may support viral proliferation; in concord with this hypothesis, hyperglycemia or a history of diabetes were revealed to be an independent factor of mortality and morbidity in COVID-19 patients.6
Hyperglycemia is a powerful indicator of prognosis in COVID-19-hospitalized patients, and recent studies revealed that hyperglycemia in patients with COVID-19 displays a higher cumulative incidence of severe disease than persons without hyperglycemia.7 Isolation from clinicians missed blood glucose monitoring, inappropriate discontinuation of angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, hyperglycemia-induced changes in the immune system, and elevation of inflammatory cytokines are all possible reasons for higher mortality in diabetic patients.8
Individuals with diabetes, hypertension, CVD, and severe obesity are at a greater risk for poor COVID-19 outcomes; people who have diabetes and/or obesity have an impaired innate and adaptive immune response characterized by a condition of chronic low-grade inflammation, which may result in systemic metabolic alteration, such as elevated leptin level (a pro-inflammatory adipokine) and decreased adiponectin level (an anti-inflammatory adipokine).9
Several factors have been related to the risk and severity of COVID-19 infection in diabetic patients, including increased expression of ACE2, IL-6, and furin, as well as reduced T-cell function.6
Patients and Methods
Study Design, Setting, and Patients
The current study was a cross-sectional study that included 200 participants identified with positive SARS COV-2 RNA from Aswan Isolation Hospitals (Middle Eastern population in Egypt); we included the patients who had history of diabetes or had new-onset diabetes (based on the first glucose measurement upon admission, fasting admission glucose ≥126 mg/dL and/or HbA1c ≥6.5%) and were over the age of 18, the patients were divided into two categories: group 1 (pre-existing diabetes = 143 patients) and group 2 (new-onset diabetes = 57 patients).
According to clinical and radiological data, they were classified into critical cases with any of the following criteria: respiratory failure that requires artificial ventilation, shock, or other organs failure that require intensive care unit, severe cases with a ratio of arterial partial oxygen pressure to inspired oxygen fraction (PaO2/FiO2) <300 mmHg, lung infiltrates >50%, respiratory rate >30 breaths per minute or patients with SpO2 <94% in room air, none severe cases (patients who did not meet the criteria of the critical or severe form).
Exclusion Criteria
- Pregnant women.
- Age <18 years.
- Type 1 diabetes and pre-diabetes.
- Previous history of drugs that elevate blood sugar as steroid, thiazide, calcineurine inhibitors.
Data Collection
We collected the following data from every eligible patient: demographic data, hospital stay duration, clinical examination findings included the severity of disease and laboratory investigations that included complete blood count (CBC), kidney function tests, random blood sugar level (RBS), HBA1c, FBS, postprandial glucose level, D-Dimer, serum ferritin level, CRP, PCR for SARS CoV-2 RNA.
Study Outcomes
The primary outcome in the present study was the high prevalence of new-onset DM among hospitalized COVID-19 patients; the secondary outcome was the association between DM and higher morbidity and mortality rates in COVID-19 patients.
Ethical Statement
We confirm that this research complies with international ethical standards as well as local regulatory requirements. There are no known dangers to study participants in terms of physical, psychological, social, legal, economic, or other factors. The study’s objectives, methodology, risks, and benefits were all explained to the participants. All eligible patients in the study have given their written informed consent. Our study was conducted in accordance with the Declaration of Helsinki for studies on human subjects. The Ethics Committee of Aswan University’s Faculty of Medicine reviewed and approved the study.
Statistical Analysis
Data were collected, revised, coded, and entered into the Statistical Package for Social Science (IBM SPSS) version 20. The qualitative data were presented as numbers and percentages, while quantitative data were presented as mean, standard deviations, and ranges when their distribution was parametric. The comparison between the two groups with qualitative data was performed using the Chi-square test and/or Fisher exact test was used instead of the Chi-square test when the expected count in any cell was found less than 5. The comparison between two independent groups with quantitative data and parametric distribution was made using an independent t-test. The comparison between two independent groups with quantitative data and non-parametric distribution was performed using the Mann–Whitney Test. The confidence interval was set to 95%, and the margin of error accepted was set to 5%. So, the p-value was considered significant as the following: P > 0.05 = non-significant (NS), P < 0.05 = significant (S), P < 0.001 = highly significant (HS). Survival analysis was done using the Kaplan–Meier method.
