Back to Journals » International Journal of Women's Health » Volume 15
Dietary Supplements Intake During Pregnancy Among Pregnant Women in Ethiopia
Authors Adelo ES, Ergena AE , Emiru YK , Ayele S, Muche HA
Received 25 September 2022
Accepted for publication 24 March 2023
Published 11 April 2023 Volume 2023:15 Pages 559—569
DOI https://doi.org/10.2147/IJWH.S388656
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Elie Al-Chaer
Eyerusalem Shello Adelo,1 Asrat Elias Ergena,2 Yohannes Kelifa Emiru,3 Sileshi Ayele,4 Haymanot Alem Muche1
1Department of Midwifery, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia; 2Department of Pharmaceutical Chemistry, School of Pharmacy, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia; 3Department of Pharmacognosy, School of Pharmacy, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia; 4Department of Obstetrics and Gynecology, School of Medicine, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
Correspondence: Asrat Elias Ergena, Tel +251 926 301599, Email [email protected]
Introduction: Pregnant women are expected to take one or more dietary supplements (DS) like iron, folic acid, zinc, calcium, magnesium, prenatal vitamins, etc. for maternal and child health during pregnancy. Despite its growing use in Ethiopia, data concerning currently marketed maternal DS products have not been intensively investigated so far. Taking into consideration the existing problem, this study was set out to assess the prevalence and commonly used DS during pregnancy in a referral hospital in Ethiopia.
Methods: A facility based cross-sectional study was employed to conduct this study from November 2020 to January 2021. The sample size was obtained by using the single population proportion formula and participants were selected and approached by using a systematic random sampling technique. Data were collected through a semi-structured interviewer-administered questionnaire. Descriptive statistics including frequencies and percentages were used to describe continuous and categorical variables and multivariate logistic regression was used to observe the association of the independent variables to the dependent variable.
Results: The overall prevalence of DS use was 84.2% and the most used product was Fefol (iron and folate supplement) (62.4%). A majority (87.8%) of DS products were obtained by prescription. In multivariate regression analysis, DS use during pregnancy was significant among nulliparous women and women who went to college and above [adjusted odds ratio (AOR): 8.142, 95% confidence interval (CI) (1.298– 51.070)] and [AOR: 9.259, 95% CI (1.998– 42.906)], respectively.
Conclusion: Even though the prevalence of DS practice showed improvement among the study participants, the duration of the DS intake is less than that recommended by the WHO. Pregnant women who did not have birth before and who went to college or above showed significant association with the use of DS.
Keywords: dietary supplements, prenatal nutrition, prenatal vitamins, Fefol, pregnant women
Introduction
Adequate nutrition is an important health care package for maternal and child health in the course of pregnancy. Pregnant women are susceptible to nutritional deficiencies due to an increment in nutrient needs to provide increased nutritional demands due to fetal growth and development, and changes in maternal metabolism such as pregnancy induced development of tissues.1 Additionally, nutritional requirements for healthy women can be increased based on the trimesters of the fetus.2,3 Although this pregnancy associated increased nutritional demand can be met by an appropriate consumption of food in a balanced diet, additional use of dietary supplements (DS) has been thought a key nutritional product with a crucial role in the maintenance of adequate nutritional status.4
According to the World Health Organization (WHO), the prevalence of preterm and underweight babies delivered are about 15 million and 20 million respectively.5,6 Women from low- and middle- income countries suffer from multiple-micronutrient deficiencies due to inadequate intake of animal products, fruits, vegetables and fortified foods7 and consequently, more than 95% of those born with low birth weight are occurring in these countries.8
Furthermore, studies show that globally, around 9.8 million women are at risk for vitamin A deficiency. African and South-East Asian countries are reported as nations with the highest burden of prenatal deficiencies of dietary supplements (9.8% and 9.9% respectively). So, low socio-economic status, compromised health conditions and dietary intake are commonly reported attributes of deficiencies of DS during pregnancy.9
