Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 18
Differences in Characteristics Between Physical Frailty Assessments in Chronic Obstructive Pulmonary Disease: A Multicenter Cross-Sectional Observational Study
Authors Tanaka Y , Hanada M , Kitagawa C, Suyama K, Shiroishi R, Rikitomi N, Tsuda T , Utsunomiya Y, Tanaka T , Shingai K, Yanagita Y , Kozu R
Received 27 January 2023
Accepted for publication 14 May 2023
Published 22 May 2023 Volume 2023:18 Pages 945—953
DOI https://doi.org/10.2147/COPD.S405894
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Min Zhang
Yasutomo Tanaka,1,2 Masatoshi Hanada,1,2 Chika Kitagawa,3 Kazuaki Suyama,4 Ryota Shiroishi,5 Naoto Rikitomi,3 Toru Tsuda,6 Yoshiaki Utsunomiya,5 Takako Tanaka,1 Kazuya Shingai,1 Yorihide Yanagita,7 Ryo Kozu1,2
1Department of Physical Therapy Science, Nagasaki University Graduate School of Biomedical Sciences, Nagasaki, Japan; 2Department of Rehabilitation Medicine, Nagasaki University Hospital, Nagasaki, Japan; 3Nagasaki Pulmonary Rehabilitation Clinic, Nagasaki, Japan; 4Tagami Hospital, Nagasaki, Japan; 5Utsunomiya Medical Clinic, Fukuoka, Japan; 6Kirigaoka Tsuda Hospital, Fukuoka, Japan; 7Department of Physical Therapy, School of Health Science, Toyohashi Sozo University, Aichi, Japan
Correspondence: Ryo Kozu, Department of Physical Therapy Science, Nagasaki University Graduate School of Biomedical Sciences, 1-7-1 Sakamoto, Nagasaki, 852-8520, Japan, Tel/Fax +81 95 819 7963, Email [email protected]
Purpose: Assessment for frailty is important as it enables timely intervention to prevent or delay poor prognosis in chronic obstructive pulmonary disease (COPD). The aims of this study, in a sample of outpatients with COPD, were to (i) assess the prevalence of physical frailty using the Japanese version of the Cardiovascular Health Study (J-CHS) criteria and the Short Physical Performance Battery (SPPB) and the degree of agreement between the findings of the two assessments and (ii) identify factors associated with the disparity in the results obtained with these instruments.
Patients and Methods: This was a multicenter cross-sectional study of individuals with stable COPD enrolled in four institutions. Frailty was assessed using the J-CHS criteria and the SPPB. Weighted Cohen’s kappa (k) statistic was performed to investigate the magnitude of agreement between the instruments. We divided participants into two groups depending on whether there was agreement or non-agreement between the results of the two frailty assessments. The two groups were then compared with respect to their clinical data.
Results: A total of 103 participants (81 male) were included in the analysis. The median age and FEV1 (%predicted) were 77 years and 62%, respectively. The prevalence of frailty and pre-frail was 21% and 56% with the J-CHS criteria and 10% and 17% with the SPPB. The degree of agreement was fair (k = 0.36 [95% CI: 0.22– 0.50], P< 0.001). There were no significant differences in the clinical characteristics between the agreement group (n = 44) and the non-agreement group (n = 59).
Conclusion: We showed that the degree of agreement was fair with the J-CHS criteria detecting a higher prevalence than the SPPB. Our findings suggest that the J-CHS criteria may be useful in people with COPD with the aim of providing interventions to reverse frailty in the early stages.
Keywords: cardiovascular health study, Short Physical Performance Battery, chronic lung disease, physical function
Plain Language Summary
Illness and age-related physical function decline increase risk of mortality or hospitalization. Detecting and intervening the low physical function in the early stages are important for health promotion. This study compared the prevalence of low physical function using two major assessments, Japanese version of the Cardiovascular Health Study (J-CHS) criteria and the Short Physical Performance Battery (SPPB), in individuals with COPD that is one of the chronic lung disease. Our findings showed that the J-CHS criteria detected higher prevalence of low physical function than SPPB. Therefore, we suggest that the J-CHS criteria may be useful in people with COPD with the aim of providing interventions to reverse low physical function in the early stages.