Results
Our study included 200 patients from Aswan Isolation Hospitals diagnosed with positive SARS COV-2 RNA. Most of the patients were females (53%) with a mean age of 64.43 ± 13.62 years. The average body mass index (BMI) of patients was 24.96 ± 2.33 kg/m2. All COVID-19 patients received I.V steroid and 19 of them (9.5%) received interleukin-6 inhibitor (tocilizumab) and 37 (18.5%) cases were on mechanical ventilator, 93 (46.5%) cases were on continuous positive airway pressure (CPAP), 23 (11.5%) cases were on reservoir oxygen mask and 47 (23.5%) cases were on nasal oxygen mask.
According to disease severity, there were 96 (48.0%) critical patients, 57 (28.5%) severe patients and 47 (23.5%) non-severe patients. The incidence of new-onset DM in COVID-19 patients was 57 new cases (28.5%), the incidence of acute kidney injury (AKI) between COVID-19 patients was 64 cases (32.0%), with a mortality rate of 84 cases (42.0%).
Regarding glycemic control, we found that there was a highly significant increase in the mean ± SD of FBS of group 1 patients 244.32 ± 54.41 mg/dl when compared with group 2 203.51 ± 33.82 mg/dl (P < 0.01) as presented in Table 1.
 |
Table 1 Comparison Between Preexisting DM (N =143) and New-Onset DM (N =57) Regarding Glycemic Finding |
In comparing glycemic control between groups of the study according to the severity of disease, there was a highly significant increase in the mean ± SD of FBS in the critical group 245.02 ± 58.28 when compared with severe 221.56 ± 47.37 and non-severe group 221.00 ± 40.62 (P 0.006), as illustrated in Table 2.
 |
Table 2 Relation Between Disease Severity and Glycemic Finding |
Table 3 compares between preexisting and new onset diabetes regarding laboratory finding and outcome and severity that reveal a significant difference between two groups regarding RBG, HBA1c, creatine and Na.
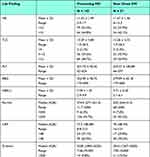 |
Table 3 Comparison Between Preexisting DM (N = 143) and New-Onset DM (N = 57) Regarding Laboratory Finding and Outcome and Severity |
 |
Table 4 Relation Between Patients’ Outcome and Glycemic Finding |
We also conducted the relation between the patient’s outcome and glycemic findings, which revealed a highly significant increase in the mean ± SD of FBS in the dead group 245.42 ± 56.88 when compared with the survived group 215.11 ± 40.41 (P = 0.00), as displayed in Table 4.
A significant difference was found between the two groups regarding hospital stay (days), we found that the median (IQR) of hospital stay was higher in group 2 [10 (7–15]) when compared with group 1 [7 (5–13)] (P 0.015).
Regarding outcome (Recovery and Death), no significant difference was found between studied groups. In group 1, there were 80 (55.9%) died case and 63 (44.1%) survived case, while in group 2, there were 36 (63.2%) died case and 21 (36.8%) survived case (P = 0.351).
We also compared two groups regarding disease severity, no significant difference was found between two groups. In group 1, there were 67 (46.9%) critical case, 40 (28.0%) severe case and 36 (25.2%) non-severe case, while in group 2, there were 29 (50.9%), critical case, 17 (29.8%) severe case and 11 (19.3%) non-severe case (P 0.675).
