Back to Journals » Journal of Pain Research » Volume 15
Differences in Structural Brain Characteristics Between Individuals with Chronic Nonspecific Neck Pain and Asymptomatic Controls: A Case–Control Study
Authors de Zoete RMJ , Stanwell P , Weber KA 2nd, Snodgrass SJ
Received 20 October 2021
Accepted for publication 18 December 2021
Published 18 February 2022 Volume 2022:15 Pages 521—531
DOI https://doi.org/10.2147/JPR.S345365
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Houman Danesh
Rutger MJ de Zoete,1,2 Peter Stanwell,2 Kenneth A Weber 2nd,3 Suzanne J Snodgrass2
1School of Allied Health Science and Practice, The University of Adelaide, Adelaide, SA, Australia; 2School of Health Sciences, Faculty of Health and Medicine, The University of Newcastle, Newcastle, NSW, Australia; 3Department of Anesthesiology, Perioperative and Pain Medicine, Stanford University, Palo Alto, CA, USA
Correspondence: Rutger MJ de Zoete, School of Allied Health Science and Practice, The University of Adelaide, North Terrace, Adelaide, SA, 5005, Australia, Email [email protected]
Background: Neck pain is a prevalent and costly problem, but its underlying mechanisms are poorly understood. Neuroimaging studies show alterations in brain morphometry in chronic musculoskeletal pain, but reports on neck pain are scarce.
Objective: This study investigates (1) differences in brain morphometry between individuals with chronic nonspecific neck pain and asymptomatic individuals and (2) associations between brain morphometry and patient-reported outcomes.
Methods: Sixty-three participants (33 pain, 11 female, mean [SD] age 35 [10] years; 30 control, 12 female, age 35 [11] years) underwent magnetic resonance imaging. Brain regions of interest (ROIs) were determined a priori, outcomes included cortical thickness and volume. Between-group differences were determined using cluster-wise correction for multiple comparisons and analyses of pain-related ROIs.
Results: Between-group differences in volume were identified in the precentral, frontal, occipital, parietal, temporal, and paracentral cortices. ROI analyses showed that parahippocampal cortical thickness was larger in the neck pain group (p=0.015, 95% CI: − 0.27 to − 0.03). Moderate to strong associations between volume and thickness of the cingulate cortex, prefrontal cortex, and temporal lobe and neck pain duration, pain intensity, and neck disability were identified (p-values 0.006 to 0.048).
Conclusion: Alterations in brain morphology that are associated with clinical characteristics inform the mechanisms underlying chronic nonspecific neck pain and may guide the development of more effective treatment approaches.
Keywords: neck pain, chronic pain, neuroimaging, brain, MRI
Introduction
Neck pain and back pain are the highest ranked conditions in terms of years-lived-with-disability among 367 conditions studied in the Global Burden of Disease study.32 Neck pain is responsible for a substantial burden to individuals and society.12 In up to 85% of the cases, neck pain becomes recurrent within 5 years of initial onset.54 Nonspecific neck pain of insidious onset, without a known cause and also known as idiopathic neck pain, is the most common type of neck pain.38 Current treatment options for chronic nonspecific neck pain, whilst consistent with clinical guidelines,8 have not been effective in reducing the personal and societal burden.60
Contributing to poor outcomes may be a lack of understanding of the mechanisms underlying chronic neck pain.60 Indeed, a broad range of possible mechanisms, including neurological, genetic, immunologic, are being explored across various pain presentations.21,25,60 A possible association between the brain and chronic pain may be investigated with neuroimaging technologies, allowing for investigation of the structural characteristics of brain tissue. Through T1-weighted magnetic resonance imaging (MRI), implications of the central nervous system have been found through a range of morphological alterations, and these may inform on the potential cause of chronic pain, and provide potential targets for treatment.
In chronic pain conditions, including low back pain,2,36,47,55,69 fibromyalgia,10,41 musculoskeletal pain,15,35,68 and chronic pain in general,57 alterations in structural properties of the brain are observed. These alterations, apparent in both cortical and subcortical brain regions, have been identified particularly in regions involved in the central processing of pain,43 such as the prefrontal cortex,10,15,19,35,36,47,57,69 cingulate cortex,10,15,19,47,57,69 insula,10,15,36,47,57,69 superior temporal gyrus,15,57 and thalamus.10,47,57 Individuals with neck pain appear to demonstrate similar alterations in brain morphometry19; however, brain morphometry has not been comprehensively investigated in individuals with neck pain.19 Potential differences between traumatic and non-traumatic neck pain have been identified based on few studies,5,16,17 yet little information on nonspecific neck pain is available, requiring further investigation.