Recent evidence suggests so far the particular importance of DS10 and in fact, available information indicates that 14 of the 21 essential micronutrients’ (seven vitamins, five minerals and choline) requirements increase with the trimester of the pregnancy.11 In the United States of America, 97% of pregnant women are advised to take DS as part of their prenatal care regimen whereas only 67% of African-American and 84% of Caucasian women of the population showed adherence for this recommendation.12 Apart from these DS, some clinicians consider single nutritional preparations to benefit some pregnant women with certain needs. For instance, iron and folic acid (folate) have long been prescribed or recommended during pregnancy by virtue of their beneficial roles to prevent or treat particular pregnancy-related health conditions.13
Along with this growth in prenatal nutrition, however, there is increasing concern over pregnant women at risk of dietary deficiency in several key nutrients such as calcium, iron, folic acid and vitamin D.13 A considerable amount of literature has been supporting the link between inadequate maternal nutritional status and adverse pregnancy outcomes,14,15 poor infant survival,16 congenital anomalies17 and risk of chronic diseases and impaired mental development in later life.18
The high prevalence of micronutrient deficiencies among pregnant women is associated with an increased demand of the nutrients because of fetal and maternal tissue growth and development.19 Any compromised intake of these nutrients in both quality and quantity during pregnancy results in failure to fulfill the increased demand of essential nutrients which would result in some adverse pregnancy outcomes.20,21 Nutritional deficiencies during pregnancy increase the vulnerability of the mothers to different disease, increase the rate of miscarriage, and the babies delivered will be underweight whose survival is at risk.18 Moreover, the low weight gain of mothers due to compromised nutrition during pregnancy is a risk factor for the delivery of infants too small for their gestational age, which in turn leads to neonatal morbidity and mortality,22 growth failure, retarded cognitive development and adulthood chronic diseases.23
Being one of the developing countries, Ethiopia has a high burden of malnutrition, morbidity as well as mortality of both mothers and children. According to Ethiopian demographic and health survey data of recent years, around 22.9% of the women with reproductive ages (15–49 years) were malnourished and the prevalence of malnutrition in Amhara region (the site of the current study) was 29%.24 However, much of previous studies undertaken in Ethiopia demonstrated that the prevalence of undernutrition among pregnant women in different parts of the country varies from 15.2 to 35.5%.25–27
Despite supplement use becoming a common practice, safety and efficacy data concerning currently marketing maternal DS products have not been intensively investigated. In Ethiopia, previous studies on DS use in pregnancy mainly focused on prevalence, adherence and associated factors of folic acid and iron supplement use during pregnancy so far.27–29 However, to the best of our knowledge, there has been no reliable evidence that explored the prevalence and potential associates of demographic characteristics of pregnant women who use newly emerging DS preparations in Ethiopia. This study therefore will set out to assess the use prevalence and types of DS products in pregnant women visiting University of Gondar Comprehensive Specialized Hospital (UOGCSH) ANC clinic and to ascertain the factors associated with their use.
Methods and Materials
Study Design, Period and Setting
An institution-based cross-sectional study was conducted in UOGCSH from November, 2020 to January, 2021. The hospital is found in Amhara region, at 738 km in a northwestern direction from Addis Ababa, the capital of Ethiopia. It is one of the teaching hospitals of the country at tertiary level and it is a referral center for district hospitals serving more than seven million population.
The ANC clinic of the hospital provides services including identification and follow up of the pregnant women and the fetus, early assessment of pregnancy related complications such as preeclampsia, anemia, and symptoms of stress and other mental health problems. It is also engaged in the screening and treatment of some concurrent diseases such as sexually transmitted diseases and human immune virus. Moreover, the pregnant women get health education to develop healthy home behaviors and have full support and counseling to prepare both emergency and birth plans as well as to promote postnatal family planning/birth spacing.
Population
Source Population
Includes all pregnant women attending the ANC clinic of UOGCSH.
Study Population
Those pregnant women who were visiting the ANC clinic of UOGCSH from November, 2020 to January, 2021 and attained the study inclusion criteria.
Inclusion and Exclusion Criteria
Inclusion Criteria
Inclusion criteria requirements were those who women accepted the consent form to take part in the study, pregnant women who lived for at least 3 months in the town and age of 18 years or older, healthy and Ethiopian citizen women.