Aging is considered an independent risk factor that is affecting the severity of disease and mortality rate among COVID-19 patients; thus, we detected a significant increase in the mean ± SD of age in the critical group (67.17 ± 13.16) when compared with severe (62.81 ± 12.82) and non-severe groups (60.79 ± 14.56) (P = 0.017) (Figure 1), with a highly significant difference in the mean ± SD of age in the dead group (67.04 ± 13.30) when compared with the survived group (60.81 ± 13.29) (P = 0.001).
 |
Figure 1 Shows the difference between disease severity and Age. |
Inflammatory markers and their relation to disease severity were also studied in our patients, and we found that the median (IQR) of ferritin was higher in the critical group [419.5 (317–566)] when compared with severe [370 (228–521)] and non-severe groups [315 (215–421)] (P = 0.001). Also, the median (IQR) of CRP was higher in the critical group [78.5 (48.5–97)] when compared with severe [74 (48–89)] and non-severe groups [48 (38–78)] (P = 0.000). In the same way, the median IQR of D-dimer was highly significant in the critical group [3750 (2522–5827)] when compared with severe 3100 (1500–5031) and non-severe groups [2050 (780–3100)] (P = 0.000) Table 5.
 |
Table 5 Relation Between Disease Severity and Inflammatory Markers |
Moreover, inflammatory markers were investigated to detect their relation to the patient’s outcome, which revealed a highly significant increase in median (IQR) of Ferritin in the dead group [417.5 (316–573.5)] when compared with the survived group [322.5 (218.5–506)] (P = 0.001). Figure 2, with the increase in the median (IQR) of CRP in the dead group 78 (48–96) when compared with the survived group [56.5 (42.5–84.5)] (P = 0.002). Also, the median (IQR) of D-dimer was higher in the dead group 3650 (2522–5810) when compared with the survived group [2307.5 (660–3674)] (P = 0.000) Figure 3.
 |
Figure 2 Shows the difference between Ferritin and patients’ outcome (Recovery - death) (P 0.001). |
 |
Figure 3 Shows the difference between D-dimer and patients’ outcome (Recovery - death) (P 0.000). |
Tables 6 and 7 show multiple factors that affected the presence of new-onset diabetes mellitus among the hospitalized patients with COVID-19 that included age, BMI, lymphocyte and inflammatory markers.
 |
Table 6 Correlation Between Presence of Newly Diagnosed DM and Different Clinical and Laboratory Parameters (n = 57) |
 |
Table 7 Logistic Regression Analysis for Predictors of Presence of Newly Diagnosed DM Among COVID-19 Patients (n = 57) |
Kaplan–Meier survival analysis curve showed that the patients with pre-existing DM had no significantly shorter survival time than patients with new-onset DM (P = 0.47) (Table 8, Figure 4).
 |
Table 8 Overall Survival Between Pre-Existing DM and New-Onset DM |
 |
Figure 4 Kaplan–Meier survival analysis according to diabetes status, p-value obtained from Log Rank Mantel–Cox test. |
Discussion
COVID-19 is a worldwide epidemic produced by SARS-CoV-2. It began in Wuhan, China, and swiftly expanded to over 180 countries. Older adults and individuals of any age with underlying medical conditions, such as diabetes, chronic obstructive pulmonary disease (COPD) and hypertension, have shown poor prognosis. Recurrent hospitalization and intensive care unit (ICU) admissions raise the risk of morbidity and mortality in diabetic patients.10
Our study showed that the frequency of newly diagnosed diabetes in COVID-19 individuals was 57 new cases (28.5%). In China, a nationwide study reported a higher prevalence of diabetes among patients with severe COVID-19 as compared to patients with non-severe disease (16.2% vs 5.7%).11 Yang et al also found that the prevalence of diabetes is about 9% by a meta-analysis that included eight studies. The differences between these studies might be related to the number of studies included, sample size, and characteristics of the included patients.12
In our study, a comparison between pre-existing and new-onset DM regarding disease severity revealed no significant difference between the two groups. On the contrary, the study by Farag et al13 reported that in terms of COVID-19 severity, there was a significant difference between newly diagnosed and pre-existing DM groups. This discrepancy could be due to sample size and inclusion criterion differences.