Altered brain morphometry may be related to patient symptoms. For example, increased pain intensity and sensitivity were associated with reduced grey matter in temporal gyrus and cingulate, prefrontal, and insular cortices in a mixed chronic musculoskeletal pain sample.15 Longitudinal investigation of grey matter in individuals with low back pain found a reduction in volume of the insula, primary motor cortex, and somatosensory cortex, as sub-acute pain transitioned into chronic pain.4 Successful treatment of chronic low back pain was associated with increased cortical thickness of the dorsolateral prefrontal cortex, which was also correlated with improvements in pain intensity and pain-related disability.55 These findings suggest that treatments for low back pain may consider incorporating treatment that addresses central maladaptive processes, and that brain morphometry may be a useful biomarker to evaluate the success of intervention approaches. Yet few studies have examined brain morphometry in individuals with chronic neck pain.
The aims of this study were to investigate (1) differences in structural brain characteristics between individuals with chronic nonspecific neck pain and asymptomatic individuals and (2) associations between structural brain characteristics and pain intensity, pain-related disability, pain duration and depression. Based on available literature in other chronic pain conditions, we hypothesize that differences in structural brain characteristics will be present between individuals with chronic nonspecific neck pain and asymptomatic individuals.
Methods
Design
This article reports data compiled from two pilot studies approved by the Human Research Ethics Committee at The University of Newcastle (H-2013-0416, H-2014-0233) and complies with the Declaration of Helsinki. All participants provided written informed consent prior to their participation. Participants with chronic neck pain, as well as age- and sex-matched asymptomatic control participants, were included after providing written informed consent. This article follows the STROBE reporting guidelines.65
Participants
Participants with chronic nonspecific neck pain (≥12 weeks’ duration) were recruited from the community and considered for inclusion if they reported neck pain as primary complaint. Neck pain intensity (assessed on a 0–10 numeric rating scale [NRS]) had to be at least 4/10 at time of the screening, disability (Short-Form 12, question 5: “During the past 4 weeks, how much did your pain interfere with your normal work [including both work outside the home and housework]?”) had to be at least “moderate”.66 Eligible participants were between 18 and 55 years old. The lower age limit was chosen because the brain in children or adolescents is still in development,62 the upper age limit was chosen because degenerative brain changes associated with aging may influence outcomes.28
Asymptomatic participants were considered for the control group if they had no current neck pain, nor had sought treatment for neck/shoulder complaints in the past 5 years. Control participants were not to have had a history of injury or trauma to the neck or head, and no current chronic musculoskeletal pain in any body area. Similar to the neck pain group, participants in the control group had to be between 18 and 55 years old. Participants in the control group were individually matched to participants in the neck pain group based on sex and age to ensure equal groups.
Individuals with neck pain were excluded if their primary complaint was headaches or dizziness, if they reported (a history of) migraine headaches, had trauma/surgery to the neck, or had diabetes, peripheral vascular disease or inflammatory disease. Neuropathic pain (score of ≥10 on the Self-Reported Leeds Assessment of Neuropathic Symptoms and Signs [S-LANSS])7 was also excluded. Exclusion criteria for both groups included pregnancy/breastfeeding, claustrophobia, and ferromagnetic implants or other contraindications to MRI. People reporting long-term steroid use or current use of anticoagulant medication were excluded. People were not excluded if using pain medication, rather, they were asked to continue their normal use of pain relief as prescribed.
This was a convenience sample, and sample size calculations were not performed a priori. Previous studies found statistically significant between-group differences in sample sizes ranging 18 to 46 per group in similar populations. We estimated that we could detect a between-group difference using a sample size of 30 per group.19
Data Collection
All participants completed the following questionnaires: the Short Form 12 (SF-12),14 the Godin-Shephard Leisure-Time Physical Activity Questionnaire assessed physical activity levels,26 and the Center for Epidemiologic Studies Depression Scale (CESD-10) assessed the potential presence of depression.51 Data from the Godin-Shephard Leisure-Time Physical Activity Questionnaire were categorized into “insufficient”, “moderate”, and “active”,26 and CESD-10 outcomes were dichotomized into either depressed (“having depressive symptoms”, scores ≥10) or not depressed (“not having depressive symptoms”).1 Pain duration data was collected for the neck pain group.
Participants with neck pain also provided their neck pain intensity using a 0–100mm Visual Analogue Scale (VAS) anchored by “no pain” on the left and “worst pain imaginable” on the right. Three recall periods (current, 24 hours, and 4 weeks) were used because individuals with neck pain have shown to complete the VAS differently with different recall periods.34 Disability due to neck pain was assessed with the Neck Disability Index (NDI), a reliable and valid tool.64 For the description of the sample of participants with neck pain, we also report the Pain Management Inventory (assesses both active and passive pain coping),18 the Pain Catastrophizing Scale (assessed rumination, magnification, and helplessness),61 and cervical range of motion. Cervical range of motion was assessed using a Cervical Range of Motion (CROM) device (CROM, Performance Attainment Associates, Minnesota, IL, USA),3 for movement in four directions: flexion, extension, left rotation, and right rotation. Three measures per direction were taken, then averaged.
Magnetic Resonance Imaging Acquisition and Processing
All scans were performed using a Siemens Magnetom Prisma 3-Tesla scanner equipped with a 64-channel head coil. For all participants, structural images were examined by a radiologist to confirm the absence of abnormalities. Structural T1-weighted images were acquired using sagittal volumetric magnetisation prepared rapid gradient echo (MP-RAGE) with the following settings: voxel size = 1.0mm3, repetition time (TR) = 2530.0ms, echo time (TE) = 3.5ms, field of view (FOV) = 256mm, slice thickness = 1.0mm, 176 slices per slab, flip angle = 7°.