Exclusion Criteria
Pregnant women with pregnancy related complication which require special follow up and dietary modifications were excluded from the study. So, women with excessive nausea and/or vomiting, both gestational and chronic diabetes mellitus, hypertensive disorders, liver and renal diseases, gastrointestinal disorders and autoimmune diseases were not parts of this study.
Sample Size Determination and Sampling Technique
The sample size was calculated by using the single population proportion formula by considering the prevalence of DS use among the pregnant population was 18.3% as per a study done in Ethiopia,30 95% of confidence interval and 5% of marginal error. A total of 253 study participants were recruited into this study after adding a 10% non-response rate. The study participants were selected and approached by using a systematic random sampling method. The estimated number of pregnant women who visited the ANC clinic for the past three consecutive months was 2000. By using this estimate, the calculated Kth interval value to approach was 8 and so, every 8th pregnant woman was approached and interview for the study.
Data Collection Tool and Procedures
Data were collected through a semi-structured interviewer administered questionnaire by the principal investigators. The data collection questionnaire was prepared by intensive reading of relevant literatures done in the area of DS use during pregnancy.27,31–35 It comprises items focusing on socioeconomic and socio-demographic characteristics, parity and gravidity, use practice and perceived reasons for using or not using DS, types of DS products and whether or not these products are prescribed, reading practice of DS level, frequency and dosage forms of DS, occurrence of side effects and experience of discussion with health care providers, and information resources of DS. The antenatal and clinical data as well as the anthropometric measurements of the pregnant women were directly copied from the respondents’ assessment charts from the ANC clinic at the time of data collection.
Data Quality Control Technique
Before the data collection procedure was started, intensive training was given to the data collectors on the contents of the training of the instrument, the methods of data collection and handling of the collected data.
Statistical Analysis
The collected data using a quantitative method were cleaned and entered into and analyzed using IBM SPSS Statistics for Windows, version 22.0. Descriptive statistics were carried out and presented with narration and tabulation. Binary logistic regression (bivariable and multivariable binary logistic regressions) analysis was performed to identify statistically significant variables.
The variables that have statistically significant associations at p-value≤0.2 with the dependent variable in the bivariable logistic regression analysis were further considered as a candidate for a multivariable logistic regression for controlling the possible effect of confounding variables. Age, number of deliveries, gestational age, number of children, educational status, number of family and place of residence of the study participants have a p-value of less than or equal to 0.2 in bivariable logistic regression analysis and the multivariable logistic regression was computed for these variables. Lastly, variables with p-value≤0.05 in a multivariable binary logistic regression model were reported as significantly associated with the dependent variable and their adjusted odds ratio (AOR) with a 95% confidence interval was used to measure the strength of association.
Ethical Considerations
The study was conducted by following the declaration of Helsinki. The research and ethics review committee of School of Midwifery, University of Gondar, the Hospital Clinical Director and head of ANC clinic of UOGSH reviewed the study proposal, the data collection tool and the verbal informed consent statement, and provided the ethical clearance letter and approved the study. The purpose of the study was explained to the study participants and a verbal informed consent was obtained from each of the respondents. The names and other identifiers of participants were not documented and their data will never be shared for third parties.
Definition of Terms and Operational Definitions
The Dietary Supplement Health and Education Act (DSHEA) of 1994 issued by FDA defines the term “dietary supplement” as a product that collectively meets the following requirements.36
- A product (other than tobacco) intended to supplement the diet or contain one or more of the following: vitamin, mineral, herb or other plant-derived substance (eg, ginseng, garlic), amino acid, concentrate, metabolite, constituent or extract.
- A product intended for ingestion in pill, capsule, tablet or liquid form.
- A product not represented for use as a conventional food or as the sole item of a meal or diet.
Prenatal vitamins are preparations made for supplementation of pregnant women to meet the needs of vitamins and minerals for healthy pregnancy. The preparations contain 400 µg folic acid, 6 µg vitamin B12, 2 mg riboflavin, 70 mg vitamin C, 400 IU vitamin D, 20 mg niacin, 3 mg thiamine, 10 mg vitamin E, 17 mg iron, 200 to 300 mg calcium, 15 mg zinc, and 150 µg iodine.