Introduction
The prevalence of chronic obstructive pulmonary disease (COPD) is projected to increase over the coming decades due to continued exposure to risk factors such as tobacco smoking and aging of the world’s population.1 COPD and age-related decline precipitates frailty, defined as a state of increased vulnerability from age-associated decline in reserve and function, resulting in reduced ability to cope with daily or acute stressors.2 A previous study showed the prevalence of frailty to be as high as 19% in people with COPD.3 Those with COPD who are frail have increased mortality, hospitalization and worse health-related quality of life (HRQoL) when compared to individuals with COPD who are not frail.4 Frailty is a reversible condition and is improved by pulmonary rehabilitation (PR); however, frailty is associated with high rates of non-completion of PR.5 Assessment for frailty has attracted increasing attention in people with chronic lung disease and early recognition is important as it enables timely intervention to prevent or delay functional deterioration, institutionalization, disability, and death.2,6
Although there are many instruments available to assess frailty, including the physical, psychological and social aspects, as yet the gold standard method is unknown.7 In this paper, we focused on two instruments that assess physical frailty in outpatients: namely the Cardiovascular Health Study (CHS) criteria and the Short Physical Performance Battery (SPPB).8,9 The CHS criteria is the most frequently used instrument to evaluate physical frailty in older people and in those with COPD.2,7,10 Moreover of relevance to the present study, Satake et al11 developed a Japanese version (the J-CHS criteria). The SPPB is used to assess frailty in older people and as an assessment of physical function in people with COPD.12,13 Both instruments are simple to use, valid and reliable.14–16 The use of instruments that are more sensitive at detecting frailty may contribute to intervention at an earlier stage. However, there are few reports comparing frailty prevalence rates obtained using different instruments and the optimal instrument for assessment of physical frailty in individuals with COPD is unknown.
The aims of this study, in a sample of outpatients with COPD, were to (i) assess the prevalence of physical frailty using the J-CHS criteria and the SPPB, and the degree of agreement between the findings of the two assessments and (ii) identify factors associated with the disparity in the results obtained with these instruments.
Materials and Methods
Study Design
This was a multicenter, cross-sectional, observational study.
Participants
Individuals with stable COPD, defined as the absence of an exacerbation in the previous 4 weeks, who were receiving PR at any of four Japanese institutes (Nagasaki Pulmonary Rehabilitation Clinic, Tagami Hospital, Kirigaoka Tsuda Hospital, Utsunomiya Medical Clinic) between September 2018 and September 2019 were considered for inclusion in this study. People were excluded if they had comorbid conditions that affected exercise performance such as musculoskeletal, neurological or cognitive impairment. The study was approved by the Ethics Review Committee of Nagasaki University Graduate School of Biomedical Sciences (approval number: 18080901–2), and complies with the Declaration of Helsinki. Individuals gave written informed consent prior to participation.