Morphometric segmentation and parcellation were performed using structural T1-weighted images with the Freesurfer image analysis suite version 6, which is documented and freely available for download (http://surfer.nmr.mgh.harvard.edu/). The longitudinal image analysis pipeline is an accurate procedure in quantifying volumetric properties,44 has been validated against histological53 and manual37 analyses, and shows good test–retest reliability.30,52 The quality of the outputs of the Freesurfer processing pipeline were manually checked for errors using Freeview. Based on the quality check, none of the outputs were manually adjusted. This approach ensures consistency across the sample, and it has been demonstrated that there are no differences between the automated method and the automated method plus manual edits.11,27,44,67
Based on a literature search, we selected the following brain regions for analysis a priori: thalamus, prefrontal cortex (subregions: medial and lateral orbitofrontal, pars opercularis, pars triangularis, rostral middle frontal, superior frontal cortex), insula, cingulate cortex (subregions: caudal anterior cingulate, isthmus cingulate, posterior cingulate, rostral anterior cingulate), temporal lobe (subregions: parahippocampus, superior temporal), cuneus, primary somatosensory cortex, and primary motor cortex.
Statistical Analyses
All results are presented as mean (SD). Using Freesurfer, cluster-wise correction for multiple comparisons was used for group analysis of volume and cortical thickness.29 The following parameters were used: vertex-wise threshold: p=0.05; cached cluster-wise correction threshold: p=0.05; inclusion of correction for left and right hemispheres; and two-step cluster-smoothing. Then, volume and cortical thickness measures were extracted for secondary analysis of individual ROIs. For these ROIs, t-tests were used to investigate between-group differences. Stepwise linear regression was used to investigate correlations between volume and thickness measures and pain intensity (VAS), duration (months), disability (NDI scored out of 50), and depression (dichotomized). For the regression analysis, firstly the interrelation between covariates was examined using Pearson r. Interdependent covariates were removed. After this, a linear regression model was fitted with all remaining independent covariates included. The least significant covariate was omitted from the model, and repeated until only statistically significant covariates remained, or until all had been eliminated. Stepwise linear regressions were only performed for the neck pain group because several outcomes, including pain intensity, neck disability, were not assessed in the control group. Sex and age were included as covariates in all analyses. All statistical analyses were conducted with IBM SPSS Statistics version 27 (IBM Corporation, Armonk, NY).
Results
Participants
Between May 2015 and May 2017, 151 volunteers with neck pain were screened for inclusion, and 33 enrolled. Thirty asymptomatic volunteers were enrolled as control participants when their sex matched a participant with neck pain and their age was within 5 years of that participant. Participant characteristics for all 63 participants are presented in Table 1. For the statistical analyses presented in this study, we have used the VAS for a 4-week recall period, as this outcome had the most variability among the sample. This is consistent with other research reported elsewhere.9,20,22,58,59
 |
Table 1 Participant Characteristics. Outcomes are Reported as Mean (SD) |
Between-Group Differences
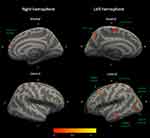
The cluster-wise corrected group analyses revealed significant differences in cortical volumes between the two groups. No significant cluster-wise corrected group differences were identified for cortical thickness. For the ROI thickness analysis, parahippocampal cortical thickness at the right hemisphere was larger (p=0.015, 95% CI: −0.27 to −0.03) in the neck pain group (2.83±0.23 mm) compared to the control group (2.68±0.25 mm). The effect size (Cohen’s d) for this between-group difference was −0.631 (95% CI −1.14 to −0.12) Table 2 presents all significant between-group volumetric differences identified through the cluster-wise corrected group analysis, also illustrated in Figure 1.
 |
Table 2 Significant Between-Group Differences in Cortical Volumetric Properties Identified Through Cluster-Wise Corrected Group Analysis Using Freesurfer |
Associations with Clinical Characteristics
Most structural brain characteristics (including volume and cortical thickness) were associated with sex (ROI volume/thickness greater in males: 50 out of 66 regression models, 76%) and/or negatively associated with age (ROI volume/thickness smaller in older age: 53/66 regression models, 80%). Therefore, age and sex were included as covariates in all subsequent models examining relationships between brain volume/thickness and pain intensity, pain duration, pain-related disability, and depression. Table 3 provides all associations between ROIs and clinical outcomes.