Fefol is maternal nutritional supplement comprising 150 mg ferrous sulphate and 0.5 mg folic acid.
Prescription DS refers to supplement that are safe and effective that used under health care providers, whereas nonprescription DS are safe and effective supplement for use without health care provider prescription.
Untoward effects: Unintended and harmful response experienced after the intake of supplement or the combinations of supplements under suggested conditions and suspected to be associated with the supplement.
Results
Socio-Demographic Characteristics
All pregnant women approached agreed to participate in the study with a response rate of 100%. From 253 study participants more than half (53%) are found between age of 25 and 34 years and most (89.3%) of the study participants are married. More than 90% of the study participants have normal body mass index and 45.8% of the respondents came the ANC visit for their first child. More than two-thirds of the respondents came from urban areas while the rest are from rural side of the country (Table 1).
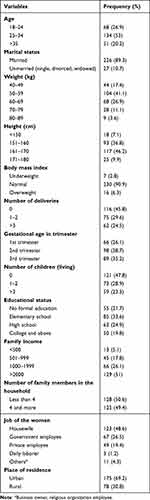 |
Table 1 Socio-Demographic Characteristics of Women Who Came to ANC, Gondar, Ethiopia, 2021 |
The Practice of Dietary Supplements Intake Among Pregnant Mothers
The reported practice to take dietary supplements by the study participants was 84.2%. Among those who reported that they are taking dietary supplement, about 76.1% women take in order to prevent anemia and delivery of babies with low birth weight. The fear of side effects of the supplements (35%) and the harmful effects they could result on the fetus (32.5%) were the frequent reasons to not take dietary supplements. Among the women who take dietary supplements, most (76.1%) take Fefol (iron + folic acid) and most (87.8%) of them are obtained by prescription (Table 2).
 |
Table 2 The Practice of Dietary Supplements Intake Among Pregnant Women, Gondar, Ethiopia, 2021 |
Magnitude of Dietary Supplement Practice and Associated Factors
In multivariable logistic regression, to assess the practice of dietary supplementation, variables fitting the chi-square assumption directly undergwent bivariate, and then multivariate regression analysis. In multivariate regression analysis, number of deliveries and educational status of the women were significantly associated with dietary supplements use. The odds of taking dietary supplements were 8.142 times higher among those who have not gave birth before. And, the dietary supplementation women who went to college and above are more tended to use dietary supplementation than the women who did not get formal education [AOR: 9.259, 95% CI (1.998–42.906)] (Table 3).
 |
Table 3 Magnitude of Dietary Supplement Practice and Associated Factors, Gondar, Ethiopia, 2021 |
Discussion
At the times of pregnancy and lactation, increased nutritional supplementation is expected to meet the increased needs in order to support and enhance the growth and development at both fetal and infant stages of development. So, every pregnant woman is expected to have a healthy and balanced diet during the period of pregnancy and this specified maternal diet plays a critical role in the fetal health, growth and development. Health professionals also should take pregnancy as an opportunity to encourage women to make dietary improvements as this time gives motivation for the women and their caregivers to change their aspects of nutrition and diet.
In the present study, 84.2% of the respondents take DS at some point of their pregnancy period. This result showed better finding than some similar studies conducted in Colombia (68.6%),33 the United States of America (69.8%)37 and Denmark (77%).38 The observed increment in the current study could be due to differences among the study setting. The data in our study were obtained from women who came to ANC follow up, which have access to DS and the information regarding pregnancy. This could be supported by 87.8% of the pregnant women who take DS in our study use prescription supplements from health institutions. However, the result goes in line with the finding of study conducted in Israel, which reported the DS utilization is 85%.39 The Poland study also reported that the use of DS was 81.2%.39 And, the study conducted in Jordan showed higher prevalence of DS practice (96.8%) than the current study.32
The practice of dietary supplementation is good during pregnancy among the study participants and showed significant association with number of deliveries and educational status of the study participants as compared to non-users of the dietary supplements. Women who went to college and above had odds of 9.259, 95% CI (1.998–42.906) to take dietary supplements than women who did not have formal education. Other studies conducted in different population and study settings also showed the same result with our study.31,33,38,40,41 The best way to explain this finding could be that higher education increases the awareness as well as acceptability of the role of nutrition in good health. We also found the dietary supplement intake was 8.142 times higher among women with their first pregnancy than women who were pregnant for the second time or more. Some studies also reported similar results which showed the association of nulliparity with high dietary supplement intake.35,40,42 This could possibly be explained as the adherence of the pregnant women to the health care professional recommendation could increase as they do not have self-experience on pregnancy.