Assessment
Assessments were undertaken within one visit during the PR program. We collected data on COPD exacerbations requiring hospitalization within the past year, from interviews with the participants and from the medical notes. Spirometry and static lung volumes were measured according to international guidelines.17 COPD severity was determined based on the Global Initiative for Chronic Obstructive Lung Disease (GOLD).18 We assessed dyspnea using the modified Medical Research Council (mMRC) dyspnea scale,19 and HRQoL was evaluated using the Japanese version of the COPD Assessment Test (CAT).20 Falls history was obtained by asking participants “Have you ever fallen within the past year?”.21 A fall was defined as an unexpected event in which the participant comes to rest on the ground, floor, or other lower level. Additionally, we used the Hospital Anxiety and Depression Scale (HADS) to assess anxiety and depression.22
Physical Frailty
Frailty was assessed using the J-CHS criteria and the SPPB. The former comprises five characteristics that reduce physiological reserve and precipitate a vulnerable state: unintentional weight loss of 2 kg or more in the past 6 months, self-reported exhaustion, low activity evaluated by interview, weakness (handgrip strength [<28 kg in men or <18 kg in women]), and slowness (gait speed <1.0 m/s). Each of these criteria score 1 point, providing a total score ranging from 0–5. Handgrip strength was measured in kilograms using a Smedley-type handheld dynamometer (GRIP-D; Takei Ltd, Niigata, Japan). The participant sat with the wrist in a neutral position and the elbow flexed to 90 degrees. Handgrip strength was measured twice on each hand, and the highest value was used in the analysis.23 Gait speed was measured with the 4m usual walk test, in which the participant was asked to walk at their usual pace for 5m, of which the first 4m was timed. The participant was permitted to use walking aids if usually used. The test was completed twice with the faster speed used in the analysis. Scores obtained using the J-CHS criteria were used to define frail; 3 or more points: pre-frail; 1–2 points, and robust; 0 points.11
The SPPB evaluates lower limb function and mobility based on three tests: static balance (standing postures with legs closed, semi tandem, tandem for up to 10 seconds for each posture), 4m usual gait speed (4MGS), and time taken to perform five sit to stands (5STS) without upper extremity assistance.9 Performance on each test is scored from 0 to 4 (total score of 12) with a higher score indicating better function. The cut-off points for frailty with the SPPB are: frail; 0–6: pre-frail; 7–9: robust; 10–12.24
Statistical Analysis
Weighted Cohen’s kappa coefficient (k) and calculation of 95% confidence intervals were performed to investigate the magnitude of agreement between the two frailty assessments. Interpretation of kappa values was based on Viera and Garrett25 (k<0: less than chance agreement, 0.01–0.20: slight agreement, 0.21–0.40: fair agreement, 0.41–0.60: moderate agreement, 0.61–0.80: substantial agreement, 0.81–0.99: almost perfect agreement). The clinical characteristics from participants were compared between frail and pre-frail or robust for each of the J-CHS criteria and the SPPB. We divided participants into two groups depending on whether there was agreement or non-agreement between the results of the J-CHS criteria and the SPPB (eg, participants were assigned to the non-agreement group if the J-CHS criteria identified them as frail; however, from the SPPB, they were identified as being either pre-frail or robust). The two groups were then compared with respect to their clinical data using the Mann–Whitney U-test for continuous variables and chi-square test for categorical variables. All analyses were performed using IBM SPSS Statistics V.25.0, and a P value of less than 0.05 was considered to indicate statistical significance. Data are presented as the median [interquartile range: IQR] for continuous variables and percentages for categorical variables.
Results
Characteristics of the Participants
In total, 113 individuals were screened of which 3 declined to participate. Of the 110 COPD participants recruited to the study, 7 had incomplete data thus data from 103 participants were included in the analysis (Figure 1). Table 1 shows the characteristics of the participants. The median age was 77 years, and the majority (79%) of participants were male, of normal body weight, had mild or moderate airflow limitation and 27% had been hospitalized for an exacerbation in the previous year. The median CAT score was 11 indicating poor health status, and 17 participants (17%) had experienced a fall in the past year. A small percentage (8.7%) of participants had depression or anxiety.