 |
Table 3 Significant Associations Between Structural Properties of Regions of Interest and Clinical Characteristics in the Neck Pain Group |
Discussion
This study investigated structural brain properties between individuals with chronic nonspecific neck pain and asymptomatic controls, as well as the associations between brain properties and clinical outcomes in those with neck pain. Cluster-wise corrected group analysis demonstrated cortical volume differences between the two groups in the precentral, frontal, parietal, occipital, and temporal cortices, and further ROI analysis indicated that right parahippocampal cortical thickness was larger in the neck pain group. Significant associations were found between morphology of the prefrontal cortex, cingulate cortex, temporal lobe, and clinical outcomes including neck pain intensity, pain-related disability, pain duration, and the presence of depression. Negative correlations between brain volume or thickness and pain intensity or disability demonstrate a relationship between brain regions with less volume or thickness and worse symptoms. As the brain is plastic it can change in response to inputs. This may suggest that brain morphometry (volume or thickness) may be a useful biomarker for evaluating the severity of a neck pain condition. Longitudinal studies could determine if brain morphometry can predict chronicity of neck pain, and whether it is useful as an objective assessment tool to evaluate clinical improvement. These results support preliminary findings suggesting that brain morphology may be altered in individuals with chronic nonspecific neck pain and that there may be a relationship between these alterations and symptom presentation.16,17
Cluster-wise group analysis showed that several brain regions were smaller in individuals with chronic neck pain: the frontal cortex, parietal cortex, occipital cortex, precentral cortex, paracentral cortex, temporal cortex, and precuneus. More between-group differences were found in the left hemisphere compared to the right hemisphere, the reason for the observed laterality is unknown. Through the ROI analysis, however, within the temporal lobe, right parahippocampal cortical thickness was larger in the neck pain group compared to the asymptomatic control group. The temporal lobe may be relevant to chronic pain as this brain region is involved with the encoding of memory information and the processing of emotions. Interestingly, this between-group difference reflected larger cortical thickness in the neck pain group compared to the control group. This contrasts with studies in fibromyalgia, where typically smaller morphometric properties of brain regions involved with the central processing of pain are observed. For example, a systematic review on brain structural brain imaging in individuals with fibromyalgia found that parahippocampal grey matter volume was decreased.10 Similarly, a meta-analysis of voxel-based morphometric studies in individuals with fibromyalgia found decreased grey matter properties in the parahippocampus.56 A possible explanation for this, noted by Cagnie et al,10 might be that the hippocampus is associated with the central processing of stress. Implication of this ROI is therefore more likely in those chronic pain conditions in which stress, anxiety, and depression are more prevalent. In addition, due to its neuropathic nature, fibromyalgia may not be a suitable comparison to our nonspecific neck pain sample, in which fibromyalgia and any type of neuropathic pain were excluded. A third review article with meta-analysis found increased parahippocampal volume in a mixed chronic pain cohort (including a variety of musculoskeletal, rheumatoid, and neuropathic pain).57 Lastly, parahippocampus morphometry has been shown to be affected by long-term alcohol use, which was not assessed in this study and may therefore influence these findings if pain participants perhaps used alcohol as a coping mechanism more often than asymptomatic participants.45
Most brain characteristics were associated with age and sex. All associations indicated that at an older age, brain regions presented smaller volumes and thickness. In addition, males in the current study had larger volumes and thickness of brain regions than females. This indicates that findings from this study are consistent with published literature with regards to age and sex differences. This was the reason for matching participants based on sex and age, and for including these covariates in analyses. Through the linear regression models, we identified several associations between brain characteristics and clinical outcomes. We found that cingulate, prefrontal cortex, and temporal lobe were associated with neck pain intensity, pain-related disability, and the presence of depression. This is in line with recently reported associations between neuroimaging findings and cognition, central sensitisation symptoms, and hyperalgesia in people with traumatic neck pain.15 Some evidence is available suggesting that hippocampal, striatal, and limbic functional and structural connectivity can predict risk for chronic back pain, yet evidence for neck pain is lacking.4,46,63 Associations found in this study are outlined below in the context of available literature.
Within the cingulate cortex, a brain region part of the limbic system and responsible for the processing of emotions, learning, and memory, we found that smaller volume of the left rostral anterior cingulate cortex was associated with greater pain intensity. A larger right caudal anterior cingulate cortex volume was associated with higher pain intensity – which is a correlation in the opposite direction from those found for the rostral anterior cingulate cortex Most available studies report a decreased volume in the cingulate cortex in those with various chronic pain conditions,6,10,13,19,23,31,33,39,40,47,56,57,68–70 though one study reports inconclusive findings.36 Whilst this study did not find between-group differences in cingulate cortex volume, the associations between smaller thickness of the right rostral anterior cingulate cortex and greater pain intensity and depression fit previous findings.
Associations between characteristics of the prefrontal cortex and clinical outcomes were found in the smaller volume of the left medial orbitofrontal cortex and smaller thickness of the right lateral orbitofrontal cortex, both associated with greater neck disability (β= −0.309, p=0.048 and β= −0.359, p=0.041, respectively), and associated with the presence of depression (β = 0.402, p=0.024). The negative associations, indicating that a smaller volume is associated with higher perceived disability, are in line with available literature indicating smaller morphometric properties of the prefrontal cortex in those with chronic pain.6,10,13,19,31,33,36,39,40,47,49,56,57,68,69 While these associations are unable to determine cause or effect, there is some evidence that brain volume changes in response to intervention. Hippocampus volume has been shown to increase following exercise24 with associated increases in memory. This suggests that some types of clinical interventions may result in changes to brain volume. Future studies are needed to show if clinical interventions for chronic pain may affect brain volume.