Preventing maternal anemia and low birth weight of the fetus was the most commonly reported (76.1%) reason to take dietary DS in the current study. This finding does not go in line with the result from a Saudi Arabian study on which the most reported reason was to meet the elevated fetal dietary requirement.31 This discrepancy could be due to the guidelines for health education and treatment of pregnant women in low-income countries mainly focusing on maternal health issues such as anemia and maternal malnutrition. While the fear of adverse effects both for themselves and the fetus were the most reported reasons to not take dietary supplements among the non-users. But only half of the women who took the dietary supplements experienced some kinds of adverse effects. Based on this finding, it is reasonable to suggest that increasing the awareness of the pregnant women regarding the possible risks and benefits of the supplements could increase the practice.
Most (62.4%) of the study participants from the current study use Fefol (iron + folic acid) as DS during pregnancy. This could be due to the fact that using a combination formulation would be easier than using two independent formulations. This could imply having combination formulations may increase the practice due to their convenience to use.
Among those who take the DS, almost all reported that they took the supplements for less than three months. A prospective cohort study conducted in China showed same results with current study which says the average usage duration of the DS was 2.5 months.35 But the findings are not in line with the WHO recommendation which suggests the dietary supplementations should be taken throughout pregnancy.43
Conclusion
This study addressed information on the prevalence of the DS practice, the types of supplements commonly used among pregnant women at the ANC clinic of UOGCSH and factors associated with DS use. Even though the prevalence of the dietary supplementation practice is good among the study participants, the duration of the DS intake is less than that recommended by the WHO. Pregnant women who did not have birth before and who went to college or above showed significant association with the use of DS. The investigators recommend that the general population should be given health education on proper dietary supplements’ use through different media outlets. Health practitioners also should be guided to follow the WHO recommendation of dietary supplement use during pregnancy. Future guidelines and policies should give emphasis on articles that encourage the health care practitioners and the community to follow the standardized recommendations.
Limitations of the Study
Despite the fact that this study was able to provide useful information on dietary supplements practice among pregnant women, several limitations are noted. First, the study participants were women who came to health facility for their ANC visit. This may not give the whole picture of the practice in the community since there might be women who do not attend their ANC visit properly. Second, misreporting due to a recall bias may happen as mothers were asked to record all types of DS products, frequency and dosage forms of DS and other questions which need a recall ability. Thirdly, the nature of the data obtained by a cross-sectional study design limits the reader to establish a temporal relationship between the causes and the effects. Despite these limitations, we feel the study provide a reasonably accurate assessment of the dietary supplements practice among pregnant women and it is a very first kind in the study area.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Morrison JL, Regnault TR. Nutrition in Pregnancy: Optimising Maternal Diet and Fetal Adaptations to Altered Nutrient Supply. Multidisciplinary Digital Publishing Institute; 2016:342.
2. Agostoni C, Bresson J, Fairweather-Tait SJS, Pison L. EFSA Panel on dietetic products, nutrition and allergies (NDA). Heart Rhythm. 2010;8(9):1459. doi:10.1016/j.hrthm.2010.06.024
3. Wang L, Mei Z, Li H, Zhang Y, Liu J, Serdula MK. Modifying effects of maternal Hb concentration on infant birth weight in women receiving prenatal iron-containing supplements: a randomised controlled trial. Br J Nutr. 2016;115(4):644–649. doi:10.1017/S0007114515004870
4. Medicine Io, Status IoMSoN, Pregnancy WGd, Pregnancy CoNSD, Lactation, Intake IoMSoD. Nutrition During Pregnancy: Part I: Weight Gain, Part II: Nutrient Supplements. National Academy Press; 1990.