 |
Table 1 Characteristics of the Participants |
 |
Figure 1 Flow diagram. |
Agreement Between J-CHS and SPPB
Table 2 shows the prevalence of frailty and degree of agreement between the J-CHS criteria and the SPPB. The prevalence of frailty and pre-frail was 21% and 56% with the J-CHS criteria, and 10% and 17% with the SPPB. The degree of agreement between the two methods of assessment was fair (k=0.36 [95% CI: 0.22–0.50], P<0.001). The frailty characteristics of the participants are shown in Table 3. Using the J-CHS criteria the numbers of participants reporting exhaustion and low activity were 30 (29%) and 33 (32%) respectively. Thirty-eight (37%) participants achieved the total possible score on the SPPB indicating full function. Table 4 shows the clinical characteristics of those characterized as frail, pre-frail or robust for each of the J-CHS criteria and the SPPB. Participants characterized as frail using either the J-CHS criteria or the SPPB had worse dyspnea, poorer HRQoL and a higher prevalence of falls; however, age, BMI, and severity of airflow limitation were not different compared with those characterized as pre-frail or robust. Moreover, frail participants, identified using the J-CHS criteria, had a high prevalence of history of COPD exacerbations and higher levels of anxiety or depression.
 |
Table 2 Agreement Between J-CHS Criteria and SPPB |
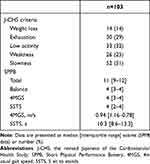 |
Table 3 Frailty Characteristics of the Participants |
 |
Table 4 Comparison of Clinical Characteristics Between Frail and Pre-Frail or Robust for Each of the J-CHS Criteria and the SPPB |
Factors Associated with the Disparity Between J-CHS and SPPB
Comparison of clinical characteristics between the participants assigned to the agreement group (n = 44) and the non-agreement group (n = 59) are shown in Table 5. There were no significant differences found.
 |
Table 5 Comparison of Clinical Characteristics Between the Agreement Group and the Non-Agreement Group |
Discussion
In the current study, we showed that; (i) the degree of agreement between the assessment of frailty obtained using the J-CHS criteria and SPPB was fair in people with COPD and (ii) no clinical factors were associated with the disparity in the results obtained between these instruments. To our knowledge, this is the first multicenter, cross-sectional, observational study to report the degree of agreement and investigate factors associated with the disparity between the J-CHS criteria and SPPB as instruments to assess frailty in people with COPD.
A systematic review3 found the estimates for the prevalence of frailty and pre-frail to be 19% (95% CI: 14% to 24%) and 56% (95% CI: 52% to 60%) among people with COPD. Studies included in this review almost exclusively used the CHS criteria to assess frailty. Consistent with this review, we found a similar prevalence for frailty and pre-frail assessed using the J-CHS criteria; however, the rates were lower when frailty was assessed using the SPPB. Our findings are not consistent with a study undertaken in COPD participants from community respiratory and pulmonary rehabilitation assessment clinics that reported moderate agreement (frail: k = 0.46) between the CHS criteria and the SPPB.26 The other previous study undertaken in a sample from a geriatric outpatient clinic that reported the degree of agreement with pre-frail was lower compared with frail [pre-frail: fair (k = 0.27), frail: moderate (k = 0.48)] between the CHS criteria and the SPPB.27 Therefore, our findings may have been influenced by the definition of frailty divided into frailty and pre-frailty in our study.
Frail participants, identified using the two frailty assessments, had worse dyspnea and HRQoL, and a higher falls history. Moreover, participants assessed as frail using the J-CHS criteria but not the SPPB showed a high prevalence of history of COPD exacerbations and higher levels of anxiety or depression. This finding is consistent with other studies in those with COPD or in elderly people that showed frailty was associated with higher levels of anxiety or depression (HADS), as well as a higher prevalence of COPD exacerbations and falls, greater dyspnea and worse HRQoL (mMRC dyspnea scale, St. George’s Respiratory Questionnaire, Medical Outcomes Survey Short Form-36).4,8,26,28 The presence of anxiety or depression may impact subjective measurements in the J-CHS criteria such as the reporting of exhaustion.26 History of COPD exacerbations may have influenced unintentional weight loss or low activity in the J-CHS criteria which are characteristics of prior physical performance.