In terms of glycemic findings among the groups tested, we identified a significant difference between the two groups in terms of RBG, FBS, and HBA1c. Online with our results, the study by Farag et al13 revealed that a highly significant difference was found between the studied groups regarding HBA1c, FBS, and fasting insulin.
Regarding hospital stay among the studied groups, we detected a significant difference between the two groups in terms of hospitalization (Day), Wang et al, 202014 reported that there was a positive correlation between the patient’s outcomes and duration of hospital stay, 133 of the 344 patients died on the 28th day with a median survival of 25 days. The median length of hospitalization for a negative test response was 12 days among survival.
We observed no significant difference between the two groups regarding Recovery-Death, the study by Singh and Singh15 reported that in patients with COVID-19, either new-onset diabetes or new-onset hyperglycemia without diabetes is associated with a poor prognosis when compared with persons with normal blood sugar and individuals with old diabetes, another study by Fadini et al, 202016 reported that Patients with diabetes had greater comorbidity burden, the primary outcome occurred in 37.4% of the patients with diabetes compared to 20.3% in those without (RR 1.85; 95%CI 1.33–2.57; p < 0.001), the association was stronger for newly diagnosed compared to pre-existing diabetes (RR 3.06 vs 1.55; p = 0.004). Higher glucose level at admission was associated with COVID-19 severity, with a stronger association among patients without as compared to those with pre-existing diabetes; admission glucose was correlated with most clinical severity indexes.
Limited studies found in literature reported the correlation between hospital stay and the outcome in pre existing and new-onset diabetes in COVID-19 patients.
DM was found to be a predictor of an elevated risk of mortality in individuals with COVID-19, with a mortality rate of 40%, the study by Islam et al17 reported that DM was non-significantly associated with reduced outcomes but highly significantly associated with increased mortality, another study by Bode et al18 found that patients with diabetes or uncontrollable hyperglycemia who were hospitalized with COVID-19 had prolonged hospital stays and a higher risk of death.
In terms of age, our research found a significant difference between the dead and the survivors’ groups; our results were supported by Islam et al,17 who found that older age was significantly associated with poor outcomes and higher mortality rate in Logistic regression analysis of the risk factors associated with mortality and morbidity of COVID-19 patients.
Our findings also revealed a highly significant difference between the three groups (critical, severe, and non-severe) regarding inflammatory markers (ferritin, CRP, and D-dimer); we also found higher inflammatory markers in the dead group than in the survived group. These results were supported by the study of Guo et al, 2020,19 who revealed that inflammatory markers, such as IL-6, CRP, serum ferritin, D-dimer, and coagulation index, were markedly greater in patients with diabetes when compared to non-diabetic persons, implying that the diabetic patients are more liable to an inflammatory storm that leads to deterioration of COVID-19 patients; our result supported by the study of Zhou et al,2 who reported a highly significant difference between two groups (Recovery and Death groups) as regard ferritin and D-dimer. As well, Alguwaihes et al20 revealed that the prevalence of bilateral lung infiltrates, aspartate aminotransferase (AST), mean D-dimer, ferritin, CRP, and lactate dehydrogenase (LDH) were all substantially greater in the severe than in non-severe group when the patients were categorized regarding disease severity.
Conclusion
Finally, in Egypt, the prevalence of new-onset DM is high in hospitalized COVID-19 cases. COVID-19 new-onset DM cases require significantly more hospitalization than pre-existing DM patients, and new-onset DM cases had no significantly higher mortality rate and less recovery rate than pre-existing DM patients. COVID-19 disease was severe in older individuals who had lower recovery and higher mortality. Inflammatory markers, such as serum ferritin, CRP, and D-dimer were strongly linked to COVID-19 severity and clinical outcome, including recovery and mortality.