5. World Heath Organization. WHO Recommendations on Interventions to Improve Preterm Birth Outcomes. World Heath Organization; 2015.
6. Gebremedhin S, Enquselassie F, Umeta MJ. Independent and joint effects of prenatal zinc and vitamin A deficiencies on birthweight in rural Sidama, Southern Ethiopia: prospective cohort study. PLoS One. 2012;7(12):e50213. doi:10.1371/journal.pone.0050213
7. World Heath Organization. Vitamin and Mineral Requirements in Human Nutrition. World Health Organization; 2004.
8. Koletzko B, Bauer C-P, Bung P, et al. German national consensus recommendations on nutrition and lifestyle in pregnancy by the “healthy start-young family network”. Ann Nutr Metab. 2013;63(4):311–322. doi:10.1159/000358398
9. Baytekus A, Tariku A, Debie AJB. Clinical vitamin-A deficiency and associated factors among pregnant and lactating women in Northwest Ethiopia: a community-based cross-sectional study. BMC Pregnancy Childbirth. 2019;19(1):1–8.
10. Brown ML. Present knowledge in nutrition; 1990.
11. Yu SM, Keppel KG, Singh GK, Kessel WJA. Preconceptional and prenatal multivitamin-mineral supplement use in the 1988 National Maternal and Infant Health Survey. Am J Public Health. 1996;86(2):240–242. doi:10.2105/ajph.86.2.240
12. Tamura T, Picciano MFJTA. Folate and human reproduction. Am J Clin Nutr. 2006;83(5):993–1016. doi:10.1093/ajcn/83.5.993
13. Health UDo, Services H. Dietary Guidelines for Americans 2015–2020. Skyhorse Publishing Inc; 2017.
14. Abu-Saad K, Fraser D. Maternal nutrition and birth outcomes. Epidemiol Rev. 2010;32(1):5–25. doi:10.1093/epirev/mxq001
15. Scholl TJ. Maternal nutrition before and during pregnancy. In: The Window of Opportunity: Pre-Pregnancy to 24 Months of Age. Nestlec Ltd; 2008:79–89.
16. Black RE, Allen LH, Bhutta ZA, et al. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet. 2008;371(9608):243–260. doi:10.1016/S0140-6736(07)61690-0
17. Taye M, Afework M, Fantaye W, Diro E, Worku AJB. Factors associated with congenital anomalies in Addis Ababa and the Amhara Region, Ethiopia: a case-control study. BMC Pediatr. 2018;18(1):1–11.
18. Victora CG, Adair L, Fall C, et al. Maternal and child undernutrition: consequences for adult health and human capital. Lancet. 2008;371(9609):340–357. doi:10.1016/S0140-6736(07)61692-4
19. Black RE, Victora CG, Walker SP, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013;382(9890):427–451. doi:10.1016/S0140-6736(13)60937-X
20. Berti C, Biesalski H, Gärtner R, et al. Micronutrients in pregnancy: current knowledge and unresolved questions. Clin Nutr. 2011;30(6):689–701. doi:10.1016/j.clnu.2011.08.004
21. Bhutta ZA, Das JK, Rizvi A, et al. Evidence-based interventions for improvement of maternal and child nutrition: what can be done and at what cost? Lancet. 2013;382(9890):452–477. doi:10.1016/S0140-6736(13)60996-4
22. Christian P, West JKP, Khatry SK, et al. Night blindness of pregnancy in rural Nepal—nutritional and health risks. Int J Epidemiol. 1998;27(2):231–237. doi:10.1093/ije/27.2.231
23. Kramer MSJ. Determinants of low birth weight: methodological assessment and meta-analysis. Bull World Health Organ. 1987;65(5):663.
24. CSA-Ethiopia I. International. Ethiopia Demographic and Health Survey 2016: Key Indicators Report. USA: CSA and ICF; 2016.
25. Kuche D, Singh P, Moges D, Belachew TJJ. Nutritional status and associated factors among pregnant women in Wondo Genet District, Southern Ethiopia. J Food Sci. 2015;5(2):85–94.