Within the temporal lobe, larger thickness of the right parahippocampus was associated with greater duration of neck pain, and reduced thickness of the left superior temporal cortex was associated with having depressive symptoms. This is consistent with recent literature, indicating that individuals with various chronic pain conditions demonstrated smaller morphometric properties in the temporal cortex when depression was present.6,31,36,47,57,68
The clinical implications of neuroimaging investigations in chronic neck pain are multifold. Firstly, if patients present with pain related to altered central processes, clinicians should choose management strategies to address central mechanisms rather than merely peripheral structures. If successful, apparent associations between brain structure and patient-reported outcomes would mean pain intensity, pain-related disability, and psychological status might improve. Secondly, perhaps neuroimaging could be used to investigate which acute pain patients might be susceptible to chronicity,42 or to predict which pain patients might respond well to a proposed intervention – and which will not. More work will be required to answer these questions, however the potential impact is promising.
Limitations
Whereas other studies frequently report on retrospectively created samples from mixed populations, our prospectively designed study recruited a specific sample for the sole purpose of research with strict inclusion and exclusion criteria. This means the included participants are a homogenous representation of nonspecific neck pain. We did not ask participants stop taking medication ahead of their clinical assessment or acquisition of their MRI scan, hence this may have affected the results. Whilst we selected the VAS using a 4-week recall period for statistical analyses, we note that the VAS for current pain and the 24-hour recall period returned lower pain intensities (30.2±18.8 and 38.0±20.3, respectively). This may indicate that pain intensities were lower at the time of clinical testing and MRI acquisition and should therefore be taken in consideration in interpreting the findings.
We note that the number of brain regions and secondary outcomes included in the statistical analyses introduces the risk of a Type I error; however, the cluster-wise analysis in Freesurfer corrects for multiple comparisons. Furthermore, the results are consistent with previously reported findings. Whilst the brain regions were selected a priori informed by available literature, this was based on studies investigating various pain populations. As the selected brain regions showed promising results here, these regions should be included in future studies of nonspecific neck pain.
Whilst valid comparisons with available literature were made, it should be recognized that interpretation and comparisons of outcomes from different neuroimaging studies incurs technical challenges. At the acquisition stage, potential differences in magnetic field strength, voxel resolution, and other parameters may be responsible for differences in images. For example, our study using a voxel size of 1.0mm3 found a between-group difference in parahippocampal thickness of 0.15mm. With a less precise voxel size, these findings might have been overlooked. At image processing stage, differences may be introduced through software modalities used, eg, Freesurfer has shown most accuracy in parcellation of cortical structures, but less accuracy in segmentation of subcortical structures.50 The deep positioning of the parahippocampus, combined with its susceptibility to imaging artefacts,48 should also be considered. These combined limitations, as well as retrospective studies often including heterogeneous samples from different clinical works, may result in studies not being able to detect small differences, if present, and could contribute to different results.
Conclusion
We found differences in precentral, frontal, parietal, occipital, and temporal cortical volume and thickness between individuals with chronic nonspecific neck pain and asymptomatic controls. Within the neck pain group, we also found several associations between volume and thickness of the cingulate cortex, prefrontal cortex, temporal lobe, and neck pain intensity, pain-related disability, pain duration, and the presence of depression. These results demonstrate that brain neuroimaging may provide relevant information on central processes, potentially representing mechanisms underlying chronic nonspecific neck pain. Management approaches addressing central maladaptive processes could improve treatment effectiveness for individuals with chronic neck pain.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was funded by the Ramaciotti Foundation and the Australian Spinal Research Foundation.