Further multi-center studies with a larger sample size are needed to study the correlation between diabetes duration and COVID-19 severity and outcome.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Andersen KG, Rambaut A, Lipkin WI, et al. The proximal origin of SARS-CoV-2. Nat Med. 2020;26(4):450–452. doi:10.1038/s41591-020-0820-9
2. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062.
3. Chow N, Fleming-Dutra K, Gierke R, et al. Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019 - United States, February 12-March 28, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(13):382–386. doi:10.15585/mmwr.mm6913e2
4. Chee YJ, Ng SJH, Yeoh E. Diabetic ketoacidosis precipitated by Covid-19 in a patient with newly diagnosed diabetes mellitus. Diabetes Res Clin Pract. 2020;4:108166.
5. Chee YJ, Tan SK, Yeoh E. Dissecting the interaction between COVID‐19 and diabetes mellitus. J Diabetes Investig. 2020;11(5):1104. doi:10.1111/jdi.13326
6. Sardu C, D’onofrio N, Balestrieri ML, et al. Outcomes in patients with hyperglycemia affected by COVID-19: can we do more on glycemic control? Diabetes Care. 2020;43(7):1408–1415. doi:10.2337/dc20-0723
7. Liu S-P, Zhang Q, Wang W. Hyperglycemia is a strong predictor of poor prognosis in COVID-19. Diabetes Res Clin Pract. 2020;167:108338. doi:10.1016/j.diabres.2020.108338
8. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934–943. doi:10.1001/jamainternmed.2020.0994
9. Luzi L, Radaelli MG. Influenza and obesity: its odd relationship and the lessons for COVID-19 pandemic. Acta Diabetol. 2020;57(6):759–764. doi:10.1007/s00592-020-01522-8
10. Singh AK, Gupta R, Ghosh A, et al. Diabetes in COVID-19: prevalence, pathophysiology, prognosis and practical considerations. Diabetes Metab Synd. 2020;14(4):303–310. doi:10.1016/j.dsx.2020.04.004
11. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi:10.1056/NEJMoa2002032
12. Yang J, Zheng Y, Gou X, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. doi:10.1016/j.ijid.2020.03.017
13. Farag AA, Hassanin HM, Soliman HH, et al. Newly diagnosed diabetes in patients with COVID-19: different types and short-term outcomes. Trop Med Infect Dis. 2021;6(3):142. doi:10.3390/tropicalmed6030142
14. Wang Y, Lu X, Li Y, et al. Clinical course and outcomes of 344 intensive care patients with COVID-19. Am J Respir Crit Care Med. 2020;201(11):1430–1434. doi:10.1164/rccm.202003-0736LE
15. Singh AK, Singh R. Hyperglycemia without diabetes and new-onset diabetes are both associated with poorer outcomes in COVID-19. Diabetes Res Clin Pract. 2020;167:108382. doi:10.1016/j.diabres.2020.108382
16. Fadini GP, Morieri ML, Boscari F, et al. Newly-diagnosed diabetes and admission hyperglycemia predict COVID-19 severity by aggravating respiratory deterioration. Diabetes Res Clin Pract. 2020;168:108374. doi:10.1016/j.diabres.2020.108374
17. Islam MZ, Riaz BK, Islam AN, et al. Risk factors associated with morbidity and mortality outcomes of COVID-19 patients on the 28th day of the disease course: a retrospective cohort study in Bangladesh. Epidemiol Infect. 2020;148:546.
18. Bode B, Garrett V, Messler J, et al. Glycemic characteristics and clinical outcomes of COVID-19 patients hospitalized in the United States. J Diabetes Sci Technol. 2020;14(4):813–821. doi:10.1177/1932296820924469
19. Guo W, Li M, Dong Y. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev. 2020;36(7):e3319. doi:10.1002/dmrr.3319
20. Alguwaihes AM, Al-Sofiani ME, Megdad M, et al. Diabetes and Covid- 19 among hospitalized patients in Saudi Arabia: a single-centre retrospective study. Cardiovasc Diabetol. 2020;19(1):1–12. doi:10.1186/s12933-020-01184-4
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