26. Moges M, Worku A, Loha E. Nutritional status and associated factors among pregnant women in Boricha Woreda, Sidama Zone, Southern Ethiopia; 2015.
27. Nana A, Zema TJ. Dietary practices and associated factors during pregnancy in northwestern Ethiopia. BMC Pregnancy Childbirth. 2018;18(1):1–8.
28. Alemayehu MS, Tesema EM. Dietary practice and associated factors among pregnant women in Gondar town north west, Ethiopia, 2014. Int J Nutr Food Sci. 2015;4(6):707–712.
29. Gebremariam AD, Tiruneh SA, Abate BA, Engidaw MT, Asnakew DT. Adherence to iron with folic acid supplementation and its associated factors among pregnant women attending antenatal care follow up at Debre Tabor General Hospital, Ethiopia, 2017. PLoS One. 2019;14(1):e0210086. doi:10.1371/journal.pone.0210086
30. Mohammed MA, Aljadhey HS, Ahmed JH, Science R. Supplement use among pregnant women in Ethiopia: prevalence and predictors. Ther Innov Regul Sci. 2013;47(4):416–423. doi:10.1177/2168479013485078
31. Alfawaz HA, Khan N, AlOteabi N, Hussain SD, Al-Daghri NM. Factors associated with dietary supplement use in Saudi pregnant women. Reprod Health. 2017;14(1):1–6.
32. Asali FF, Tayyem RF, Allehdan SS, Mahfouz IA, Bawadi HAJ. Use of dietary supplements among pregnant women in the center of Jordan. NFS J. 2020;20:43–47.
33. Ramírez-Vélez R, Correa-Bautista JE, Triana-Reina HR, et al. Use of dietary supplements by pregnant women in Colombia. BMC Pregnancy Childbirth. 2018;18(1):1–8.
34. Saldanha LG, Dwyer JT, Andrews KW, et al. Is nutrient content and other label information for prescription prenatal supplements different from nonprescription products? J Acad Nutr Diet. 2017;117(9):1429–1436. doi:10.1016/j.jand.2017.04.002
35. Tang L, Lee AH, Yau KK, Hui YV, Binns CW, Nutrition C. Consumption of dietary supplements by Chinese women during pregnancy and postpartum: a prospective cohort study. Matern Child Nutr. 2017;13(4):e12435. doi:10.1111/mcn.12435
36. US Food and Drug Administration. Center for food safety and applied nutrition overview; 2001.
37. Branum AM, Bailey R, Singer BJ. Dietary supplement use and folate status during pregnancy in the United States. J Nutr. 2013;143(4):486–492. doi:10.3945/jn.112.169987
38. Knudsen VK, Hansen HS, Ovesen L, Mikkelsen TB, Olsen SF. Iron supplement use among Danish pregnant women. Public Health Nutr. 2007;10(10):1104–1110. doi:10.1017/S136898000769956X
39. Moran-Lev H, Bauer S, Farhi A, et al. Nutrition and the use of supplements in women during pregnancy: a cross-sectional survey. Food Nutr Bull. 2019;40(2):231–240. doi:10.1177/0379572119833857
40. Bärebring L, Mullally D, Glantz A, et al. Sociodemographic factors associated with dietary supplement use in early pregnancy in a Swedish cohort. Br J Nutr. 2018;119(1):90–95. doi:10.1017/S0007114517003270
41. Knapik A, Kocot K, Witek A, et al. Dietary supplementation usage by pregnant women in Silesia—population based study. Ginekol Pol. 2018;89(9):506–512. doi:10.5603/GP.a2018.0086
42. Aronsson CA, Vehik K, Yang J, et al. Use of dietary supplements in pregnant women in relation to sociodemographic factors–a report from The Environmental Determinants of Diabetes in the Young (TEDDY) study. Public Health Nutr. 2013;16(8):1390–1402. doi:10.1017/S1368980013000293
43. World Health Organization. WHO Recommendations on Antenatal Care for a Positive Pregnancy Experience. World Health Organization; 2016.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