Disclosure
Dr Kenneth A Weber 2nd reports grants from NIH, during the conduct of the study. Dr Suzanne J Snodgrass reports grants from Ramaciotti Foundation (Australia) and Australian Spinal Research Foundation, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D. Am J Prev Med. 1994;10(2):77–84. doi:10.1016/S0749-3797(18)30622-6
2. Apkarian AV, Sosa Y, Sonty S, et al. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci. 2004;24(46):10410–10415. doi:10.1523/JNEUROSCI.2541-04.2004
3. Audette I, Dumas J-P, Côté JN, De Serres SJ. Validity and between-day reliability of the Cervical Range of Motion (CROM) device. J Orthop Sports Phys Ther. 2010;40(5):318–323. doi:10.2519/jospt.2010.3180
4. Baliki MN, Petre B, Torbey S, et al. Corticostriatal functional connectivity predicts transition to chronic back pain. Nat Neurosci. 2012;15(8):1117–1119. doi:10.1038/nn.3153
5. Baliki MN, Mansour AR, Baria AT, Apkarian AV. Functional reorganization of the default mode network across chronic pain conditions. PLoS One. 2014;9(9):e106133. doi:10.1371/journal.pone.0106133
6. Bashir A, Lipton RB, Ashina S, Ashina M. Migraine and structural changes in the brain: a systematic review and meta-analysis. Neurology. 2013;81(14):1260–1268. doi:10.1212/WNL.0b013e3182a6cb32
7. Bennett MI, Smith BH, Torrance N, Potter J. The S-LANSS score for identifying pain of predominantly neuropathic origin: validation for use in clinical and postal research. J Pain. 2005;6(3):149–158. doi:10.1016/j.jpain.2004.11.007
8. Bier JD, Scholten‐Peeters WGM, Staal JB, et al. Clinical practice guideline for physical therapy assessment and treatment in patients with nonspecific neck pain. Phys Ther. 2018;98(3):162–171. doi:10.1093/ptj/pzx118
9. Blyton SJ, Edwards S, Moghaddas D, et al. A pilot longitudinal study of 3-dimensional head and neck kinematics during functional tasks in individuals with chronic idiopathic neck pain either wait-listed for or receiving chiropractic spinal manipulative therapy with exercise. J Manipulative Physiol Ther. 2020;43(5):490–505. doi:10.1016/j.jmpt.2019.01.003
10. Cagnie B, Coppieters I, Denecker S, Six J, Danneels L, Meeus M. Central sensitization in fibromyalgia? A systematic review on structural and functional brain MRI. Semin Arthritis Rheum. 2014;44(1):68–75. doi:10.1016/j.semarthrit.2014.01.001
11. Canna A, Russo AG, Ponticorvo S, et al. Automated search of control points in surface-based morphometry. Neuroimage. 2018;176:56–70. doi:10.1016/j.neuroimage.2018.04.035
12. Carroll LJ, Hogg-Johnson S, Côté P, et al. Course and prognostic factors for neck pain in workers. Eur Spine J. 2008;17(S1):S93–S100. doi:10.1007/s00586-008-0629-6
13. Cauda F, Palermo S, Costa T, et al. Gray matter alterations in chronic pain: a network-oriented meta-analytic approach. Neuroimage Clin. 2014;4:676–686. doi:10.1016/j.nicl.2014.04.007
14. Cheak-Zamora NC, Wyrwich KW, McBride TD. Reliability and validity of the SF-12v2 in the medical expenditure panel survey. Qual Life Res. 2009;18(6):727–735. doi:10.1007/s11136-009-9483-1
15. Coppieters I, Meeus M, Kregel J, et al. Relations between brain alterations and clinical pain measures in chronic musculoskeletal pain: a systematic review. J Pain. 2016;17(9):949–962. doi:10.1016/j.jpain.2016.04.005
16. Coppieters I, De Pauw R, Caeyenberghs K, et al. Differences in white matter structure and cortical thickness between patients with traumatic and idiopathic chronic neck pain: associations with cognition and pain modulation? Hum Brain Mapp. 2018;39(4):1721–1742. doi:10.1002/hbm.23947
17. Coppieters I, De Pauw R, Caeyenberghs K, et al. Decreased regional grey matter volume in women with chronic whiplash-associated disorders: relationships with cognitive deficits and disturbed pain processing. Pain Physician. 2017;20:E1025–E51. doi:10.36076/ppj/2017.7.E1025
18. Davis GC, Atwood JR. The development of the pain management inventory for patients with arthritis. J Adv Nurs. 1996;24(2):236–243. doi:10.1111/j.1365-2648.1996.tb02865.x
19. De Pauw R, Coppieters I, Meeus M, Caeyenberghs K, Danneels L, Cagnie B. Is traumatic and non-traumatic neck pain associated with brain alterations? – a systematic review. Pain Physician. 2017;20:245–260. doi:10.36076/ppj.2017.260
20. de Zoete RMJ, Osmotherly PG, Rivett DA, Snodgrass SJ. Cervical sensorimotor control does not change over time and is not related to chronic idiopathic neck pain characteristics: a 6-month longitudinal observational study. J Orthop Sports Phys Ther. 2020;100(2):268–282.
21. de Zoete RMJ, Chen K, Sterling M. Central neurobiological effects of physical exercise in individuals with chronic musculoskeletal pain: a systematic review. BMJ Open. 2020;10(7):e036151. doi:10.1136/bmjopen-2019-036151
22. de Zoete RMJ, Osmotherly PG, Rivett DA, Snodgrass SJ. No differences between individuals with chronic idiopathic neck pain and asymptomatic individuals on 7 cervical sensorimotor control tests: a cross-sectional Study. J Orthop Sports Phys Ther. 2020;50(1):33–43. doi:10.2519/jospt.2020.8846
23. Dehghan M, Schmidt-Wilcke T, Pfleiderer B, et al. Coordinate-based (ALE) meta-analysis of brain activation in patients with fibromyalgia. Hum Brain Mapp. 2016;37(5):1749–1758. doi:10.1002/hbm.23132
24. Erickson KI, Voss MW, Prakash RS, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci USA. 2011;108(7):3017–3022. doi:10.1073/pnas.1015950108
25. Farrell SF, de Zoete R, Cabot PJ, Sterling M. Systemic inflammatory markers in neck pain: a systematic review with meta-analysis. Eur J Pain. 2020;24(9):1666–1686. doi:10.1002/ejp.1630