We did not find any clinical factors such as participant demographics or pulmonary function associated with the disparity between results of frailty using the J-CHS criteria and the SPPB. Whilst many of our participants reported weight loss, low activity and exhaustion (J-CHS criteria), their physical performance was well preserved when assessed using the SPPB. With the exception of the frailty characteristic of exhaustion, the prevalence of malnutrition and low activity in our sample was similar to previous studies, and both are important characteristics of frailty together with gait speed in people with COPD.2,5,29,30 The CHS criteria reflect the pathophysiology of frailty caused by several factors in COPD and include a mixture of both subjective self-reported and objective measures.2 Whereas, the SPPB provides only an objective assessment of physical performance that includes balance, 4MGS and 5STS. A likely explanation for the disparity in the results for frailty between the two instruments is the different components of each assessment. Our findings are consistent with a previous study which showed that disparity in the results for frailty between the CHS criteria and SPPB in participants with COPD may have arisen particularly from the weight loss and exhaustion components of the CHS criteria.26
This is the first study to report the degree of agreement between the J-CHS criteria and the SPPB, instruments that are simple to use, and produce valid and reliable results in people with COPD.14–16 Our results showed the J-CHS criteria was more sensitive to assess frailty than the SPPB in our sample. A previous study suggested that use of the assessment that poor detection of frailty to screen for frailty may result in important missed opportunities to identify and provide early intervention such as PR.31 Based on these findings, we recommend using the J-CHS criteria to assess physical frailty over the SPPB in people with COPD as this may lead to better clinical outcomes as it would permit earlier intervention.
This study has several limitations. First, gait speed in the J-CHS criteria was measured from a static start in contrast to an earlier study14 in which gait speed was measured from a dynamic start with the participant walking 2 m before measurement commenced. The use of a static start is associated with a lower gait speed and thus may have contributed to the higher prevalence of frailty assessed using the J-CHS criteria.32 Second, the frailty instruments we used are restricted to the assessment of physical frailty and do not assess cognitive, psychological and social domains, deficits in these domains increase risk of adverse outcomes in older people.33 Finally, participants comprised people with COPD attending PR and thus may have had better physical function that a sample with similar COPD severity who have not undergone PR. Moreover, assessments may have been conducted at the immediately prior or after the PR program, not only during the PR program, and this may have had impact on the prevalence of frailty. However, PR is a comprehensive intervention that comprises exercise training, education, and behavior change.34 Thus, all components of the J-CHS and the SPPB may have been favorably affected by PR, and therefore may not have impacted significantly on the disparity in the findings obtained using two assessments. A strength of our study included the study setting, ie, multicenter and the recruitment of a sample with COPD that had a high prevalence of frailty and pre-frail. Therefore, our findings are representative of outpatients with stable COPD. Studies are required to assess frailty in acute settings such as in patients with COPD exacerbation as well as in those with stable COPD at an early stage of COPD.
Conclusion
In this study, we assessed the degree of agreement and factors associated with the disparity between J-CHS criteria and SPPB as instruments for assessing frailty in people with COPD. The degree of agreement was fair with the J-CHS criteria detecting a higher prevalence than the SPPB. Our findings suggest that the J-CHS criteria may be useful for assessing physical frailty in people with COPD with the aim of providing interventions to reverse frailty in the early stages.
Data Sharing Statement
Data used to support the findings of this study are included within the manuscript in Tables 1–4. Raw data are available from the corresponding author upon reasonable request.
Acknowledgments
The authors are grateful to the staff physical therapists and all the staff who acquired data for study. In addition, we are grateful to Dr Sue Jenkins, Institute for Respiratory Health and Physiotherapy Department, Sir Charles Gairdner Hospital, for her help in reviewing our manuscript. Finally, we thank Dr Shuntaro Sato, Clinical Research Center, Nagasaki University Hospital, Nagasaki, for his support with statistical analysis.