26. Godin G. The godin-shephard leisure-time physical activity questionnaire. Health Fit J Can. 2011;4(1):18–22.
27. Guenette JP, Stern RA, Tripodis Y, et al. Automated versus manual segmentation of brain region volumes in former football players. Neuroimage Clin. 2018;18:888–896. doi:10.1016/j.nicl.2018.03.026
28. Haga KK, Khor YP, Farrall A, Wardlaw JM. A systematic review of brain metabolite changes, measured with 1H magnetic resonance spectroscopy, in healthy aging. Neurobiol Aging. 2009;30(3):353–363. doi:10.1016/j.neurobiolaging.2007.07.005
29. Hagler DJ
30. Han X, Jovicich J, Salat D, et al. Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. Neuroimage. 2006;32(1):180–194. doi:10.1016/j.neuroimage.2006.02.051
31. Henssen D, Dijk J, Knepflé R, Sieffers M, Winter A, Vissers K. Alterations in grey matter density and functional connectivity in trigeminal neuropathic pain and trigeminal neuralgia: a systematic review and meta-analysis. Neuroimage Clin. 2019;24:102039. doi:10.1016/j.nicl.2019.102039
32. Hurwitz EL, Randhawa K, Yu H, Côté P, Haldeman S. The global spine care initiative: a summary of the global burden of low back and neck pain studies. Eur Spine J. 2018;27(Suppl 6):796–801. doi:10.1007/s00586-017-5432-9
33. Jia Z, Yu S. Grey matter alterations in migraine: a systematic review and meta-analysis. Neuroimage Clin. 2017;14:130–140. doi:10.1016/j.nicl.2017.01.019
34. Kamper SJ, Grootjans SJ, Michaleff ZA, Maher CG, McAuley JH, Sterling M. Measuring pain intensity in patients with neck pain: does it matter how you do it? Pain Pract. 2015;15(2):159–167.
35. Kregel J, Coppieters I, DePauw R, et al. Does conservative treatment change the brain in patients with chronic musculoskeletal pain? A systematic review. Pain Physician. 2017;20(3):139–154.
36. Kregel J, Meeus M, Malfliet A, et al. Structural and functional brain abnormalities in chronic low back pain: a systematic review. Semin Arthritis Rheum. 2015;45(2):229–237. doi:10.1016/j.semarthrit.2015.05.002
37. Kuperberg GR, Broome MR, McGuire PK, et al. Regionally localized thinning of the cerebral cortex in schizophrenia. Arch Gen Psychiatry. 2003;60(9):878–888. doi:10.1001/archpsyc.60.9.878
38. L’Hereux-Lebeau B, Godbout A, Berbiche D, Saliba I. Evaluation of paraclinical tests in the diagnosis of cervicogenic dizziness. Otol Neurotol. 2014;35(10):1858–1865. doi:10.1097/MAO.0000000000000506
39. Lin C, Lee SH, Weng HH. Gray matter atrophy within the default mode network of fibromyalgia: a meta-analysis of voxel-based morphometry studies. Biomed Res Int. 2016;2016:7296125. doi:10.1155/2016/7296125
40. Lin CS, Costigan M. Brain signature of chronic orofacial pain: a systematic review and meta-analysis on neuroimaging research of trigeminal neuropathic pain and temporomandibular joint disorders. PLoS One. 2014;9(4):e94300. doi:10.1371/journal.pone.0094300
41. Lutz J, Jager L, de Quervain D, et al. White and gray matter abnormalities in the brain of patients with fibromyalgia: a diffusion-tensor and volumetric imaging study. Arthritis Rheum. 2008;58(12):3960–3969. doi:10.1002/art.24070
42. Mansour AR, Baliki MN, Huang L, et al. Brain white matter structural properties predict transition to chronic pain. Pain. 2013;154(10):2160–2168. doi:10.1016/j.pain.2013.06.044
43. Martucci KT, Mackey SC. Neuroimaging of pain: human evidence and clinical relevance of central nervous system processes and modulation. Anesthesiology. 2018;128(6):1241–1254. doi:10.1097/ALN.0000000000002137
44. McCarthy CS, Ramprashad A, Thompson C, Botti JA, Coman IL, Kates WR. A comparison of FreeSurfer-generated data with and without manual intervention. Front Neurosci. 2015;9:379. doi:10.3389/fnins.2015.00379
45. Meda SA, Hawkins KA, Dager AD, et al. Longitudinal effects of alcohol consumption on the hippocampus and parahippocampus in college students. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3(7):610–617. doi:10.1016/j.bpsc.2018.02.006
46. Mutso AA, Petre B, Huang L, et al. Reorganization of hippocampal functional connectivity with transition to chronic back pain. J Neurophysiol. 2014;111(5):1065–1076. doi:10.1152/jn.00611.2013
47. Ng SK, Urquhart DM, Fitzgerald PB, Cicuttini FM, Hussain SM, Fitzgibbon BM. The relationship between structural and functional brain changes and altered emotion and cognition in chronic low back pain brain changes: a systematic review of MRI and fMRI Studies. Clin J Pain. 2018;34(3):237–261. doi:10.1097/AJP.0000000000000534
48. Olman CA, Davachi L, Inati S. Distortion and signal loss in medial temporal lobe. PLoS One. 2009;4(12):e8160. doi:10.1371/journal.pone.0008160
49. Pan PL, Zhong JG, Shang HF, et al. Quantitative meta-analysis of grey matter anomalies in neuropathic pain. Eur J Pain. 2015;19(9):1224–1231. doi:10.1002/ejp.670
50. Perlaki G, Horvath R, Nagy SA, et al. Comparison of accuracy between FSL’s FIRST and Freesurfer for caudate nucleus and putamen segmentation. Sci Rep. 2017;7(1):2418. doi:10.1038/s41598-017-02584-5
51. Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. doi:10.1177/014662167700100306
52. Reuter M, Schmansky NJ, Rosas HD, Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage. 2012;61(4):1402–1418. doi:10.1016/j.neuroimage.2012.02.084
53. Rosas HD, Liu AK, Hersch S, et al. Regional and progressive thinning of the cortical ribbon in Huntington’s disease. Neurology. 2002;58(5):695–701. doi:10.1212/WNL.58.5.695
54. Safiri S, Kolahi AA, Hoy D, et al. Global, regional, and national burden of neck pain in the general population, 1990–2017: systematic analysis of the global burden of disease study 2017. BMJ. 2020;368:m791. doi:10.1136/bmj.m791
55. Seminowicz DA, Wideman TH, Naso L, et al. Effective treatment of chronic low back pain in humans reverses abnormal brain anatomy and function. J Neurosci. 2011;31(20):7540–7550. doi:10.1523/JNEUROSCI.5280-10.2011
56. Shi H, Yuan C, Dai Z, Ma H, Sheng L. Gray matter abnormalities associated with fibromyalgia: a meta-analysis of voxel-based morphometric studies. Semin Arthritis Rheum. 2016;46(3):330–337. doi:10.1016/j.semarthrit.2016.06.002
57. Smallwood R, Laird A, Ramage A, et al. Structural brain anomalies and chronic pain: a quantitative meta-analysis of gray matter volume. J Pain. 2013;14(7):663–675. doi:10.1016/j.jpain.2013.03.001
58. Snodgrass SJ, de Zoete RMJ, Croker C, Yerrapothu M, Elliott JM. Reliability of cervical muscle volume quantification using magnetic resonance imaging. Musculoskelet Sci Pract. 2019;44:102056. doi:10.1016/j.msksp.2019.102056
59. Snodgrass SJ, Croker C, Yerrapothu M, et al. Cervical muscle volume in individuals with idiopathic neck pain compared to asymptomatic controls: a cross-sectional magnetic resonance imaging study. Musculoskelet Sci Pract. 2019;44:102050. doi:10.1016/j.msksp.2019.102050
60. Sterling M, de Zoete RMJ, Coppieters I, Farrell SF. Best evidence rehabilitation for chronic pain part 4: neck pain. J Clin Med. 2019;8(8):1219. doi:10.3390/jcm8081219
61. Sullivan MJL, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess. 1995;7(4):524–532. doi:10.1037/1040-3590.7.4.524
62. Tau GZ, Peterson BS. Normal development of brain circuits. Neuropsychopharmacology. 2010;35(1):147–168. doi:10.1038/npp.2009.115
63. Vachon-Presseau E, Tétreault P, Petre B, et al. Corticolimbic anatomical characteristics predetermine risk for chronic pain. Brain. 2016;139(Pt 7):1958–1970. doi:10.1093/brain/aww100
64. Vernon H, Mior S. The neck disability index: a study of reliability and validity. J Manipulative Physiol Ther. 1991;14(7):409–415.
65. von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–349. doi:10.1016/j.jclinepi.2007.11.008
66. Ware J
67. Waters AB, Mace RA, Sawyer KS, Gansler DA. Identifying errors in Freesurfer automated skull stripping and the incremental utility of manual intervention. Brain Imaging Behav. 2019;13(5):1281–1291. doi:10.1007/s11682-018-9951-8
68. Yin Y, He S, Xu J, et al. The neuro-pathophysiology of temporomandibular disorders-related pain: a systematic review of structural and functional MRI studies. J Headache Pain. 2020;21(1):78. doi:10.1186/s10194-020-01131-4
69. Yuan C, Shi H, Pan P, et al. Gray matter abnormalities associated with chronic back pain: a meta-analysis of voxel-based morphometric studies. Clin J Pain. 2017;33(11):983–990. doi:10.1097/AJP.0000000000000489
70. Zhang C, Hu H, Das SK, et al. Structural and functional brain abnormalities in trigeminal neuralgia: a systematic review. J Oral Facial Pain Headache. 2020;34(3):222–235. doi:10.11607/ofph.2626
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.