Author Contributions
YT and RK contributed significantly to conception and design, or analysis and interpretation of data; and have been involved in drafting and reviewing the manuscript. MH and YY contributed significantly to analysis and interpretation of data and have been involved in drafting and revised it critically for important intellectual content. All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
The study design, collection and analysis of the data, and preparation of the manuscript were not funded by any public, commercial, or not-for-profit sectors.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3(11):e442. doi:10.1371/journal.pmed.0030442
2. Guan C, Niu H. Frailty assessment in older adults with chronic obstructive respiratory diseases. Clin Interv Aging. 2018;13:1513–1524. doi:10.2147/CIA.S173239
3. Marengoni A, Vetrano DL, Manes-Gravina E, Bernabei R, Onder G, Palmer K. The relationship between COPD and frailty: a systematic review and meta-analysis of observational studies. Chest. 2018;154(1):21–40. doi:10.1016/j.chest.2018.02.014
4. Kennedy CC, Novotny PJ, LeBrasseur NK, Wise RA, Sciurba FC, Benzo RP. Frailty and clinical outcomes in chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2019;16(2):217–224. doi:10.1513/AnnalsATS.201803-175OC
5. Maddocks M, Kon SS, Canavan JL, et al. Physical frailty and pulmonary rehabilitation in COPD: a prospective cohort study. Thorax. 2016;71(11):988–995. doi:10.1136/thoraxjnl-2016-208460
6. Luckhardt T, Thannickal VJ. Measures of frailty in chronic lung diseases. Ann Am Thorac Soc. 2017;14(8):1266–1267. doi:10.1513/AnnalsATS.201706-420ED
7. Bouillon K, Kivimaki M, Hamer M, et al. Measures of frailty in population-based studies: an overview. BMC Geriatr. 2013;13:64. doi:10.1186/1471-2318-13-64
8. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol a Biol Sci Med Sci. 2001;56(3):M146–56. doi:10.1093/gerona/56.3.M146
9. Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–M94. doi:10.1093/geronj/49.2.M85
10. Walston J, Buta B, Xue QL. Frailty screening and interventions: considerations for clinical practice. Clin Geriatr Med. 2018;34(1):25–38. doi:10.1016/j.cger.2017.09.004
11. Satake S, Arai H. The revised Japanese version of the Cardiovascular Health Study criteria (revised J-CHS criteria). Geriatr Gerontol Int. 2020;20(10):992–993.
12. Pavasini R, Guralnik J, Brown JC, et al. Short physical performance battery and all-cause mortality: systematic review and meta-analysis. BMC Med. 2016;14(1):215. doi:10.1186/s12916-016-0763-7
13. Lage VKS, Silva GP, Lacerda ACR, et al. Functional tests associated with sarcopenia in moderate chronic obstructive pulmonary disease. Expert Rev Respir Med. 2020;2020:1–8.
14. Makizako H, Shimada H, Doi T, Tsutsumimoto K, Suzuki T. Impact of physical frailty on disability in community-dwelling older adults: a prospective cohort study. BMJ Open. 2015;5(9):e008462. doi:10.1136/bmjopen-2015-008462
15. Dogan Varan H, Deniz O, Coteli S, Tuna Dogrul R, Kizilarslanoglu MC, Goker B. Validity and reliability of fried frailty phenotype in Turkish population. Turk J Med Sci. 2022;52:323–328. doi:10.55730/1300-0144.5318
16. Gomez JF, Curcio CL, Alvarado B, Zunzunegui MV, Guralnik J. Validity and reliability of the Short Physical Performance Battery (SPPB): a pilot study on mobility in the Colombian Andes. Colomb Med. 2013;44(3):165–171. doi:10.25100/cm.v44i3.1181
17. Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi:10.1183/09031936.05.00034805
18. Grobal Initiative for Chronic Obstructive Lung Disease. Global strategy for prevention, diagnosis and management of COPD: 2023 report. Available from: https://goldcopd.org/2023-gold-report-2/.
19. Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54(7):581–586. doi:10.1136/thx.54.7.581
20. Tsuda T, Suematsu R, Kamohara K, et al. Development of the Japanese version of the COPD assessment test. Respir Investig. 2012;50(2):34–39. doi:10.1016/j.resinv.2012.05.003
21. Lamb SE, Jorstad-Stein EC, Hauer K, Becker C; Prevention of Falls Network E, Outcomes Consensus G. Development of a common outcome data set for fall injury prevention trials: the prevention of falls network Europe consensus. J Am Geriatr Soc. 2005;53(9):1618–1622. doi:10.1111/j.1532-5415.2005.53455.x
22. Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi:10.1111/j.1600-0447.1983.tb09716.x
23. Bianchi L, Ferrucci L, Cherubini A, et al. The predictive value of the EWGSOP definition of sarcopenia: results from the InCHIANTI study. J Gerontol a Biol Sci Med Sci. 2016;71(2):259–264. doi:10.1093/gerona/glv129
24. Subra J, Gillette-Guyonnet S, Cesari M, Oustric S, Vellas B, Platform T. The integration of frailty into clinical practice: preliminary results from the Gerontopole. J Nutr Health Aging. 2012;16(8):714–720. doi:10.1007/s12603-012-0391-7
25. Viera AJ, Garrett JM. Understanding interobserver agreement: the kappa statistic. Fam Med. 2005;37(5):360–363.
26. Brighton LJ, Nolan CM, Barker RE, et al. Frailty and mortality risk in COPD: a cohort study comparing the fried frailty phenotype and short physical performance battery. Int J Chron Obstruct Pulmon Dis. 2023;18:57–67. doi:10.2147/COPD.S375142
27. Pritchard JM, Kennedy CC, Karampatos S, et al. Measuring frailty in clinical practice: a comparison of physical frailty assessment methods in a geriatric out-patient clinic. BMC Geriatr. 2017;17(1):264. doi:10.1186/s12877-017-0623-0
28. Hanlon P, Lewsey J, Quint JK, et al. Frailty in COPD: an analysis of prevalence and clinical impact using UK Biobank. BMJ Open Respir Res. 2022;9(1):e001314. doi:10.1136/bmjresp-2022-001314
29. Blodgett J, Theou O, Kirkland S, Andreou P, Rockwood K. The association between sedentary behaviour, moderate-vigorous physical activity and frailty in NHANES cohorts. Maturitas. 2015;80(2):187–191. doi:10.1016/j.maturitas.2014.11.010
30. Verlaan S, Ligthart-Melis GC, Wijers SLJ, Cederholm T, Maier AB, de van der Schueren MAE. High prevalence of physical frailty among community-dwelling malnourished older adults-a systematic review and meta-analysis. J Am Med Dir Assoc. 2017;18(5):374–382. doi:10.1016/j.jamda.2016.12.074
31. Chin M, Kendzerska T, Inoue J, et al. Comparing the hospital frailty risk score and the clinical frailty scale among older adults with chronic obstructive pulmonary disease exacerbation. JAMA Netw Open. 2023;6(2):e2253692. doi:10.1001/jamanetworkopen.2022.53692
32. Krumpoch S, Lindemann U, Rappl A, Becker C, Sieber CC, Freiberger E. The effect of different test protocols and walking distances on gait speed in older persons. Aging Clin Exp Res. 2021;33(1):141–146. doi:10.1007/s40520-020-01703-z
33. Lee Y, Kim E, Yun J, Chuck KW. The influence of multiple frailty profiles on institutionalization and all-cause mortality in community-living older adults. J Cachexia Sarcopenia Muscle. 2022;13(5):2322–2330. doi:10.1002/jcsm.13033
34. Spruit MA, Singh SJ, Garvey C, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188(8):e13–64. doi:10.1164/rccm.201309-1634ST
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
